Abstract
Background
Optimizing preoperative anemia is a required component of the Joint Commission Patient Blood Management Certification and an important component of Enhanced Recovery After Surgery.
Objective
To describe a preoperative anemia protocol developed and implemented at the Kaiser Permanente San Jose Medical Center in California to facilitate preoperative identification and treatment of anemia.
Methods
The protocol included all operations at risk of causing substantial blood loss. It excluded emergent operations and those for which the patient had a normal last hemoglobin value within the prior 12 months unless newly developed anemia was suspected. Eligible patients were screened for laboratory evaluation, and those with anemia were treated for reversible causes. Consistency was ensured by physician, staff, and patient education, and by use of electronic health records. Administration of intravenous iron and erythropoietin and consultation with specialists were expedited as part of a management algorithm.
Results
Among 510 patients enrolled during 1 year, 442 (87%) received anemia screening laboratory tests. Half of those with laboratory results were eligible for further optimization: 207 had anemia and 21 had iron deficiency without anemia. Among the 228 patients eligible for optimization, 189 (83%) had anemia addressed preoperatively. Of 129 patients with iron deficiency anemia, 102 (79%) received intravenous iron preoperatively, with a mean preoperative increase in hemoglobin level by 0.98 g/dL (n = 79).
Conclusion
Integration of specialty services, optimization of technology, and consistency across practitioners were crucial for successful implementation and sustainability of a preoperative anemia protocol developed to expedite and enhance best practices.
Keywords: anemia, best practices, blood conservation, enhanced recovery after surgery, ERAS, intravenous iron, iron deficiency, National Surgical Quality Improvement Program, NSQIP, patient blood management, perioperative anemia management, protocol, surgery
INTRODUCTION
Preoperative anemia is a common yet modifiable condition associated with increased perioperative morbidity, mortality, and blood transfusions.1,2 In the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database of more than 200,000 patients undergoing major noncardiac operations, the incidence of preoperative anemia was 30%.3 Postoperative mortality and morbidity at 30 days were higher in patients with anemia, with consistent differences for varying degrees of anemia. Anemia, when present along with any other known preoperative risk factor, led to a synergistically greater than 10-fold negative effect on outcome.3 The European Surgical Outcomes Study group, in a secondary analysis study of more than 39,000 patients, showed that those with severe or moderate preoperative anemia had significantly longer length of stay and higher rates of inhospital mortality and postoperative admission to the intensive care unit.4
Patient blood management consists of the following 3 pillars: First, identification and treatment of preoperative anemia; second, minimization of surgical blood loss; and third, consideration for the appropriateness of transfusions. Preoperative anemia is associated with poor patient outcomes independent of blood transfusions, and poor outcomes are amplified in transfusion recipients. Prior randomized clinical trials of red blood cell transfusion strategies have demonstrated improved mortality and morbidity with restrictive strategies in medical patients, including patients with upper gastrointestinal (GI) tract bleeding,5,6 and the noninferiority of restrictive strategies in patients with hip fracture and known cardiovascular risk factors.7 Successfully addressing the first pillar with an associated reduction in blood transfusion would support the utility of a preoperative anemia protocol.
Although intravenous (IV) iron has been suggested as a transfusion-sparing strategy in surgical patients,8 it was previously not a part of routine preoperative practice. IV iron infusions, especially the newer-generation formulations, have previously been shown to be safe and efficacious. A recent large systematic review and meta-analysis of all IV iron preparations did not show any increase in severe adverse events or infection rates.9 When high-molecular-weight iron dextran is avoided, IV iron is safe, with an estimated serious adverse event incidence of less than 1:200,000.10 Oral iron often does not improve anemia in preoperative patients because of the high prevalence of functional iron deficiency, poor compliance owing to its common GI side effects, and poor absorption caused by inflammatory bowel disease, celiac disease, Helicobacter pylori infection, history of gastric bypass or small-bowel resections, or concomitant use of antacids or proton pump inhibitors. Even in cases of good oral iron absorption, oral repletion generally takes 3 to 6 months,8 a lengthy time frame often not feasible in the preoperative patient. Additionally, oral iron replacement may not be adequate for those with ongoing bleeding issues before the operation.8
Although evidence points to a need for comprehensive preoperative anemia programs, few such programs exist in the US. Efficient methods for early identification and timely treatment of preoperative anemia are lacking. Many patients with anemia proceed directly to an operation with minimal to no optimization.1 Mild anemia, especially when chronic, is often not recognized because of a tendency to normalize these frequently seen but abnormal laboratory findings. Moderate to severe anemia is also frequently not addressed preoperatively because of the lack of lead time between identification and the operation date, resulting in few options for optimization other than last-minute surgery cancellation. To address these gaps, we have developed and implemented a preoperative anemia protocol to enhance the early recognition and timely treatment of anemia.
METHODS
Setting
Kaiser Permanente (KP) is a large integrated health care delivery system in the US with a physician-led corporation, health insurance partner, and hospitals and clinics for coordinating inpatient and outpatient care. The KP San Jose Medical Center in California has 242 licensed hospital beds. In 2016, a total of 6683 Ambulatory Surgery Unit and 5048 hospital operating room cases, including a large variety of general and specialty operations, were performed. The KP San Jose site is a regional referral center for spine, colorectal, hepatobiliary, and hand reconstructive operations but does not perform bariatric or cardiothoracic operations. At the time of this study, KP San Jose’s Perioperative Medicine Clinic staffing included 3 full-time equivalent (FTE) physicians, 1 FTE nurse practitioner, 1.2 FTE registered nurses, 1.8 FTE medical assistants, and 1 FTE clerk for reception and schedule maintenance.
