Abstract
Here, we describe the use of a fluorescence based lateral flow competition assay for the screening of four classes of drugs, viz, Δ9-tetrahydrocannabinol (THC), cocaine (through the detection of benzoylecgonine, BZE), opiates (through the detection of morphine, MOR) and amphetamine (AMP) present in the sweat of a fingerprint. The Drug Screening Cartridge was specifically developed for fingerprint sample collection and analysis. For this study, the cut-offs were set at: 190, 90, 68 and 80 pg/fingerprint for THC, BZE, MOR and AMP, respectively. Working with three UK coroners, the Drug Screening Cartridge, together with its fluorescence reader, was applied to the detection of drugs in the sweat of a fingerprint from deceased individuals. The study shows that there was sufficient sweat present on the fingertips to enable analysis and that the Drug Screening Cartridge could detect the presence, or absence, of each drug. The presence of the drugs was confirmed using LC–MS-MS analysis of a second fingerprint sample collected simultaneously. Excellent correlation was achieved between the results obtained from the Drug Screening Cartridge and the LC–MS-MS analysis of the fingerprint samples obtained from 75 individuals. The accuracy of the results was: 99% for THC; 95% for BZE; 96% for MOR and 93% for AMP. The results obtained using the Drug Screening Cartridge were also compared to toxicological analysis of blood and urine samples with good correlation. The accuracy of the results between the Drug Screening Cartridge and blood was: 96%, 92%, 88% and 97% for THC, BZE, MOR and AMP, respectively. The comparison with urine showed an accuracy ranging between 86% and 92%. This fingerprint sample method has a collection time of just 5 s and a total analysis time of <10 mins. These results show that the lateral flow Drug Screening Cartridge is an excellent screening test to provide information on drug use from the sweat in a single fingerprint sample.
Introduction
Drugs of abuse taken by an individual are typically detected by measurement of the analytes within urine (1), blood (2) or, more recently, saliva (3, 4). Alternative sample matrices include hair (5) and sweat, the latter collected using patches placed on the arm or back of an individual (3, 6–10). With these samples, the drugs of abuse are typically separated from the matrix using gas or liquid chromatography and then detected using mass spectrometry, GC–MS or LC–MS, respectively (1, 3, 6–11). Recently, it has been shown that drug metabolites can be detected in the sweat deposited in a latent fingerprint using antibody-nanoparticle conjugates (12–15). Drug metabolites that have been detected using such nanoparticle conjugates include, cotinine, from tobacco users (12, 14); benzoylecgonine from cocaine users (13, 15); morphine from heroin users (15); and methadone and its metabolite 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP) from patients undergoing drug dependency treatment (13). In addition, it has been shown that two drug metabolites, benzoylecgonine and morphine, can be detected in a single fingerprint from individuals using both cocaine and heroin (15). Subsequently, it was reported by Jickells et al., that methadone and EDDP could be quantified from fingerprints, obtained from patients receiving treatment for heroin dependency, that had been dissolved and then analysed using LC–MS-MS (16). The typical concentrations found were 0.90–9.20 and 0.07–0.08 ng per fingerprint for methadone and EDDP, respectively. The same group were able to detect lorazepam, a drug associated with drug facilitated sexual assault, in the fingerprints of healthy volunteers given a single 2-mg oral dose of the drug. Since the lorazepam dose was of a low concentration, ten fingerprints from each volunteer were combined and the drug, together with its glucuronide metabolite, were detected using LC–MS-MS at a concentration of 11 and 210 pg, respectively (17). Surface-assisted laser desorption/ionization (SALDI)-MS has been used to detect illicit drugs and their metabolites in exogenously doped fingerprints and those obtained from known drug users (18). Through the use of a hydrophobic silica powder to act as a SALDI enhancer, contact residues of codeine, diacetylmorphine (heroin), cocaine and products related to opium resin have all been detected in fingerprints (18). The SALDI–MS technique has also been used to detect methadone/EDDP and nicotine/cotinine in volunteer’s fingerprints (19). Desorption electrospray ionization (DESI)–MS has been used to image fingerprints through the detection of a molecular ion associated with either endogenous or exogenous (doped) species (20, 21). Endogenous species such as cis-hexadec-6-enoic acid, steric acid, cis-octadec-8-enoic acid, palmitic acid, pentadecyclic acid, myristic acid and triacylglycerols in sebum-rich fingerprints have all been used to image fingerprints (20). Additionally, fingerprints were imaged using molecular ions from cocaine, THC and the explosive trinitrohexahydro-1,3,5-triazine (RDX) that had been used to artificially dope fingers (20). In a proof of concept study, matrix-assisted laser desorption ionization (MALDI)–MS has been used where the endogenous compound, oleic acid, has been used to image a fingerprint (22). The techniques have been developed so that fingermarks found at crime scenes have been assessed for the presence of cocaine and THC (23). In related work, it has been shown that cocaine and its metabolites, benzoylecgonine and methylecgonine, can be detected from a single fingerprint of drug users using MALDI ion mobility tandem mass spectrometry (MALDI–IMS–MS-MS) and DESI–MS (24). Most recently, it has been shown that cocaine, benzoylecgonine and methylecgonine can be identified, with limits of detection of 1, 2 and 31 ng/mL, respectively, in single fingerprint samples using paper spray mass spectrometry (25).
