Abstract
Growth factors have important roles in gastrointestinal (GI) tract development, maintenance, and response to injury. Various in vitro, in vivo, and human experiments have been used to demonstrate growth factor influence. Collectively, these studies have demonstrated enhancement of mucosal proliferation, intestinal motility, and immune modulation, decreased apoptosis, enhanced gut barrier function, and enteric nervous system (ENS) protection. Select growth factors, including epidermal growth factor (EGF) and heparin-binding EGF-like growth factor (HB-EGF), demonstrate some beneficial effects in experimental and clinical intestinal injury, including necrotizing enterocolitis (NEC). The roles of glucagon-like peptide-2 (GLP-2), insulin-like growth factor-1 (IGF-1), erythropoietin (EPO), growth hormone (GH), and hepatocyte growth factor (HGF) in NEC have also been investigated. The roles of these growth factors will be summarized in this chapter.
Keywords: Necrotizing enterocolitis, growth factors, intestinal injury
INTRODUCTION
Necrotizing enterocolitis (NEC) is the most frequent and most serious gastrointestinal (GI) surgical emergency in preterm infants.1 Despite decades of research, it remains the leading cause of morbidity and mortality in neonates, occurring predominately in premature infants weighing less than 1500 g and those born before 36 weeks gestation.2 Advances in modern medical and clinical care have improved the survival of premature infants, yet the prevalence of NEC remains high. In the US, nearly 12% of extremely premature infants, those weighing less than 1500g, develop NEC, with up to 50% succumbing to the disease.3 Severe NEC, characterized by full-thickness necrosis of the intestine with peritonitis and sepsis, occurs in up to 63% of NEC cases and requires life-saving surgical intervention.2 Patients who survive and recover require prolonged care due to associated complications including defects in growth and development, liver failure from prolonged use of total parental nutrition, and short bowel syndrome resulting in intestinal failure.1 Epidemiologic studies have identified multiple complex factors associated with the development of NEC, with gut prematurity, formula feedings, and altered gut microbiome thought to have primary roles in disease pathogenesis.1,4–6
Growth factors play important roles in the health and development of the GI tract, with many involved in intestinal growth and repair from inflammation or injury.7 It has been established that growth factors have critical effects on cellular proliferation, differentiation, and survival.8 It is hypothesized that absence or reduced levels of specific growth factors normally present during gestation contribute to the development of NEC, however this remains poorly understood. To date there have been various studies demonstrating altered levels of growth factors in injured tissues, as well as changes in the response of injured tissue in the presence or absence of a given growth factor. Continued work toward understanding these factors and their roles may be of clinical value in the future prevention and treatment of NEC.
EPIDERMAL GROWTH FACTOR (EGF)
Members of the epidermal growth factor (EGF) family are recognized as critical trophic factors for normal intestinal development.9 Most EGF family members are first synthesized as transmembrane precursors, eventually undergoing proteolysis into the mature, secreted form of the growth factor. EGF family proteins have tyrosine kinase activity and activate the EGF receptor (EGFR), which has been identified predominantly on the basolateral surface of intestinal enterocytes.9,10 EGF is a 53-amino-acid peptide with resistance to proteolytic degradation from the gastric pH normally encountered in the gastric lumen and GI tract.11 The primary source of intestinal EGF is the submandibular salivary gland.10 Human EGF expression remains poorly understood, with information on expression of salivary EGF and serum EGF levels in premature infants particularly lacking.9
EGF and the intestine: Various studies have shown that EGF given in utero accelerates the maturation of intestinal enzyme activity and intestinal growth.9 In addition, there is evidence that EGF stimulates intestinal growth. The importance of EGF has been demonstrated by the observation that EGFR knockout mice die prematurely or in utero.12 Furthermore, mice who survive postnatally demonstrate significant epithelial underdevelopment in various tissues and develop severe hemorrhagic enteritis similar to human NEC.9,13 EGF is present in various fluids that come into contact with the developing intestine, including amniotic fluid, fetal urine, breast milk, bile and saliva.9 In addition, EGF exerts potent trophic and cytoprotective effects on enterocytes, facilitating normal function, maturation, healing, and survival.11,14 After intestinal mucosal injury, EGF promotes epithelial intestinal restitution, thus restoring gut barrier function and preventing bacterial translocation and resulting sepsis.15,16 This is further enhanced by the ability of EGF to decrease apoptosis and moderate the pro-inflammatory response after injury.17