Study Cohort
The study included all adults (≥ 18 years of age) eligible for the preoperative anemia protocol in a 1-year period between June 1, 2016 and May 31, 2017 who underwent evaluation at the KP San Jose Perioperative Medicine Clinic. The protocol was first instituted on January 25, 2016 with numerous initial adjustments and standardizations made on the basis of clinical experience. An initial washout implementation period was intentionally excluded from data analysis.
Preoperative Anemia Protocol
In 2015, KP San Jose established a Preoperative Anemia Steering Committee with the objective to design and implement a protocol that would enhance the early recognition and timely treatment of anemia. Through an iterative process with input from the Surgery, Anesthesia, Perioperative Medicine, Nephrology, Gastroenterology, and Hematology Departments, a protocol was developed to identify patients with anemia preoperatively and to recommend a progression of evidence-based interventions.
Inclusion and Exclusion Criteria
Included were all operations at risk of causing substantial blood loss at KP San Jose—defined as hysterectomies, all bowel operations excluding appendectomy, all solid organ resections excluding prostate, and included Whipple procedures, mastectomies only if accompanied by reconstruction, all hip and knee arthroplasties, all multilevel spine operations, and all major vascular surgeries including carotid, femoral popliteal bypass, and abdominal aortic aneurysm (open or endovascular) procedures. Exclusion criteria were age younger than 18 years, emergent operations (within 48 hours of case request and operation during current hospitalization), and patients with a last hemoglobin value within normal limits in the past 12 months before surgical case creation.
Protocol Case Identification
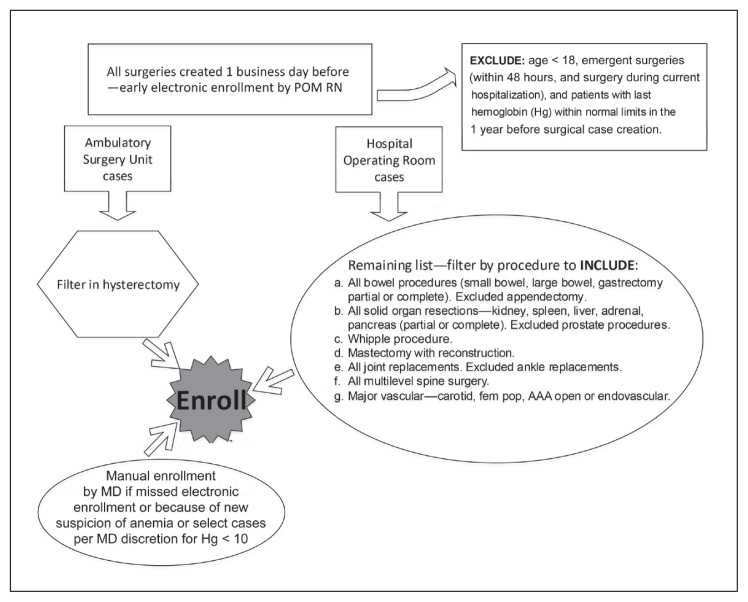
A daily, encrypted patient spreadsheet report was developed to extract surgical cases created the previous day, which were then filtered by inclusion and exclusion criteria to capture major operations at risk of causing substantial blood loss and patients who were more likely to have anemia. Filterable fields included patient’s sex and last hemoglobin value as well as operation type, location, and date (Figure 1). Electronic identification of patients was conducted each business day by a perioperative medicine nurse, and subsequent manual identification of patients was conducted by a perioperative medicine physician during routine preoperative evaluations. Manual identification included cases missed by screening because of an evaluation by the perioperative medicine physician scheduled on the day of case creation, cases missed on the spreadsheet because of case request from surgeons at a separate KP facility, and cases enrolled per physician discretion (eg, if a recent bleeding history warranted a suspicion of anemia development despite the last known hemoglobin level within 1 year to be within normal limits).
Figure 1.
Enrollment, spreadsheet report, and filters.
AAA = abdominal aortic aneurysm; fem pop = femoral popliteal; MD = physician; POM RN = perioperative medicine registered nurse.
Protocol Interventions
At the time that the preoperative anemia protocol went live on January 25, 2016, all KP San Jose perioperative medicine physicians, nurses, and staff were trained regarding implementation. Committee members held periodic meetings to discuss any protocol issues and revisions. Ongoing continuing medical education and electronic health record (EHR) training sessions were held to improve physician education and adherence to the protocol.
Protocol definitions of anemia and iron deficiency, and recommendations for IV iron therapy, were adopted from previously published guidelines.11,12 The World Health Organization definition of anemia for men is hemoglobin level below 13 g/dL and for women is hemoglobin level below 12 g/dL. A trial of IV iron intervention is indicated in both of the following groups: 1) absolute iron deficiency: Transferrin saturation less than 20% and/or ferritin level below 30 ng/mL and 2) functional iron deficiency: Transferrin saturation less than 20% or ferritin level below 100 ng/mL. Absolute iron deficiency refers to the presence of low iron stores, and functional iron deficiency refers to insufficient mobilization of iron in the presence of normal or elevated iron stores. Iron deficiency without anemia refers to a reduction in total body iron with normal hemoglobin level and was defined as the absence of anemia, hemoglobin level of 13 g/dL and higher for men and hemoglobin level of 12 g/dL and higher for women, in addition to a ferritin level below 22 ng/mL or transferrin saturation below 14% using KP San Jose laboratory references of lower than normal range.