An indirect competition immunoassay has been reported for the detection of cocaine in drug user samples (26). In this instance, the assay provided a quantitative measure of the concentration of cocaine in a fingerprint sample (ca. 0.3–0.9 ng/mL cocaine in a dissolved fingerprint). This concentration was then compared to the concentration found in saliva samples from the same individuals. Individuals with high concentrations of cocaine in saliva were found to have high concentrations present in the sweat deposited in a fingerprint, suggesting a direct relationship of the concentration of the cocaine in the two matrices (26). The methods used for the detection of drugs of abuse, their metabolites and other species in fingerprints have been reviewed (27–29).
In this present study we show, for the first time, that an innovative lateral flow device can enable the detection of four different drug classes from a single fingerprint sample in <10 mins. Working with three UK H.M. Coroners, the lateral flow device was applied to the detection of drugs of abuse in the sweat of a fingerprint from deceased individuals. The study shows that not only was there sufficient sweat present on the fingertips of the deceased individual to perform the analytical measurements but that the lateral flow device could detect the presence, or absence, of the four classes of drugs of abuse. The presence of the drugs was confirmed using LC–MS-MS analysis of a second fingerprint sample from the same individual. Finally, these analyses are compared to analysis of other bodily fluid samples, viz., blood and urine. The results highlight that the lateral flow device can rapidly screen for drug use in individuals using a fingerprint sample that is easy to collect and does not generate potentially toxic waste disposal problems. The study also shows that the lateral flow device can be used by coroners and their staff to assist in understanding the possible cause of death and to inform potential further post-mortem activities. Most importantly, this study shows that the lateral flow device can be used in a variety of situations to readily detect drugs of abuse in the sweat of a fingerprint from an individual.
Methods
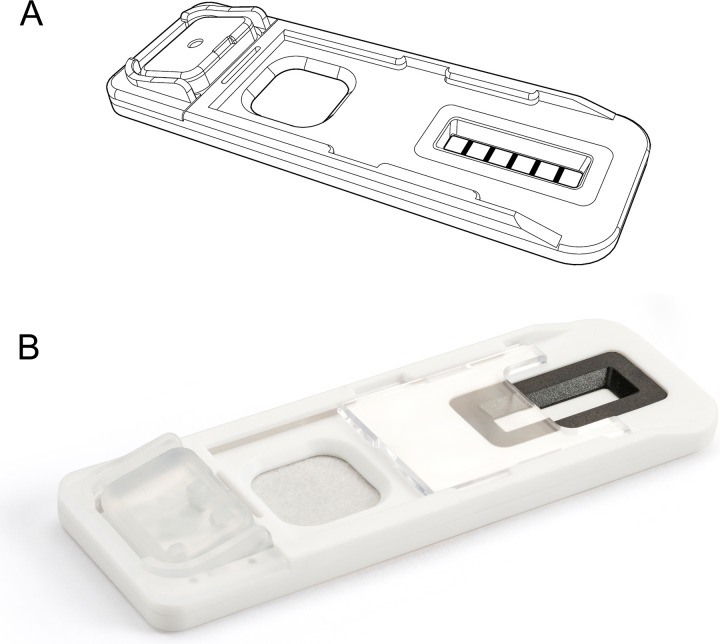
The lateral flow device is a screening test that can provide information on drug use from the analysis of the sweat from a collected fingerprint sample. Sweat samples were collected using a single use, disposable plastic Drug Screening Cartridge (Intelligent Fingerprinting Drug Screening Cartridge DOA114, Cambridge, UK; Figure 1).
Figure 1.
(A) Schematic diagram and (B) image showing the fingerprint Drug Screening Cartridge.
Sample collection and ethics
Each fingerprint sweat sample was collected by a mortuary staff member by pressing, using firm pressure, a fingertip of an individual onto the sample collection area of the cartridge for a period of 5 s. This time period enables transfer of the sweat from the fingertip onto the test collection area. The anti-tamper cover was used to protect the sample from contamination or adulteration. The Drug Screening Cartridges were configured to detect four drug classes: Δ9-tetrahydrocannabinol, cocaine (through detection of benzoylecgonine), opiates (through the detection of morphine), and amphetamine from a single fingerprint sample. Once collected, the sample was then analysed for information concerning drug use by fluorescence analysis using the Intelligent Fingerprinting Reader 1000 unit (IFR001; Intelligent Fingerprinting Ltd., Cambridge, UK) (Figure 2). A potential concern when detecting drugs in the sweat of a fingerprint is that the person may have handled or accidentally come into contact with a trace residue of a particular drug. When live subjects are tested, simply washing the hands with soap and water and drying them with paper towel removes trace residues. Sufficient sweat then appears after 10–15 min to obtain a sweat sample, which is not adulterated with contact residues, that can be used to detect whether the person has used one, or more, of the four drug classes. Obviously the hand-washing strategy is not possible with deceased individuals. By detecting the drug metabolite rather than the parent molecule partially overcomes the issue of a trace residue within the fingerprint sweat. Consequently, the metabolites of cocaine and heroin, BZE and morphine respectively, were detected. For THC and amphetamines the parent molecule was detected so accidental contact with these drugs prior to death may give a positive result. In all instances, the detection of the presence of drugs in the fingerprint sweat should be used as a screen, with all positive results confirmed using LC–MS-MS.