EGF and necrotizing enterocolitis: Various investigations have highlighted the importance of EGF in the pathogenesis and treatment of NEC. Immaturity of the developing gut leading to decreased intestinal mucosal barrier function, diminished restitution, impaired intestinal motility and bacterial translocation are believed to contribute to NEC.18–20 Premature infants have immature intestinal defenses and are often exposed to antibiotics and formula feedings that alter the intestinal microbiome. These factors are thought to increase pathogenic bacterial translocation.21 Neonatal rat models have shown significantly decreased EGFR expression in the injured ilium of animals with NEC.22 Furthermore, administration of EGF led to decreased incidence and severity of NEC through accelerated goblet cell maturation, mucin production and normalization of enterocyte tight junction protein expression.17 While there is minimal human data, an association between EGF and human disease has been demonstrated.23 Salivary EGF is significantly reduced in infants with NEC compared to premature infants who do not develop NEC,9 with lower salivary EGF levels correlating with increasing prematurity.24 Formula feeding is a well-known risk factor for NEC, one that can be mitigated to a degree with breast milk (BM). BM and colostrum are the primary source of EGF postnatally, with EGF concentration being inversely proportional to the gestational age of the infant.25 Commercial infant formulas contain no EGF, which may explain why formula feeding in premature infants is considered a leading risk factor for the development of NEC.25,26
EGF and the inflammatory response: Another process thought to have significance in the pathogenesis of NEC is the altered inflammatory response, with a primarily proinflammatory cascade thought to have a major role.25 In a neonatal rat model of NEC, elevated expression of pro-inflammatory cytokines IL-18 and IL-12 were directly associated with injury severity.26 Furthermore, infants with NEC have high levels of proinflammatory platelet-activating factor (PAF), tumor necrosis factor-alpha (TNF-a), IL-8, and nitric oxide (NO).27 Interestingly, enteral EGF administration results in decreased IL-18 and increased anti-inflammatory IL-10 expression during experimental NEC.28
EGF and apoptosis: Another potential mechanism in the pathogenesis of NEC is enterocyte apoptosis. Apoptosis, mediated through BAX, is observed in rat models of NEC. It is thought to occur prior to the onset of fulminant disease and is influenced by EGF. EGF administration leads to decreased BAX and increased anti-apoptotic Bcl-2 levels.16
Clinical studies of EGF for NEC: Clinical studies of EGF as a treatment for NEC have been limited and have not provided much advance in its treatment or prevention. However, further investigation is warranted given that EGF has distinct roles in pathogenesis of experimental NEC.
HEPARIN-BINDING EGF-LIKE GROWTH FACTOR (HB-EGF)
HB-EGF is a glycoprotein member of the EGF family, with expression triggered by hypoxia and oxidative stress. HB-EGF induces wound healing and tissue regeneration in response to tissue damage.29 In addition to binding to the EGFR, HB-EGF also binds and signals through a specific receptor known as N-arginine dibasic convertase (NRDc), resulting in increased chemoattractant and migration activities.30 It is thought that the EGFR-binding specificities of HB-EGF, along with its ability to signal though its specific NRDc receptor and its ability to bind to cell-surface heparin-sulfate proteoglycans (HSPGs), may combine to confer a particularly important functionality of HB-EGF compared with other members of the EGF family. HB-EGF is found in amniotic fluid (AF) and BM, allowing its continuous exposure to fetal and newborn intestine.31 Endogenous HB-EGF is expressed in many cell types, and is a potent mitogen for a number of those cells, including cells of the intestinal mucosa.32 Various animal models of intestinal injury have been utilized to demonstrate the effects of HB-EGF, with a significant amount of knowledge gained from work in our laboratory.