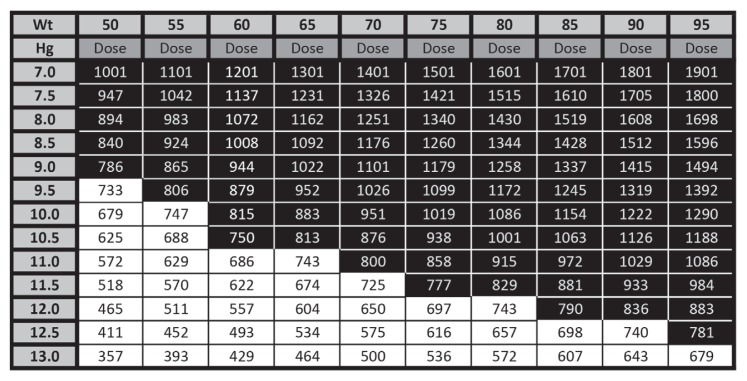
Patients with abnormal laboratory findings according to the anemia protocol were scheduled for an anemia evaluation with a perioperative medicine physician. These anemia-focused appointments were generally up to 30-minute telephone evaluations done before and in addition to the patient’s usual preoperative evaluations. Spreadsheet filters and electronic patient lists were developed to enroll and track patients. EHR tools, including order preference lists and electronic note templates with built-in decision guidance, were developed to document and guide anemia management to ensure consistency and completeness. Creation of standing orders for staff helped with physician workload. An IV iron table was created using the Ganzoni formula to simplify iron replacement calculations (Figure 2).
Figure 2.
Intravenous (IV) iron table to calculate dose of ferric carboxymaltose on the basis of body weight (Wt) and starting hemoglobin (Hg).a
a Black shading indicates 2 doses of 750 mg of IV ferric carboxymaltose administered at 1 week apart. White shading indicates 1 dose of 750 mg of IV ferric carboxymaltose. Hemoglobin (Hg) concentration to be corrected to 14 g/dL. Iron to replenish stores = 5 mg/kg. Iron deficit (mg) = body weight (kg) × (14 − Hg) × (2.145) + iron to replenish stores (mg).
The IV iron infusions took place in our infusion center. Perioperative medicine physicians ordered ferric carboxymaltose and electronically routed the patient’s chart to the infusion center. The infusion center was committed to schedule and administer IV iron within 2 business days of request, sometimes on the same day. For monitoring of erythropoiesis, all patients who received IV iron were to have a complete blood cell count and reticulocyte count obtained on the day of operation, just before surgical incision. The minimum target hemoglobin level was 11 g/dL for total joint replacements and 10 g/dL for all other operations in both men and women. These cutoffs were selected per facility agreements and were supported by a prior NSQIP study, which showed that moderate to severe anemia, defined as hematocrit below 29%, was associated with worse outcomes than mild anemia.3 For patients with a hemoglobin level below target with elective procedures, a follow-up hemoglobin value and reticulocyte count were tracked every 2 weeks until the minimum hemoglobin target was reached before the surgeon proceeded with the operation.
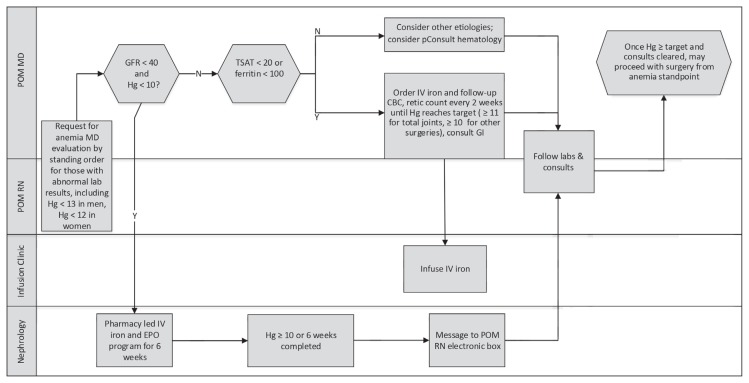
The preoperative anemia protocol included algorithms to identify and optimize preoperative anemia expeditiously to avoid surgery delay. Interventions included IV iron therapy, erythropoietin injections, gastroenterology and hematology consultations, and a consideration for the delay of elective operation when indicated (Figures 3–5). Erythropoietin was used only in anemic patients with a glomerular filtration rate less than 40 and in those with anemia of chronic disease with a hemoglobin level below 11 g/dL undergoing joint replacement or those with a hemoglobin level below 10 g/dL undergoing multilevel spine operations. A pharmacy-led erythropoietin and IV iron sucrose program for treatment of anemia of renal disease was condensed to 6 weeks. Gastroenterology consultants were committed to provide expedited evaluations, including endoscopy if needed, within 1 week of referral for suspected GI tract blood loss.
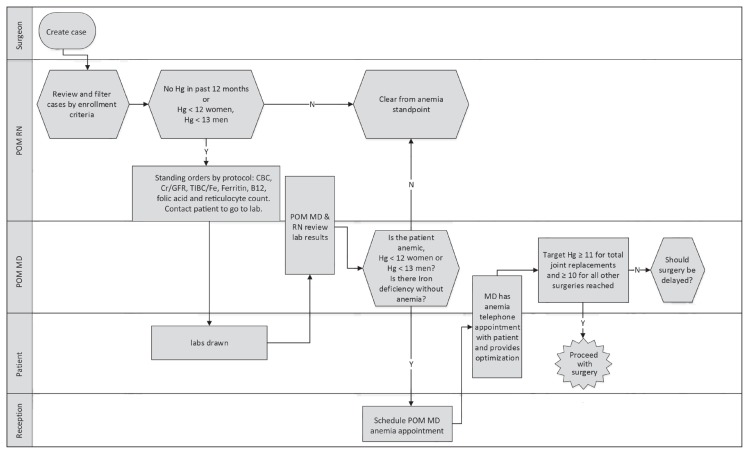
Figure 3.
Swim-lane diagram of preoperative anemia screen workflow.