Figure 2.
The Intelligent Fingerprinting Reader 1000 Unit and the Drug Screening Cartridge.
The process for analysis of the collected fingerprint sweat sample is as follows. The Drug Screening Cartridge activation buffer clip is depressed (see Figure 1)—this releases buffer solution into the sample collection cartridge. The sample collection cartridge is then inserted into the Reader 1000 analysis tray, as shown in Figure 2, and after following instructions, as directed by the Reader 1000 touch-screen, the results of the analysis of the test are displayed on the screen within 8 min of initiating the test. The result of each test gives a pass/fail for the presence of each drug, based on a cut-off for each analyte. For this study, the cut-offs for each drug detected using the lateral flow device were set at 190 pg for THC, 90 pg for BZE, 68 pg for morphine and 80 pg for amphetamine. The results obtained using the Drug Screening Cartridge and Reader 1000 can be printed to provide a permanent record.
Fingerprint sweat samples were collected from three UK coroner areas involved in the study: Plymouth, Torbay & South Devon; South Yorkshire (West) and Staffordshire (South). Under the appropriate consented jurisdiction of each coroner, fingerprint sweat samples were collected by mortuary staff from routine mortuary case submissions. Mortuary staff were asked not to preselect cases to ensure a wide cross-section of individuals. The collected samples and all test results were anonymised, and a full chain of evidence continuity was maintained during sample collection and analysis. The time since death and sample collection varied between cases.
Each sample was analysed for the following drug groups: cannabis; cocaine; opiates and amphetamines. A total of three sets of analyte data were collected from each individual: (1) results of screening tests carried out within the mortuary, by mortuary staff, using the fingerprint Drug Screening Cartridge (in situ tests); (2) results of quantitative tests carried out by an independent third-party analytical laboratory on fingerprint samples collected in parallel with the screening tests—the confirmatory fingerprint was collected on a Collection Cartridge (Intelligent Fingerprinting DOA150, Cambridge UK) which was specifically designed for ease of analysis of a fingerprint using LC–MS-MS; (3) Results of quantitative tests carried out as part of post-mortem examination toxicology tests. The results of these three sets of data were used to assess the accuracy of the fingerprint Drug Screening Cartridge and reader.
Confirmatory analysis
As stated above, for confirmatory analysis of the presence of drugs in a fingerprint, a second fingerprint was collected from each individual. The second, confirmatory, fingerprint was collected on a Collection Cartridge by mortuary staff. This sample was then sealed and sent to an independent laboratory (LGC Ltd, Fordham, UK) for analysis. The confirmation analysis of the drugs present in the fingerprint samples was performed using a UHPLC–MS-MS system consisting of a Xevo-TQS mass spectrometer and Acquity I-class UPLC system, both from Waters Ltd (Elstree, UK). Mass spectral analysis was performed in positive electrospray mode (ESI) with analysis performed in multiple reaction monitoring mode (MRM) with a minimum of two MRM transitions selected for each analyte(s).
Results and Discussion
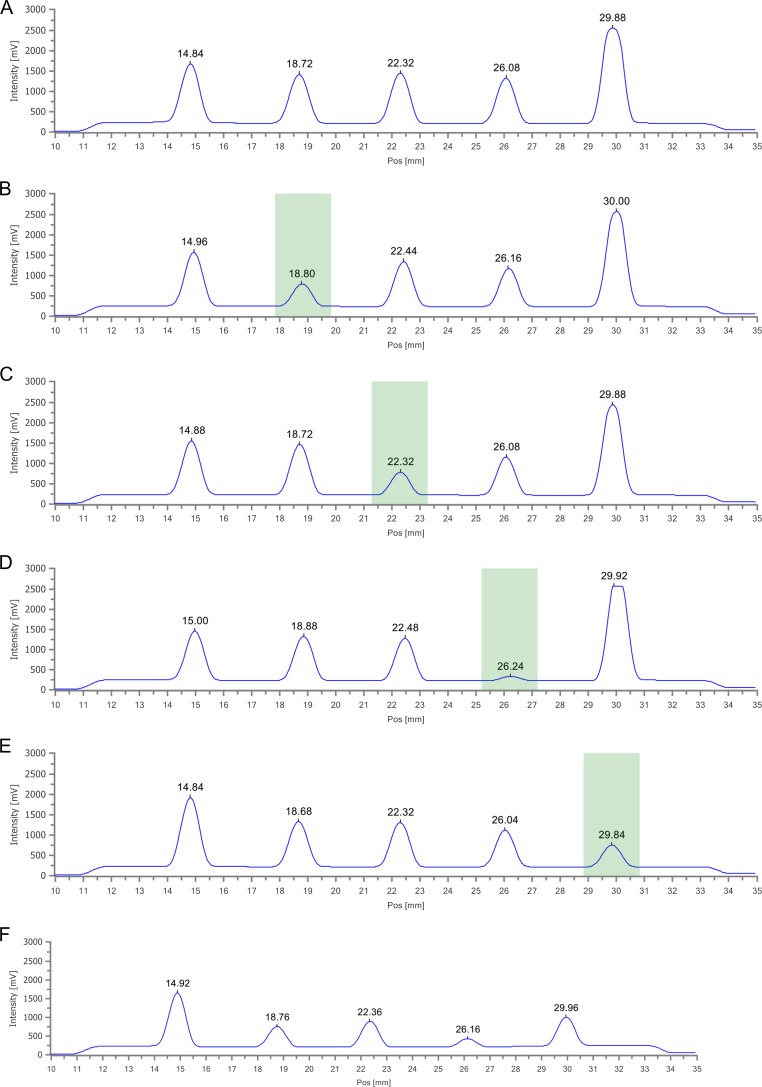