HB-EGF and intestinal restitution: Endogenous HB-EGF plays an important role in restitution.33 Administration of HB-EGF activates the ErbB-1 receptor and subsequent signaling increases the rate of IEC migration, proliferation, and restitution after intestinal ischemia/reperfusion (I/R) injury33 and experimental NEC.34 HB-EGF also affects restitution by altering cell adhesion and by decreasing intercellular adhesion molecules.35
HB-EGF and intestinal stem cells (ISC): The intestinal epithelium is the most rapidly renewing tissue in mammals and is fueled by intestinal stem cells (ISC) that reside at the base of the crypts. Intestinal injury of various forms damages not only differentiated intestinal epithelial cells (IEC), but also the ISC required for restitution.36 Enteral administration of HB-EGF protects ISC and enterocytes from injury in experimental NEC.37
HB-EGF and mesenchymal stem cells (MSC): HB-EGF protects MSCs from hypoxia-induced apoptosis.38 In models of NEC, intravenous (IV) and intraperitoneal (IP) administration of MSC protects the intestines from injury, with simultaneous administration of HB-EGF enhancing the beneficial effects of MSC.39 HB-EGF likely protects not only injured ISC, but also preserves the viability of transplanted MSC, thus allowing these cells to play a greater role in intestinal healing.
HB-EGF and the enteric nervous system (ENS): The premature GI tract has poor motility, likely from underdevelopment of the ENS, which is critical for coordinating peristalsis.40 ENS abnormalities involving both neurons and glial cells exist in human intestine resected for NEC compared to age-matched intestine resected for non-NEC conditions.41 HB-EGF has been shown to improve post-NEC intestinal motility by promotion of neural stem cell (NSC) proliferation and migration.42 Enteral administration of HB-EGF in rats exposed to NEC results in preservation of enteric neuronal nitric oxide synthase (nNOS) levels with concomitant improvement in post-injury intestinal motility.41
HB-EGF-mediated protection from apoptosis: Apoptosis is triggered by the generation of reactive oxygen species (ROS) after oxidative stress, which is commonly seen in NEC. Apoptosis is thought to play either an etiologic role or to represent a final cellular pathway in the pathogenesis of NEC.43 HB-EGF decreases IEC apoptosis after exposure to hypoxia in vitro44 and reduces ROS production following enteral administration in a rat model of I/R injury.45 Furthermore, HB-EGF decreases IEC apoptosis in a rat model of NEC.46
HB-EGF and inflammatory mediators: Immune dysregulation is one potential etiologic factor associated with the development of NEC. In a rat model of I/R injury, HB-EGF administration decreased the serum levels of the pro-inflammatory cytokines TNF-alpha, IL-6, and IL-1β after injury.47 During intestinal injury, inflammatory cytokines trigger an upregulation of inducible NOS (iNOS), which generates large amounts of NO that is oxidized into reactive nitrogen species. Intraluminal administration of HB-EGF after intestinal I/R injury significantly decreases iNOS gene expression and protein production,48 with subsequent decreased intestinal damage.
HB-EGF and gut barrier function: NEC is associated with decreased gut barrier function, increased intestinal permeability, and resultant bacterial translocation.49 HB-EGF decreases bacterial translocation across IEC monolayers after I/R exposure.50 In HB-EGF KO mice, HB-EGF is shown to be essential for preservation of gut barrier function, in part due to inhibition of neutrophil-endothelial cell interactions.51 In experimental models of NEC and other intestinal injury, exogenous enteral HB-EGF preserves gut barrier function.48
HB-EGF and human NEC: HB-EGF can protect the intestines from various forms of intestinal injury including NEC. The beneficial effects observed in animal models may be applicable to humans. HB-EGF mRNA levels in human small bowel resected for NEC were higher at the resection margins adjacent to NEC-afflicted tissue, suggesting that lack of HB-EGF expression may play a role in the pathogenesis of NEC, or that its expression may play a role in healing after injury.52
Clinical studies of HB-EGF for NEC: As of yet, there have been no clinical trials investigating the effects of HB-EGF in NEC. Until then, further investigations of HB-EGF in animal models will continue to elucidate the mechanisms by which this growth factor exerts its beneficial effects.