B12 = vitamin B12; CBC = complete blood cell count; Cr/GFR = creatinine/glomerular filtration rate; Hg = hemoglobin (g/dL); lab = laboratory; labs = laboratory blood samples; MD = physician; N = no, POM MD = perioperative medicine physician; POM RN = perioperative medicine nurse; RN = registered nurse; TIBC/Fe = total iron-binding capacity/iron; Y = Yes.
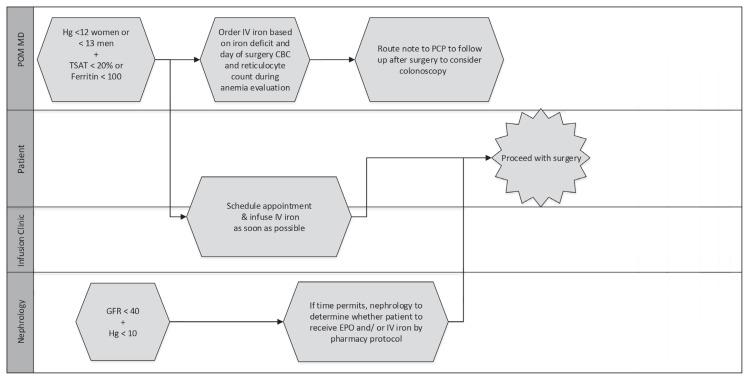
Figure 4.
Swim-lane diagram of anemia workflow—surgery cannot be delayed.
CBC = complete blood cell count; EPO = erythropoietin; GFR = glomerular filtration rate; Hg = hemoglobin (g/dL); IV = intravenous; PCP = primary care physician; POM MD = perioperative medicine physician; TSAT = transferrin saturation.
Figure 5.
Swim-lane diagram of anemia workflow—surgery can be delayed (elective).
CBC = complete blood cell count; EPO = erythropoietin; GFR = glomerular filtration rate; GI = gastroenterologist; Hg = hemoglobin (g/dL); IV = intravenous; lab = laboratory test; labs = laboratory blood samples; MD = physician; N = no; pConsult = curbside or telephone consultations; POM = perioperative medicine; retic = reticulocyte count; RN = registered nurse; TSAT = transferrin saturation (%); Y = yes.
Statistical Analysis
Continuous variables were reported as means for normally distributed variables and as median values for nonnormally distributed variables. These data were analyzed using the Student t-test, analysis of variance, or the Kruskal-Wallis equality-of-populations rank test as appropriate. Categorical variables were reported as percentages and analyzed using the χ2 or Fisher exact tests. All analyses were performed using SAS Version 9.3 (SAS Institute, Cary, NC).
Approval for the study was obtained from institutional review boards of KP Northern California (KPNC). A waiver of informed consent was obtained because this cohort study was retrospective.
RESULTS
The incidence of preoperative anemia was 20% for all operations and 22% for those who were admitted to the hospital postoperatively according to KP San Jose 2014 data. The admitted group was older, had more complex medical issues, and underwent more complex operations.
A cohort of 510 operations during 1 year, from June 1, 2016 to May 31, 2017, was enrolled in the preoperative anemia protocol. For patients with multiple qualifying operations, only the first operation was counted to avoid confounding postoperative anemia.
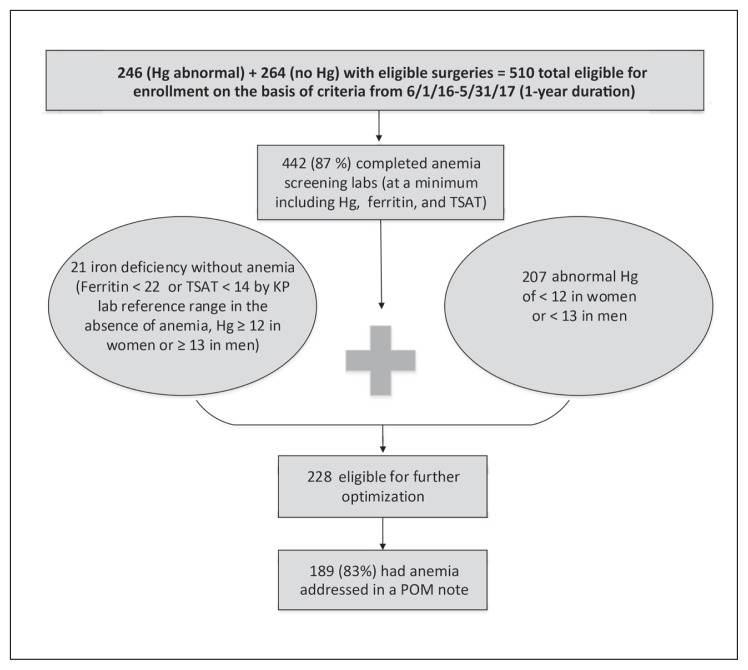
Baseline characteristics of the cohort showed a mean age of 60.2 years, median operating room time of 1.83 hours, 56.00% women, and racial distribution of 52.00% white, 5.60% African American, 22.55% Hispanic, 14.31% Asian/Pacific Islander, and 4.90% other races. American Society of Anesthesiologists classes 2 and 3 occurred in 67.45% and 27.25% of the cohort, respectively. Operations included 168 multilevel spinal operations (32.94%), 106 total knee arthroplasties (20.78%), 66 total hip arthroplasties (12.94%), 77 hysterectomies (15.10%), 24 mastectomies with reconstruction (4.71%), 8 Whipple procedures (1.57%), 5 nephrectomies (0.98%), 3 abdominal aortic aneurysm repairs (0.59%), and 53 other operations (10.39%). Anemia screening laboratory blood draw occurred at a median time of 28.5 days before the operation (Table 1). Most of the cohort, 442 patients (87%), received anemia screening laboratory tests, with half of those meeting criteria for further optimization (Figure 6). Patients counted as missing screening laboratory tests included those who had anemia-related laboratory tests outside KP such as in skilled nursing facilities, those who missed electronic and manual enrollment, and those who were enrolled but did not follow through to obtain laboratory tests or had cancellation of their operation. Non-KP laboratory results could not be extracted but were seen on chart reviews to investigate those with missing laboratory test results.