The lateral flow device is a fluorescence based competition assay. The device has four drug–bovine serum albumin (BSA) conjugate lines drawn on the nitrocellulose test strip together with a fifth line that acts as a control test to ensure the assay is functioning correctly. Fluorescently-tagged antibodies specific to each drug class are passed over the four drug-conjugate lines and the control (horse anti-mouse antibody, HAM). Upon binding to the four drug, plus the control, conjugate lines the fluorescently-tagged antibodies provide a fluorescent signal measured by the reader. A sample with no drug present gives a maximum fluorescence signal as the fluorescently-tagged antibody readily binds to the drug-conjugate line. If a drug is present in the sample, the drug will preferentially bind to the fluorescently-tagged antibody thereby preventing the antibody from binding to the drug-conjugate line. This results in a decrease in the fluorescence signal. Therefore, samples with a drug present will have a decreasing fluorescence signal with increasing concentration of the analyte. To assess the usability of the lateral flow device for the detection of the four classes of drugs, spiked solutions in 10 μl phosphate buffer solution (either individual drugs or combined) were used to challenge the lateral flow cartridge. Upon addition of the spiked solution, the test was activated, allowed to run for 8 min and then the cartridge was read using the fluorescence based reader. The results of these challenge experiments are shown in Figure 3. With each lateral flow cartridge, there are five peaks associated with the control (HAM), cannabis (THC), cocaine (BZE), opiates (MOR) and amphetamines (AMP), respectively (Figure 3a)—the respective peaks are at positions 14.8, 18.7, 22.3, 26.0 and 29.9 mm along the length of the lateral flow device. For this study, the cut-offs for each drug to be detected using the lateral flow device were set at 190 pg for THC, 90 pg for BZE, 68 pg for morphine and 80 pg for amphetamine. The cut-offs were determined by analysing the sweat of fingerprints from a total of 2,459 volunteers who were attending treatment programs at drug dependency clinics. Comparison of the data from the Drug Screening Cartridge and that from a confirmation fingerprint Collection cartridge analysed by LC–MS-MS determined the maximum efficiency to select the best assay performance. Upon applying a spiked sample to a cartridge, the respective peak decreases in fluorescence intensity. When a 240-pg THC spike was added to the phosphate buffer solution, the peak due to the THC conjugate at 18.8 mm decreased in intensity (Figure 3b). Similar decreases of specific peak intensity were observed when spiked samples of 90 pg benzoylecgonine, 70 pg of morphine and 190 pg of amphetamine were applied to separate cartridges—Figure 3C, D and E, respectively. When a buffer sample that contained all four drug spikes was applied to a cartridge, the fluorescence intensity of all four drug-conjugate lines was decreased. In all instances, the control HAM conjugate line remained at a constant fluorescence intensity. These results show that the lateral flow Drug Screening Cartridge can detect the presence of individual drugs, or all four drugs, present in a single sample. Considerable studies were undertaken to investigate the cross-reactivity of the antibodies used within the Drug Screening Cartridge. A total of 50 drug-related compounds were assessed for potential cross-reactivity with the four drug targets, while a further 44 compounds/materials that could potentially be found on fingers were assessed as possible interferences. The specificity value of > 94% that was obtained for all four drug classes with the mortuary screening tests (Table I, see below) highlights that the Drug Screening Cartridge is specific for all four drug classes.
Figure 3.
Fluorescence intensity profiles for the four drug panel lateral flow assay: Peaks due to the control (HAM), THC, BZE, MOR and AMP are at 14.8, 18.7, 22.3, 26 and 29.8 mm, respectively. (A) No spike, just buffer only, (B) 240 pg THC spike, (C) 90 pg BZE spike, (D) 70 pg MOR spike, (E) 190 pg AMP spike and (F) A combined spike showing signal decrease of all four peaks associated with the four drugs.
Table I.