GLUCAGON-LIKE PEPTIDE 2 (GLP-2)
GLP-2 is an intestinotrophic hormone that is known for its proliferative and anti-inflammatory effects in the intestine. It is secreted from the L cells of the small and large intestine in response to fatty acids and glucose in the intestinal lumen, and in response to ENS stimulation.53 GLP-2 increases crypt cell proliferation, leading to increased villus height, crypt depth, and overall intestinal length.54 There is incomplete knowledge of the downstream mechanisms of action of GLP-2, but animal models have shown promise in its ability to reduce the severity of NEC by maintaining the intestinal mucosa and reducing inflammatory cytokine production.53
GLP-2 and gut development: GLP-2 signaling is hypothesized to play an important role in the developing intestine. The GLP-2 precursor, proglucagon, and the enzyme necessary for cleaving GLP-2 from proglucagon, are found in rat fetal intestine, further supporting this theory.55 Various studies have evaluated its effects and application in multiple GI diseases. GLP-2 administration results in increased intestinal growth and improved intestinal barrier function.56 Animal studies demonstrate that it reduces histological injury in experimental models of colitis.57,58 Furthermore, there is increased mucosal villous height and proximal intestinal weight with exogenous GLP-2 administration in a premature piglet model of NEC.56
GLP-2 and Inflammation: Multiple studies have demonstrated the anti-inflammatory effects of GLP-2 in the intestine. In a rat model of intestinal inflammation, GLP-2 administration led to reduced levels of the inflammatory cytokines IFN-λ, TNF-α, IL-1β, and iNOS.59 In a rat model of NEC, GLP-2 administration resulted in decreased TNF-α and IL-6 levels that were comparable to dam-fed pups.53 Finally, addition of GLP-2 to macrophages primed with LPS resulted in decreased pro-inflammatory iNOS, COX-2, TNF-α, IL-1β, and IL-6 levels.59 Although knowledge of the exact mechanisms by which GLP-2 mediates its anti-inflammatory effects remains incomplete, there is continued study of its potential roles in inflammatory bowel disease.60,61
GLP-2 and NEC: Several studies have demonstrated the ability of GLP-2 to attenuate intestinal injury.62 However, there are few publications on its role in NEC. A neonatal piglet model of NEC in which GLP-2 was administered demonstrated delayed onset of NEC from 10 to 25 hours, and increased proximal jejunum weight, villous height and area, and decreased histological damage.56 However, in this study GLP-2 did not demonstrate a reduction in incidence of NEC or survival from NEC. Another study showed that high dose GLP-2 administration in a rat model improved incidence and survival from NEC.53 GLP-2 demonstrates potential in the treatment and prevention of NEC, however additional investigation of its role is needed.