Table 1.
Baseline characteristics of study cohort
| Characteristic | No. (%)a |
|---|---|
| Age, years, mean (SD) | 60.6 (13.4) |
| Duration of surgery, hrs, median (IQR) | 1.83 (1.53) |
| Women | 285 (55.88) |
| Men | 225 (44.12) |
| BMI, kg/m2, mean (SD) | 29 (5.7) |
| Race | |
| White | 267 (52.35) |
| African American | 30 (5.88) |
| Hispanic non-black | 115 (22.55) |
| Asian/Pacific Islander | 73 (14.31) |
| Native American/multiracial/other/unknown | 25 (4.90) |
| ASA class | |
| 1 | 23 (4.51) |
| 2 | 344 (67.45) |
| 3 | 139 (27.25) |
| 4 | 4 (0.78) |
| Type of surgery | |
| Spine | 168 (32.94) |
| Knee replacement | 106 (20.78) |
| Hysterectomy | 77 (15.10) |
| Hip replacement | 66 (12.94) |
| Mastectomy with reconstruction | 24 (4.71) |
| Whipple procedure | 8 (1.57) |
| Nephrectomy | 5 (0.98) |
| Abdominal aortic aneurysm | 3 (0.59) |
| Other | 53 (10.39) |
| Days from anemia screening lab collection date to surgery, median (IQR) | 28.5 (32.5) |
Data are No. (%) unless indicated otherwise.
ASA = American Society of Anesthesiologists; BMI = body mass index; IQR = interquartile range; SD = standard deviation.
Figure 6.
Results on feasibility of preoperative anemia protocol.
Hg = hemoglobin (g/dL); KP = Kaiser Permanente; lab = laboratory test; labs = laboratory blood samples; POM = Perioperative Medicine Clinic; TSAT = transferrin saturation (%).
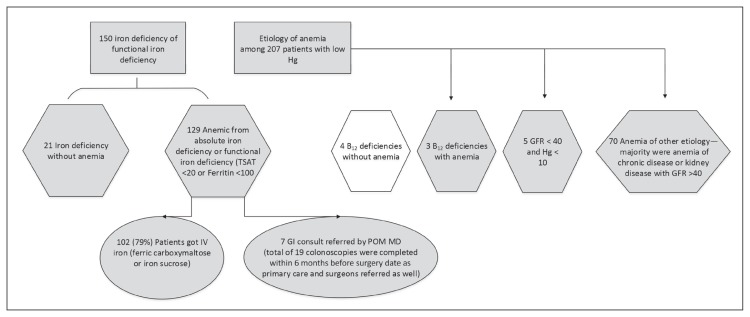
Those with normal laboratory findings were early graduators of the protocol. The remaining 228 patients were eligible for optimization, including 207 who were anemic and 21 who were iron deficient without anemia; 83% of those eligible had anemia addressed preoperatively. Of 129 patients with either classic or functional iron deficiency anemia (transferrin saturation < 20% or ferritin level < 100 ng/mL), 102 (79%) received IV iron with either ferric carboxymaltose or iron sucrose; and 7 gastroenterology consults were requested by perioperative medicine physicians (Figure 7). Because other physicians, including primary care physicians and surgeons, also requested for select gastroenterology consults, 19 patients had GI tract endoscopy completed within 6 months before the operation date. Iron deficiency without anemia, identified in 21 patients, was treated via physician discretion, receiving either or both IV iron infusion and oral iron supplementation. These iron deficiency without anemia cases were identified in patients who had no baseline hemoglobin level in the last 12 months before case creation or who were manually included in the protocol via physician discretion. Seven vitamin B12 deficiencies were identified in the entire cohort, of which 4 had vitamin B12 deficiency without anemia and 3 had vitamin B12 deficiency with anemia. All vitamin B12 deficiencies were addressed by supplementation and routing follow-up request to primary care. A total of 75 patients had anemia of other causes, including chronic disease and kidney disease.
Figure 7.
Results on cause and management of anemia.
B12 = vitamin B12; GFR = glomerular filtration rate; GI = gastroenterology; Hg = hemoglobin (g/dL); IV = intravenous; POM MD = perioperative medicine physician; preop = preoperatively; TSAT = transferrin saturation (%).
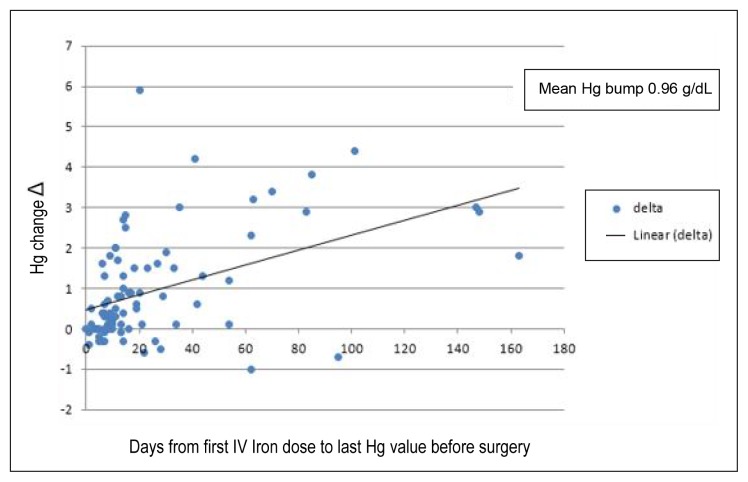
In the data analysis of 79 patients, who had received IV iron preoperatively and had hemoglobin levels measured after iron infusion but before surgical incision, the mean change in hemoglobin level was an increase of 0.98 g/dL. Most patients (72%) responded with a hemoglobin increase. A linear relationship was observed between time and delta (Δ, change in) hemoglobin, such that longer times led to a greater increase in hemoglobin (Figure 8).