Results of the mortuary screening tests using the Drug Screening Cartridge as compared to the independent laboratory LC–MS-MS detection of drugs in fingerprint samples. Results were obtained by collecting pairs of fingerprints in parallel
| Result | THC | Cocaine | Opiates | AMP |
|---|---|---|---|---|
| True Negative (TN) | 73 | 62 | 53 | 68 |
| True Positive (TP) | 1 | 9 | 19 | 2 |
| False Negative (FN) | 0 | 1 | 1 | 1 |
| False Positive (FP) | 1 | 3 | 2 | 4 |
| Number of Samples | 75 | 75 | 75 | 75 |
| Accuracy (%) | 98.7 | 94.7 | 96.0 | 93.3 |
| Sensitivity (%) | 100.0 | 90.0 | 95.0 | 66.7 |
| Specificity (%) | 98.6 | 95.4 | 96.4 | 94.4 |
| PPV (%) | 50.0 | 75.0 | 90.5 | 33.3 |
| NPV (%) | 100.0 | 98.4 | 98.1 | 98.6 |
Mortuary screening tests
The lateral flow screening device and reader were used to determine whether drugs of abuse were present, and could be detected, in the sweat that remained on the fingers of deceased individuals. For this study, the cut-offs for each drug in the fingerprint sweat detected using the lateral flow device were set at 190 pg for THC, 90 pg for BZE, 68 pg for morphine, and 80 pg for amphetamine. The tests were performed by mortuary staff who collected a fingerprint on a Drug Screening Cartridge (Intelligent Fingerprinting DOA114) from an individual and then a second fingerprint on a Collection Cartridge (Intelligent Fingerprinting DOA150), the latter being sent to an independent laboratory for confirmatory analysis using LC–MS-MS. Toxicology results were also obtained by the respective mortuary from each individual based on blood and urine samples. The results of the mortuary screening tests carried out with the fingerprint Drug Screening Cartridge in comparison with the results obtained using the LC–MS-MS analysis are summarized in Table I. In all instances, the LC–MS-MS results were assumed to be the correct result.
The equations used to calculate the analytical characterization data are (30):
Table I shows a summary of the analytical characterization data for the detection of the four classes of drugs using the lateral flow screening tests performed in the mortuary with the Drug Screening Cartridge in comparison to the results obtained from quantitative measurements of a second fingerprint, taken at the same time, that had been solubilised and then analyzed using LC–MS-MS. Samples from 75 individuals were collected by mortuary staff. In all cases, there was sufficient sweat on the fingers for staff to obtain a fingerprint and for a full analysis to be performed, using either the lateral flow device or the LC–MS-MS measurement. The subjects were chosen, at random, from routine mortuary case submissions. There was no pre-selection to bias the study towards individuals suspected to have died of an overdose. Therefore, it is not surprising that there are not a large number of positive samples. Opiates are the class of drug with the highest positive number of cases, i.e., 20. This is perhaps to be expected as morphine is invariably used for the palliative care of patients. The lateral flow Drug Screening Cartridge was able to successfully identify 19 of the 20 samples where opiates were found to be present in the sweat of a fingerprint. Nine individuals were found to have cocaine present in their fingerprint sweat using the lateral flow device, with only one false negative. THC and AMP were present in only limited numbers of samples (1 and 3, respectively) taken in this study.
For all four classes of drugs, the accuracy of the lateral flow Drug Screening Cartridge in comparison with the LC–MS-MS analysis gave high percentage values. The accuracy for THC, BZE, MOR and AMP was 98.7%, 94.7%, 96.0% and 93.3%, respectively, at the cut-offs of 190 pg/fingerprint for THC, 90 pg/fingerprint for BZE, 68 pg/fingerprint for morphine and 80 pg/fingerprint for amphetamine.
While the Drug Screening Cartridge gave an accuracy value of >93% for all four classes of drugs from the 75 individuals tested, there were some false negative results (THC, 0; cocaine, 1; opiates, 1; and amphetamines, 1) and some false positive results (THC, 1; cocaine, 3; opiates, 2; and amphetamines, 4). Lateral flow measurements are known not to be 100% accurate and therefore such measurements should only be used as a screen with LC–MS-MS confirmation of drug usage. The accuracy values obtained for the Drug Screening Cartridge do, however, compare favorably with the accuracy values obtained using lateral flow oral fluid based products. For example, the Cozart DDSTM device was reported to exhibit an accuracy that was either 80.0 or 89.9%, dependent on the cut-off used of either 30 or 10 ng/mL, respectively, for the detection of BZE in oral fluid samples (4).
The specificity of the lateral flow device gave values in excess of 94% for THC, cocaine, opiates and amphetamines showing that the Drug Screening Cartridge is specific for all four drug classes. The lateral flow device gave sensitivity values for THC (100%), BZE (90%) and opiates (95%). The sensitivity of AMP was less high, with a value of 66.7%. This lower value is likely due to the small number of true positive samples obtained for this drug. Similarly, the positive predictive value (PPV) results obtained were directly dependent on the numbers of positive samples analyzed. For the opiates, where 19 samples gave a true positive result, the PPV value was 90.5%. However, the PPV decreased for cocaine (75%), THC (50%), through to AMP (33.3%) as the numbers of true positive samples decreased (9, 1 and 2, respectively). Presumably, a larger number of positive samples for THC, BZE and AMP would provide a more accurate PPV. With large numbers of negative samples for each drug class, the negative predictive values (NPV) were high in each instance: THC (100.0%); cocaine (98.4%); opiates (98.1%) and AMP (98.6%).
The data in Table I clearly shows that the lateral flow Drug Screening Cartridge can be successfully used to detect drugs of abuse from the sweat on the fingers of deceased individuals with a strong correlation to the results obtained using LC–MS-MS analysis.
Comparison to toxicology results
The results of the mortuary screening tests performed by mortuary staff with the Drug Screening Cartridge and the fluorescence based reader as compared to the laboratory toxicology (blood) results are summarized in Table II. It should be noted that the sample numbers are variable as not all cases were submitted for blood toxicology. Table II shows that the accuracy of the fingerprint screening analysis as compared to laboratory toxicological data gives high values for all four classes of drugs. The accuracy values were: THC (96.1%); cocaine measured as BZE (92.2%); opiates measured as MOR (88.2%) and AMP (97.3%). The results show that the Drug Screening Cartridge analyses were comparable to those obtained by toxicological analysis. These results suggest that the pharmacokinetic excretion profiles of drugs and their metabolites in the sweat present on the fingertips may be similar to that in blood. A full pharmacokinetic study would be required to confirm this suggestion. However, the accuracy values shown in Table II do suggest that there is a good correlation between the data obtained with the Drug Screening Cartridge and those obtained with the blood toxicology.