INSULIN-LIKE GROWTH FACTOR-1 (IGF-1)
IGF-1 is a polypeptide that is produced primarily in the liver. Circulating IGF-1 binds to the IGF-1 receptor (IGF-1R), leading to activation of intracellular signaling pathways that result in trophic effects on tissues.63 IGF-1 is mainly present in BM and saliva. IGF-1R is present in the GI tract, with the highest concentration of the receptor found in the fetal GI tract.64 IGF-1 promotes growth of the GI tract, participates in intestinal healing, and has anti-inflammatory properties, supporting research for IGF-1 as a possible treatment or prevention for NEC.65
IGF-1 and apoptosis: IGF-1 protects IECs from oxidative-stress and apoptosis in the setting of intestinal injury.66 It also promotes cytotoxic activity of natural killer cells.67 In mouse and rat models of intestinal injury and experimental NEC, IGF-1 mitigated intestinal injury and decreased apoptosis.68,69
IGF-1 and gut barrier function: There is evidence suggesting that IGF-1 improves gut barrier function.70 Furthermore, IGF-1 decreases bacterial translocation, likely due to its effect on gut barrier function.71 When IGF-1 was administered in a rat model of small bowel transplantation, there was improvement in mucosal histology, and enhanced gut barrier function.72 In a human trial involving premature infants, formula supplemented with IGF-1 led to decreased gut permeability.73 The anti-inflammatory effect of IGF-1 along with improved gut barrier function was demonstrated in a rat model of liver cirrhosis, where IGF-1 decreased intestinal mucosal damage and bacterial translocation, with upregulation of anti-inflammatory cyclooxygenase 2 (COX-2) and downregulation of TNF-α.70
IGF-1 and NEC: Although limited, studies investigating the association of IGF-1 and NEC are promising. Early observations found that premature infants with persistently low IGF-1 levels had an increased risk of developing NEC.7 Animal studies, using a mouse model of NEC indicated that IGF-1 administration prior to injury resulted in decreased epithelial cell apoptosis and improved survival.74
ERYTHROPOIETIN (EPO)
Erythropoietin is a glycoprotein secreted by the liver prenatally and by renal peritubular cells postnatally in response to anemia.7 It is found in AF and BM, thereby providing access to its receptors in the developing GI tract, suggesting a potential role in the development, health, or proper function of the GI tract.75 Animal models have demonstrated that EPO protects the GI tract by preservation of intestinal barrier function through tight junction protein expression,79 as well as protection against I/R injury.77
EPO in experimental intestinal injury including NEC: Various animal studies have analyzed the mechanisms by which EPO affects different types of intestinal injury including I/R injury, septic- and hemorrhagic-shock, IBD, and NEC. In a rat model of intestinal I/R injury, a single dose of EPO decreased histologic injury and apoptosis, and decreased markers of oxidative stress.77 In a rat model of NEC, administration of EPO prior to injury resulted in decreased histological injury and attenuated levels of NO, suggesting that EPO provides protection from oxidative damage in NEC.78 EPO improves intestinal mucosal barrier integrity by regulating the expression of the tight junction protein zona occludens-1 (ZO-1) in a dose-dependent fashion.79 Furthermore, administration of EPO in a rat model of NEC resulted in preservation of intestinal barrier function, decreased loss of ZO-1, and reduction in the incidence of NEC.79
EPO in human NEC: Human studies of EPO in NEC are lacking. One retrospective cohort study of very low birth weight infants given recombinant EPO for prevention or treatment of anemia demonstrated a decreased incidence of NEC.80 More recently, a randomized controlled trial of EPO in 90 premature infants showed that EPO improved feeding tolerance and decreased the risk of NEC.81 While there is potential for the use of EPO in treating and preventing NEC, further investigation is necessary.
GROWTH HORMONE (GH)
Growth hormone (GH) is a protein produced by the anterior pituitary gland with systemic effects on anabolism. GH reacts with its receptor, GH receptor (GHR), a transmembrane tyrosine kinase receptor which is present in the GI tract. Upon binding to GHR, transcription of various genes occurs, resulting in glucose and lipid metabolic changes.82 GH has an effect on the release and function of downstream intestinotrophic mediators, such as insulin-like growth factor-1 (IGF-1), that have been implicated in the development of NEC.
Effects of GH on the intestine: GH is predominantly known for its impact on growth throughout the body. Short stature results from GH deficiency while gigantism and acromegaly result from GH excess. When introduced into the intestine, GH induces cell proliferation, decreased apoptosis, and preserves gut barrier function.82 Additionally, recombinant human GH (rhGH) administration to rats with GH deficiency results in increased growth of each layer of the colonic wall.83 There is ongoing investigation in the potential use of GH as a treatment or prevention of intestinal injury. Further studies to establish the role of GH in NEC are needed
HEPATOCYTE GROWTH FACTOR (HGF)
Hepatocyte growth factor (HGF) is a glycoprotein involved in angiogenesis, cellular proliferation and survival.7 Active HGF is found in the fetal GI tract as well as in BM, and is involved in epithelial migration, proliferation and repair of injured tissues.84 Once activated, HGF binds to tyrosine kinase receptors with downstream effects on cell proliferation, apoptosis, and immunity regulation.85 HGF is vital for embryonic development as shown in a study where HGF deficient mice experience embryonic demise.86 The mechanisms by which HGF exerts its effects are incompletely understood, thus further research is required.