Figure 8.
Effect of intravenous (IV) iron on preoperative hemoglobin (Hg).
DISCUSSION
The Joint Commission has recognized the importance of treating preoperative anemia, a required component since 2016 for hospitals seeking its Certification in Patient Blood Management.13 Addressing preoperative anemia is also a key component of Enhanced Recovery After Surgery (ERAS) protocols. A few preoperative anemia clinics have been created at US medical centers in recent years, including the Duke Perioperative Enhancement Team program for orthopedic operations.14 Our protocol was not limited to orthopedic or elective operations, although orthopedic operations accounted for 67% of our cohort (33% multilevel spine operations, 21% total knee arthroplasties, and 13% total hip arthroplasties). We believe these results can be extrapolated to nonorthopedic operations because preexisting medical conditions are not different enough to alter the management of preoperative anemia.
In 2014, KP San Jose’s incidence of preoperative anemia was lower than those in previously published reports possibly because of integrated health services in our patient population and increased recognition of anemia in recent years; our incidence of anemia as defined by World Health Organization criteria of hemoglobin less than 12 g/dL in women and less than 13 g/dL in men, was 22% among surgeries with at least 1 overnight stay vs 36% in a large series of patients undergoing major surgical procedures (N = 3342; 44.5% women, anemia defined as Hg < 13 g/dL in both sexes) between 2008–201415 and vs 30% in NSQIP data on patients undergoing major noncardiac surgical procedures in 2008 (N= 227,425; 57.6% women, anemia defined by World Health Organization criteria).3 Patients undergoing nonemergent operations were routinely evaluated by our Perioperative Medicine Clinic, providing a unique opportunity for us to implement the protocol. We developed algorithmic strategies to treat and optimize anemia, and these can be disseminated on a large scale to potentially benefit many patients. Without exposing patients to substantial risk or harm, this protocol enhances and expedites best practices, including IV iron therapy, erythropoietin injections, and gastroenterology and nephrology consultations to meet the timeline requirements of the surgery date. No severe adverse reactions occurred in this cohort as a result of any protocol interventions. There were no delays to operation caused by any side effects from IV iron treatment.
Integrated EHR and patient care across all specialties were crucial to the successful implementation of the protocol. Development of encrypted patient spreadsheet reports, electronic templates with built-in decision guidance, and other EHR tools helped with enrollment, tracking, and workload. KP San Jose was well positioned to garner institutional support and achieve the participation and collaboration of various internal departments, including Perioperative Medicine, Surgery, Nephrology, Gastroenterology, Hematology, and the Infusion Clinic. Although individual protocol interventions are often recommended care for anemic nonsurgical patients, they were standardized and expedited with the prevention of surgery delays in mind. Inclusion and exclusion criteria were designed to capture major operations at risk of causing substantial blood loss and patients who were most likely to have preoperative anemia. The protocol captured most patients who would likely benefit; 442 (87%) of the eligible cohort obtained anemia screening laboratory tests, and approximately half of these had abnormal laboratory results, which led to sequential considerations and interventions. Most patients with iron deficiency anemia (n = 102, 79%) received IV iron therapy, and those indicated received gastroenterology consultations and endoscopic evaluations. Iron deficiency anemia is a low-hanging fruit in terms of its prevalence and amenability to preoperative optimization, and we successfully targeted its identification and optimization among a heterogeneous cohort. Most patients responded to IV iron with an increase in hemoglobin levels. In our cohort, the most prevalent causes of anemia were classic and functional iron deficiencies, together accounting for 62.31% of anemia. Some IV iron deficiency cases likely also had concomitant anemia of chronic disease. Iron deficiency without anemia was also identified as expected, given that iron deficiency is known to precede the development of anemia, and its importance may be magnified in anticipation of substantial surgical blood loss. This subgroup was treated at the discretion of perioperative medicine physicians with either IV or oral iron supplementation and an as-needed bowel regimen.
In addition to expediting and enhancing best practices, the Preoperative Anemia Protocol has improved the early identification and management of cancer, GI tract bleeding, liver disease, and vitamin B12 and iron deficiencies. The early detection of colon cancer has been lifesaving. A woman scheduled for total knee arthroplasty was newly identified to have iron deficiency anemia via protocol screening laboratory test results. After expedition of gastroenterologic procedures within 1 week as a part of the protocol, colonoscopy revealed a cecal mass; her elective knee arthroplasty was postponed, and IV iron therapy and right hemicolectomy were expedited. Surgical pathologic findings showed adenocarcinoma, fortunately with clean margins. She eventually completed elective knee arthroplasty without complications, but this alteration in sequence of management likely avoided unnecessary metastasis, thromboembolic risk, and malignancy-related bleeding risk during postoperative deep venous thrombosis prophylaxis.