Table II.
Results of the in situ mortuary drug screening tests as compared to laboratory-based toxicology blood analysis
| Result | THC | Cocaine | Opiates | AMP |
|---|---|---|---|---|
| True Negative (TN) | 49 | 44 | 36 | 36 |
| True Positive (TP) | 0 | 3 | 9 | 0 |
| False Negative (FN) | 0 | 0 | 3 | 0 |
| False Positive (FP) | 2 | 4 | 3 | 1 |
| Number of Samples | 51 | 51 | 51 | 37 |
| Accuracy (%) | 96.1 | 92.2 | 88.2 | 97.3 |
| Sensitivity (%) | 100.0 | 75.0 | ||
| Specificity (%) | 96.1 | 91.7 | 92.3 | 97.3 |
| PPV | 42.9 | 75.0 | ||
| NPV | 100.0 | 100.0 | 92.3 | 100.0 |
Note: The number of samples are variable as not all cases were submitted for (blood) toxicology analysis.
The comparison of the results obtained using the Drug Screening Cartridge versus those of the blood toxicology gave an accuracy of >88% for all four drug classes. There were some false negative (FN) and false positive (FP) results: FN results (THC, 0; cocaine, 1; opiates, 1; and amphetamines, 1) and FP results (THC, 1; cocaine, 3; opiates, 2; and amphetamines, 4). The difference of the accuracy obtained with the Drug Screening Cartridge and the LC–MS-MS analysis of the second fingerprint versus the comparison with blood toxicology may be due to the drug excretion pathway differences between fingerprint sweat and blood. The specificity of the Drug Screening Cartridge gave values of: THC (96.1%); cocaine (91.7%); opiates (92.3%); and AMP (97.3%). For the sensitivity measurements, there were not many true positive samples—so the sensitivity of both THC and AMP could not be measured. The sensitivity for cocaine and opiates was 100% and 75%, respectively. Similarly, the PPV results of THC and AMP were affected by the absence of true positive samples. The PPV results of cocaine and opiates were 42.9% and 75%, respectively. The NPV results were good with values above 92% for all four classes of drugs.
As a further examination of the performance of the lateral flow device, a comparison was made of the analytical results obtained using the Drug Screening Cartridge with the toxicological data from urine samples. The results are shown in Table III. Fewer samples were submitted for urine toxicology analysis, for example, there were no samples analyzed for THC. However, while there are fewer samples, the analytical data, as shown in Table III, for the comparison of the Drug Screening Cartridge with the urine toxicological analysis are favorable. While the correlation of the results between the Drug Screening Cartridge and the blood toxicology is particularly strong, the comparison between the cartridge and the urine toxicology is also good for accuracy, sensitivity and specificity. It would be expected that the pharmacokinetic excretion profiles of the four classes of drugs in urine and fingerprint sweat matrices would be different. The pharmacokinetic differences between sweat and urine excretion may account for the false negative and false positive results observed in Table III. A full pharmacokinetic comparison of these four classes of drugs in fingerprint sweat in comparison to other matrices such as blood, urine and oral fluid should be performed to establish their excretion profile. However, our results show that there is a particularly strong correlation between the Drug Screening Cartridge and blood toxicology with a reasonable correlation with the data obtained using the cartridge in comparison with urine toxicology.
Table III.
Results of the in situ mortuary drug screening tests as compared to laboratory-based toxicology urine analysis
| Result | THC | Cocaine | Opiates | AMP |
|---|---|---|---|---|
| True Negative (TN) | 29 | 24 | 24 | |
| True Positive (TP) | 4 | 7 | 0 | |
| False Negative (FN) | 0 | 3 | 1 | |
| False Positive (FP) | 3 | 2 | 1 | |
| Number of Samples | 0 | 36 | 36 | 26 |
| Accuracy (%) | 91.7 | 86.1 | 92.3 | |
| Sensitivity (%) | 100.0 | 70.0 | ||
| Specificity (%) | 90.6 | 92.3 | 96.0 | |
| PPV | 57.1 | 77.8 | ||
| NPV | 100.0 | 88.9 | 96.0 |
Note: The sample numbers are variable as not all cases were submitted for (urine) toxicology analysis.