HGF and experimental models of intestinal injury: HGF has been shown to protect and repair intestinal tissue in many animal models. When given prior to I/R injury in rats, there was decreased apoptotic activity in the intestinal epithelium.87 Mice with HGF receptor deficiency that were exposed to DSS- or acetic acid-induced colitis had impaired colonic mucosal regeneration and increased mortality.88 Finally, administration of enteral HGF decreased the incidence and severity of NEC in rats.89
OTHER GROWTH FACORS IN NEC
The information presented above is a snapshot of the knowledge we gain daily about a multitude of growth factors and their roles in the development and treatment of NEC. There are other growth factors that remain under investigation that were not included in this chapter. These include keratinocyte growth factor (KGF), intestinal trefoil factor (ITF), and granulocyte colony stimulating factor (GCSF), which have been identified as having a role in NEC. These additional growth factors have been associated with stimulation of mucosal proliferation, reducing apoptosis, modulating the immune system, and enhancing gut barrier function and nutrient absorption.90,91,92
Summary/Discussion
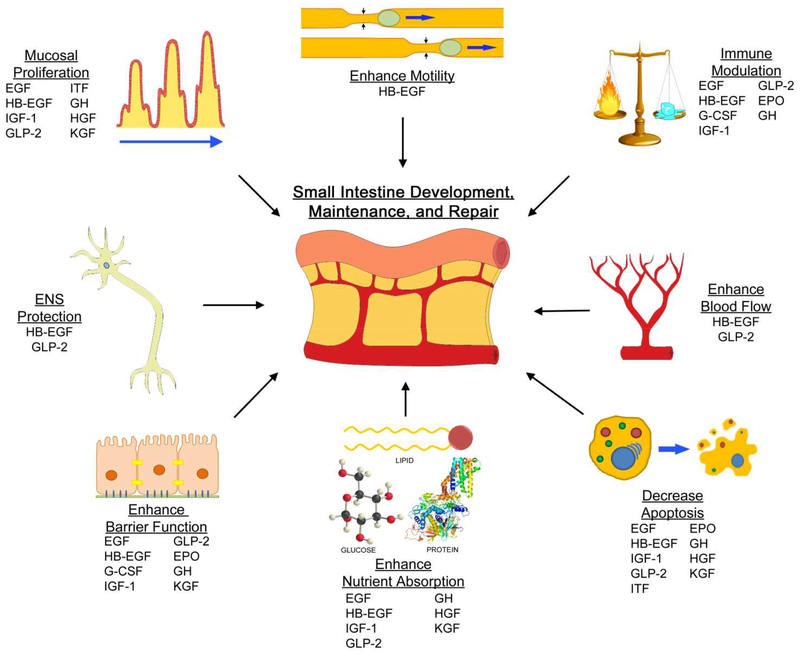
Growth factors play an important role in the development, growth and health of the GI tract (Figure 1). Through mediation of cellular activities, they have a role in cellular proliferation, migration, differentiation, and survival. Growth factors are present in AF and BM, thus having the potential to bathe intestinal cells, pre- and postnatally. A relationship has been established between the presence of growth factors and disease severity, and healing potential in intestinal injury has been observed. For each of the growth factors reviewed here, and many others not reviewed, the current body of research is varied and evolving. Some, like EGF and HB-EGF, have been a significant focus of research related to NEC, while others are only recently beginning to be reported. Additional studies are needed to further elucidate the roles of these growth factors in the pathogenesis of NEC. Eventually, growth factors may lead to the development of novel therapies for the prevention and/or treatment of clinical NEC.
Figure 1.