We would like to point out several limitations of the study. Because our Perioperative Medicine Clinic consisted of 14 rotating hospitalists who make up a total of 3 FTE physicians, we expected a number of protocol violations because of the need for more education, new physician onboarding, and variance in practice styles. A number of violations were caused by patient noncompliance with laboratory blood draws or IV iron infusions, delays in infusion scheduling where surgery delay was not warranted because the minimum hemoglobin target of either 10 g/dL or 11 g/dL was already met, and physician discretion in which oral instead of IV iron supplementation was prescribed. A few missed identifications, interventions, or follow-up laboratory tests occurred because of missed tracking, because a combination of electronic and manual tracking methods were used. Physicians were manually ordering follow-up laboratory tests for the day of surgery, and some orders were missed or done before the day of surgery; those missing Δ hemoglobin were not included in the analysis shown in Figure 8. Overall, protocol violations were low, demonstrating the feasibility and sustainability of the protocol. Greater hemoglobin response would be anticipated with earlier IV iron administration because a linear relationship was observed between time and increase in hemoglobin before operation. IV iron is expected to increase hemoglobin by 2 g/dL in a 3-week period. Among the 79 patients with hemoglobin tracking any time before surgery, including the day of surgery, 34 patients (43%) of recipients had fewer than 14 days between iron infusion and day of operation, which resulted in a mean increase in hemoglobin level of 0.98 g/dL. Although suboptimal lead time for IV iron therapy limited the preoperative hemoglobin response, iron replacement is still expected to benefit patients in the postoperative period.16
IV iron was a key intervention in our protocol given its better correction of functional iron deficiency and faster repletion of iron stores compared with oral iron.8 We chose ferric carboxymaltose because a 750-mg dose can be conveniently infused during 15 minutes at 1 to 2 doses preoperatively to replete the entire iron deficit for most patients. This choice has been reported to lower the cumulative risk of infusion reactions and extravasations, and to reduce appointments and staff utilizations.17 Ferric carboxymaltose is approved by the US Food and Drug Administration for the treatment of iron deficiency anemia in adults with intolerance or unsatisfactory response to oral iron and for the treatment of iron deficiency anemia in adults with nondialysis-dependent chronic kidney disease.18 Bypassing an oral iron trial, as we did, has been previously proposed in the literature.19 Our acquisition cost of IV iron was similar to packed red blood cell (PRBC) transfusions during the study period at $1.03 per milligram of ferric carboxymaltose vs $244 per unit of PRBCs (containing 250 mg of elemental iron at $0.97 per milligram of iron). However, IV iron infusion is more cost-effective because it requires less patient and staff time compared with PRBC infusions. IV iron infusion can replace the entire iron deficit preoperatively, whereas PRBCs are not indicated for transfusion to a normal hemoglobin level because blood transfusion is known to increase perioperative morbidity and mortality and to carry substantial risks.
Since this cohort, efforts have been ongoing to reduce variability, expedite iron administration, and increase upstream identification. KP San Jose has transitioned to a pharmacist for the ordering of IV iron, vitamin B12 and folic acid supplementations, erythropoietin injections, and follow-up laboratory studies. We have shifted to the use of low-molecular-weight iron dextran, which costs about one-fourth of ferric carboxymaltose and can be infused in 2 hours as a 25-mg test dose followed by 975 mg with similar safety, efficacy, and convenience.20 A modified version of this protocol is being evaluated for scale-up across 21 KPNC hospitals. The patient spreadsheet report initially developed at KP San Jose has been adopted regionally to help with various perioperative optimization projects. Once implemented to all KPNC hospitals serving more than 4.1 million members, and ultimately to KP across the US serving more than 11.8 million members, the KP Anemia Protocol is expected to become the largest such protocol in the US.
CONCULSION
We believe that perioperative outcomes, including the incidence of perioperative blood transfusions, will be improved by correcting hemoglobin levels preoperatively. Existing literature shows the increase in morbidity and mortality associated with preoperative anemia and indicates the need for IV iron infusion and other anemia-correcting interventions preoperatively. Outcomes data are still lacking from a preoperative anemia protocol to show increased preoperative hemoglobin levels, reduced perioperative blood transfusions, reduced length of stay, and reduced perioperative mortality and morbidity. A recent Australian study randomly assigned 72 patients with iron deficiency anemia to IV iron vs usual care.21 The IV iron group had a reduction in blood transfusions and an association with shorter hospital stay, but was limited by early termination, according to its authors. The PREVENTT trial is a currently ongoing randomized controlled trial in the UK examining clinical outcomes in the use of ferric carboxymaltose for preoperative anemia before major open abdominal operations.22 We plan to measure the important perioperative outcomes of our protocol compared with historical controls for a future publication.
Acknowledgments
From the Division of Research, Kaiser Permanente Oakland Medical Center, Oakland, CA, we thank Yun-Yi Hung, PhD, for assistance with data acquisition and statistical analysis and Nareg Roubinian, MD, MPH, for review of the manuscript. From Kaiser Permanente San Jose Medical Center, San Jose, CA, we thank Christopher L Ramm (Service Unit Manager); Georganne L Soule, RN, M-HCA (Service Line Director); and all of our Perioperative Medicine Clinic physicians, nurses, and staff for support on the protocol. We appreciate Yuhjung Tsai, MD (Local Research Chair), and Ashok Krishnaswami, MD (cardiologist), for their review of the manuscript.