Conclusions
In this study we have shown that a fluorescence based lateral flow Drug Screening Cartridge can detect four different drug classes (THC, cocaine, opiates and amphetamines) from a single fingerprint sample. Working with three UK coroners, the Drug Screening Cartridge, together with its fluorescence reader, was applied to the detection of the four drug types in the sweat of a fingerprint sample taken, by mortuary staff, from deceased individuals. The study shows that not only was there sufficient sweat present on the fingertips of deceased individuals to enable analysis but that the lateral flow device could detect the presence, or absence, of all four classes of drugs within a single fingerprint. The presence of the drugs was confirmed using LC–MS-MS analysis of a second fingerprint sample taken at the same time. Excellent correlation, in terms of accuracy, sensitivity and selectivity, was achieved between the results obtained from the Drug Screening Cartridge and the LC–MS-MS analysis of the fingerprint samples obtained from 75 deceased individuals. The accuracy of the results ranged from 93 to 99% for the four drug classes. The results obtained using the Drug Screening Cartridge analyses have been also compared to toxicological analysis of blood and urine samples. Good correlation of results was obtained using the Drug Screening Cartridge and the laboratory-based toxicology data for both blood and urine samples. The accuracy of the results between the lateral flow Drug Screening Cartridge and blood ranged from 88 to 97%, while the comparison for urine showed an accuracy of 86–92%, suggesting that there is a stronger correlation between the results obtained using the lateral flow cartridge of the fingerprint sweat and the blood toxicology. The differences between the two sample matrices is likely to reflect the differing pharmacokinetic excretion profiles of the four classes of drugs.
Overall, the results highlight that the lateral flow Drug Screening Cartridge can rapidly screen for drug use in individuals using a fingerprint sample that is easy to collect, with a sample collection time of just 5 s and a total analysis time of less than 10 min. The study shows that the lateral flow device can be used by coroners and their staff to assist in understanding the possible cause of death and to inform potential further post-mortem activities. However, with consideration of the wider impact of this research, this study shows that the lateral flow Drug Collection Cartridge can be used to readily detect drug usage by individuals through direct measurement from the sweat of a single fingerprint. The utility of this work is highlighted by the current use of Drug Screening Cartridge and reader in Coroner’s mortuaries, in drug rehabilitation centers, work place testing and within high schools. Additionally, proof of concept studies are currently being performed for airport screening and within offender management in prisons and probation services.
Acknowledgments
The authors would like to thank the H.M. Senior Coroners: Christopher Dorries, South Yorkshire (West); Andrew A. Haigh, Staffordshire (South); and Ian M. Arrow, Plymouth, Torbay and South Devon; and their mortuary staff who collected all of the fingerprint, blood and urine samples that were analyzed as part of this study. The authors acknowledge the Innovate UK Biomedical Catalyst grant scheme for financial support of the pilot phase of this work.
References
- 1. Osselton M.D. Urinalysis: the detection of common drugs in urine. In: Wolff K. (ed).. Detection of Drug Misuse - Biomarkers, Analytical Advances and Interpretation. Royal Society of Chemistry: Cambridge, U.K, 2017; pp. 3–22. [Google Scholar]
- 2. Moeller M.R., Steinmeyer S., Kraemer T. (1998) Determination of drugs of abuse in blood. Journal of Chromatography B: Biomedical Sciences and Applications, 713, 91–109. [DOI] [PubMed] [Google Scholar]
- 3. Kidwell D.A., Holland J.C., Athanaselis S. (1998) Testing for drugs of abuse in saliva and sweat. Journal of Chromatography B: Biomedical Sciences and Applications, 713, 111–135. [DOI] [PubMed] [Google Scholar]
- 4. Scherer J.N., Fiorentin T.R., Sousa T.R.V., Limberger R.P., Pechansky F. (2017) Oral fluid testing for cocaine: Analytical evaluation of two point-of-collection drug screening devices. Journal of Analytical Toxicology, 41, 392–398. [DOI] [PubMed] [Google Scholar]
- 5. Cave D.M., Kingston R. Hair testing in forensic toxicology. In: Davies S., Johnston A., Holt D. (eds).. Forensic Toxicology: Drug Use and Misuse. Royal Society of Chemistry: Cambridge, U.K, 2016; pp. 411–437. [Google Scholar]
- 6. Schwilke E.W., Barnes A.J., Kacinko S.L., Cone E.J., Moolchan E.T., Huestis M.A. (2006) Oral disposition in human sweat after controlled oral codeine administration. Clinical Chemistry, 52, 1539–1545. [DOI] [PubMed] [Google Scholar]
- 7. Cone E.J., Hillsgrove M.J., Jenkins A.J., Keenan R.M., Darwin W.D. (1994) Sweat testing for heroin, cocaine and metabolites. Journal of Analytical Toxicology, 18, 298–305. [DOI] [PubMed] [Google Scholar]