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
References
- 1.Muñoz M, Gómez-Ramírez S, Kozek-Langeneker S, et al. ‘Fit to fly’: Overcoming barriers to preoperative haemoglobin optimization in surgical patients. Br J Anaesth. 2015 Jul;115(1):15–24. doi: 10.1093/bja/aev165. . [DOI] [PubMed] [Google Scholar]
- 2.Clevenger B, Richards T. Pre-operative anemia. Anaesthesia. 2015 Jan;70(Suppl 1):20–8. e6–8. doi: 10.1111/anae.12918. . [DOI] [PubMed] [Google Scholar]
- 3.Musallam KM, Tamim HM, Richards T, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: A retrospective cohort study. Lancet. 2011 Oct 15;378(9800):1396–407. doi: 10.1016/s0140-6736(11)61381-0. [DOI] [PubMed] [Google Scholar]
- 4.Baron DM, Hochrieser H, Posch M, et al. European Surgical Outcomes Study (EuSOS) group for Trials Groups of European Society of Intensive Care Medicine; European Society of Anaesthesiology. Preoperative anaemia is associated with poor clinical outcome in non-cardiac surgery patients. Br J Anaesth. 2014 Sep;113(3):416–23. doi: 10.1093/bja/aeu098. . [DOI] [PubMed] [Google Scholar]
- 5.Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999 Feb 11;340(6):409–17. doi: 10.1056/nejm199902113400601. . [DOI] [PubMed] [Google Scholar]
- 6.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013 Jan 3;368(1):11–21. doi: 10.1056/NEJMoa1211801. doi: 10.1056/NEJMoa1211801. . Erratum in: N Engl J Med 2013 Jun 13;368(24):2341. DOI: https://doi.org/10.1056/NEJMx130015. [DOI] [PubMed] [Google Scholar]
- 7.Carson JL, Terrin ML, Noveck H, et al. FOCUS Investigators. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011 Dec 29;365(26):2453–62. doi: 10.1056/NEJMoa1012452. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015 May 7;372(19):1832–43. doi: 10.1056/NEJMra1401038. . [DOI] [PubMed] [Google Scholar]
- 9.Avni T, Bieber A, Grossman A, Green H, Leibovici L, Gafter-Gvili A. The safety of intravenous iron preparations: Systematic review and meta-analysis. Mayo Clin Proc. 2015 Jan;90(1):12–23. doi: 10.1016/j.mayocp.2014.10.007. . [DOI] [PubMed] [Google Scholar]
- 10.Chertow GM, Mason PD, Vaage-Nilsen O, Ahlmén J. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant. 2006 Feb;21(2):378–82. doi: 10.1093/ndt/gfi253. . [DOI] [PubMed] [Google Scholar]
- 11.Goodnough LT, Schrier SL. Evaluation and management of anemia in the elderly. Am J Hematol. 2014 Jan;89(1):88–96. doi: 10.1002/ajh.23598. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodnough LT, Maniatis A, Earnshaw P, et al. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesthesia. 2011 Jan;106(1):13–22. doi: 10.1093/bja/aeq361. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank S, Nunes E, Olea S. Getting ready for patient blood management certification [Internet] Oakbrook Terrace, IL: The Joint Commission; 2016. May 12, [cited 2018 Apr 16]. Available from: www.jointcommission.org/assets/1/18/Patient_Blood_Management_Certification_May-12_2016.pdf. [Google Scholar]
- 14.Guinn NR, Guercio JR, Hopkins TJ, et al. Duke Perioperative Enhancement Team (POET) How do we develop and implement a preoperative anemia clinic designed to improve perioperative outcomes and reduce cost? Transfusion. 2016 Feb;56(2):297–303. doi: 10.1111/trf.13426. . [DOI] [PubMed] [Google Scholar]
- 15.Muñoz M, Laso-Morales MJ, Gómez-Ramírez S, et al. Pre-operative haemoglobin levels and iron status in a large multicentre cohort of patients undergoing major elective surgery. Anaesthesia. 2017;72:826–34. doi: 10.1111/anae.13840. . [DOI] [PubMed] [Google Scholar]
- 16.Khalafallah AA, Yan C, Al-Badri R, et al. Intravenous ferric carboxymaltose versus standard care in the management of postoperative anaemia: A prospective, open-label, randomised controlled trial. Lancet Haematol. 2016 Sep;3(9):e415–25. doi: 10.1016/s2352-3026(16)30078-3. [DOI] [PubMed] [Google Scholar]
- 17.Auerbach M, Macdougall I. The available intravenous iron formulations: History, efficacy, and toxicology. Hemodial Int. 2017 Jun;21(Suppl 1):S83–S92. doi: 10.1111/hdi.12560. . [DOI] [PubMed] [Google Scholar]
- 18.Ferric carboxymaltose [Commercial: Non-Formulary] [Med Part D: Tiered] (Kaiser PermanenteMid-Atlantic States Drug Formulary) [Internet] Indianapolis, IN: Lexicomp, Wolters Kluwer; 2013. Feb 7, [updated 2018 Feb 21; cited 2018 May 2]. Available from: https://online.lexi.com/lco/action/doc/retrieve/docid/kaimid_f/4645946#adm. [Google Scholar]
- 19.Muñoz M, Acheson AG, Auerbach M, et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia. 2017 Feb;72(2):233–47. doi: 10.1111/anae.13773. . [DOI] [PubMed] [Google Scholar]
- 20.Wong L, Smith S, Gilstrop M, et al. Safety and efficacy of rapid (1,000 mg in 1 hr) intravenous iron dextran for treatment of maternal iron deficient anemia of pregnancy. Am J Hematol. 2016 Jun;91(6):590–3. doi: 10.1002/ajh.24361. . [DOI] [PubMed] [Google Scholar]
- 21.Froessler B, Palm P, Weber I, Hodyl NA, Singh R, Murphy EM. The important role for intravenous iron in perioperative patient blood management in major abdominal surgery: A randomized controlled trial. Ann Surg. 2016 Jul;264(1):41–6. doi: 10.1097/sla.0000000000001646. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richards T, Clevenger B, Keidan J, et al. PREVENTT: Preoperative intravenous iron to treat anaemia in major surgery: Study protocol for a randomised controlled trial. Trials. 2015 Jun 4;16:254. doi: 10.1186/s13063-015-0774-2. doi: 10.1186/s13063-015-0774-2. . Erratum in: Trials 2015 Jul 24;16:312. DOI: https://doi.org/10.1186/s13063-015-0869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]