- 8. Huestis M.A., Cone E.J., Wong C.J., Umbricht A., Preston K.L. (2000) Monitoring opiate use in substance abuse treatment patients with sweat and urine drug testing. Journal of Analytical Toxicology, 24, 509–521. [DOI] [PubMed] [Google Scholar]
- 9. Preston K.L., Huestis M.A., Wong C.J., Umbricht A., Goldberger B.A., Cone E.J. (1999) Monitoring cocaine use in substance-abuse-treatment patients by sweat and urine testing. Journal of Analytical Toxicology, 23, 313–322. [DOI] [PubMed] [Google Scholar]
- 10. Kacinko S.L., Barnes A.J., Schwilke E.W., Cone E.J., Moolchan E.T., Huestis M.A. (2005) Disposition of cocaine and its metabolites in human sweat after controlled cocaine administration. Clinical Chemistry, 51, 2085–2094. [DOI] [PubMed] [Google Scholar]
- 11. Saito T., Wtsadik A., Scheidweiler K.B., Fortner N., Takeichi S., Huestis M.A. (2004) Validated gas-chromatographic-negative ion chemical ionization mass spectrometric method for Δ9-tetrahydrocannabinol in sweat patches. Clinical Chemistry, 50, 2083–2090. [DOI] [PubMed] [Google Scholar]
- 12. Leggett R., Lee-Smith E.E., Jickells S.M., Russell D.A. (2007) “Intelligent” fingerprinting: simultaneous identification of drug metabolites and individuals by using antibody-functionalized nanoparticles. Angewandte Chemie International Edition, 46, 4100–4103. [DOI] [PubMed] [Google Scholar]
- 13. Hazarika P., Jickells S.M., Wolff K., Russell D.A. (2008) Imaging of latent fingerprints through the detection of drugs and metabolites. Angewandte Chemie International Edition, 47, 10167–10170. [DOI] [PubMed] [Google Scholar]
- 14. Hazarika P., Jickells S.M., Russell D.A. (2009) Rapid detection of drug metabolites in latent fingerprints. Analyst, 134, 93–96. [DOI] [PubMed] [Google Scholar]
- 15. Hazarika P., Jickells S.M., Wolff K., Russell D.A. (2010) Multiplexed detection of metabolites of narcotic drugs from a single latent fingermark. Analytical Chemistry, 82, 9150–9154. [DOI] [PubMed] [Google Scholar]
- 16. Jacob S., Jickells S., Wolff K., Smith N. (2008) Drug testing by chemical analysis of fingerprint deposits from methadone-maintained opioid dependent patients using UPLC-MS/MS. Drug Metabolism Letters, 2, 245–247. [DOI] [PubMed] [Google Scholar]
- 17. Goucher E., Kicman A., Smith N., Jickells S. (2009) The detection and quantification of lorazepam and its 3-O-glucuronide in fingerprint deposits by LC-MS/MS. Journal Separation Science, 32, 2266–2272. [DOI] [PubMed] [Google Scholar]
- 18. Rowell F., Hudson K., Seviour J. (2009) Detection of drugs and their metabolites in dusted latent fingermarks by mass spectrometry. Analyst, 134, 701–707. [DOI] [PubMed] [Google Scholar]
- 19. Benton M., Chua M.J., Gu F., Rowell F., Ma J. (2010) Environmental nicotine contamination in latent fingermarks from smoker contacts and passive smoking. Forensic Science International, 200, 28–34. [DOI] [PubMed] [Google Scholar]
- 20. Ifa D.R., Manicke N.E., Dill A.L., Cooks R.G. (2008) Latent fingerprint chemical imaging by mass spectrometry. Science (New York, N.Y.), 321, 805. [DOI] [PubMed] [Google Scholar]
- 21. Ifa D.R., Jackson A.U., Paglia G., Cooks R.G. (2009) Forensic applications of ambient ionization mass spectrometry. Analytical Bioanalytical Chemistry, 394, 1995–2008. [DOI] [PubMed] [Google Scholar]
- 22. Wolstenholme R., Bradshaw R., Clench M.R., Francese S. (2009) Study of latent fingermarks by matrix-assisted laser desorption/ionisation mass spectrometry imaging of endogenous lipids. Rapid Communications in Mass Spectrometry, 23, 3031–3039. [DOI] [PubMed] [Google Scholar]
- 23. Bradshaw R., Dennison N., Francese S. (2017) Implementation of MALDI MS profiling and imaging methods for the analysis of real crime scene fingermarks. Analyst, 142, 1581–1590. [DOI] [PubMed] [Google Scholar]
- 24. Bailey M.J., Bradshaw R., Francese S., Salter T.L., Costa C., Ismail M., et al. (2015) Rapid detection of cocaine, benzoylecgonine and methylecgonine in fingerprints using surface mass spectrometry. Analyst, 140, 6254–6259. [DOI] [PubMed] [Google Scholar]
- 25. Costa C., Webb R., Palitsin V., Ismail M., de Puit M., Atkinson S., et al. (2017) Rapid, secure drug testing using fingerprint development and paper spray mass spectrometry. Clinical Chemistry, 63, 1745–1752. [DOI] [PubMed] [Google Scholar]
- 26. van der Heide S., García Calavia P., Hardwick S., Hudson S., Wolff K., Russell D.A. (2015) A competitive enzyme immunoassay for the quantitative detection of cocaine from banknotes and latent fingermarks. Forensic Science International, 250, 1–7. [DOI] [PubMed] [Google Scholar]
- 27. Hazarika P., Russell D.A. (2012) Advances in fingerprint analysis. Angewandte Chemie International Edition, 51, 3524–3531. [DOI] [PubMed] [Google Scholar]
- 28. Wei Q., Zhang M., Ogorevc B., Zhang X. (2016) Recent advances in the chemical imaging of human fingermarks (a review). Analyst, 141, 6172–6189. [DOI] [PubMed] [Google Scholar]
- 29. Bécue A. (2016) Emerging fields in fingermark (meta)detection—a critical review. Analytical Methods, 8, 7983–8003. [Google Scholar]
- 30. Verstraete A.G. The results of the roadside drug testing assessment project. In: Wong R.C., Tse H.Y. (eds).. Drugs of Abuse—Body Fluid Testing. Humana Press: New Jersey, U.S.A., 2005; pp. 271–292. [Google Scholar]