Growth factors contribute to the development, maintenance, and repair of the small intestine. Research has led to the discovery of their roles in mucosal proliferation, enhancing motility, immune modulation, enhanced blood flow, decreased apoptosis, enhanced nutrient absorption, enhanced barrier function, and enteric nervous system protection. The growth factors above are listed according to their proposed general mechanisms. EGF, epidermal growth factor; EPO, erythropoietin; G-CSF, granulocyte colony stimulating factor; GH, growth hormone; GLP-2, glucagon-like peptide 2; HB-EGF, heparin-binding EGF-like growth factor; HGF, hepatocyte growth factor; IGF-1, insulin-like growth factor-1; ITF, intestinal trefoil factor; KGF, keratinocyte growth factor.
Key Points.
Necrotizing enterocolitis (NEC) remains a significant healthcare problem in the premature infant
Growth factors have a significant role in the development and maintenance of the GI tract
Select growth factors have been shown to protect the intestines in experimental models of NEC
Further investigation is required to elucidate the exact mechanisms and effects of these growth factors prior to their clinical use
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Hunter CJ, Upperman JS, Ford HR, et al. Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC). Pediatr Res 2008;63(2):117–23. [DOI] [PubMed] [Google Scholar]
- 2.Lee JS and Polin RA, Treatment and prevention of necrotizing enterocolitis. Semin Neonatol, 2003. 8(6):449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gephart SM, McGrath JM, Effken JA, Halpern MD. Necrotizing Enterocolitis Risk: State of the Science. Advances in Neonatal Care. 2012;12(2):77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caplan MS and Jilling T, Neonatal necrotizing enterocolitis: possible role of probiotic supplementation. J Pediatr Gastroenterol Nutr, 2000. 30 Suppl 2: S18–22. [PubMed] [Google Scholar]
- 5.Feng J, El-Assal ON, and Besner GE, Heparin-binding EGF-like growth factor (HB-EGF) and necrotizing enterocolitis. Semin Pediatr Surg, 2005. 14(3):167–74. [DOI] [PubMed] [Google Scholar]
- 6.Schwiertz A, et al. , Development of the intestinal bacterial composition in hospitalized preterm infants in comparison with breast-fed, full-term infants. Pediatr Res, 2003. 54(3): 393–9. [DOI] [PubMed] [Google Scholar]
- 7.Rowland KJ, Choi PM, Warner BW. The Role of Growth Factors in Intestinal Regeneration and Repair in Necrotizing Enterocolitis. Seminars in pediatric surgery. 2013; 22(2):101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMellen ME, Wakeman D, Longshore SW, et al. Growth factors: possible roles for clinical management of the short bowel syndrome. Semin Pediatr Surg. 2010; 19:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warner BW, Warner BB. Role of epidermal growth factor in the pathogenesis of neonatal necrotizing enterocolitis. Seminars in pediatric surgery. 2005; 14:175–180. [DOI] [PubMed] [Google Scholar]
- 10.Playford RJ, et al. , The epidermal growth factor receptor (EGF-R) is present on the basolateral, but not the apical, surface of enterocytes in the human gastrointestinal tract. Gut, 1996. 39(2):262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Britton JR, et al. , Minimal hydrolysis of epidermal growth factor by gastric fluid of preterm infants. Gut, 1989. 30(3):327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li AK, et al. , Hypersecretion of submandibular saliva in male mice: trophic response in small intestine. Gastroenterology, 1983. 84(5 Pt 1):949–55. [PubMed] [Google Scholar]
- 13.Miettinen PJ, et al. , Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature, 1995. 376(6538):337–41. [DOI] [PubMed] [Google Scholar]
- 14.Clark JA, et al. , Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol, 2006. 291(5):G938–49. [DOI] [PubMed] [Google Scholar]
- 15.Miller CA and Debas HT, Epidermal growth factor stimulates the restitution of rat gastric mucosa in vitro. Exp Physiol, 1995. 80(6):1009–18. [DOI] [PubMed] [Google Scholar]
- 16.Wilson AJ, Gibson PR. Role of epidermal growth factor receptor in basal and stimulated colonic epithelia cell migration in vitro. Exp Cell Research, 1999. 250:187–196. [DOI] [PubMed] [Google Scholar]
- 17.Clark JA, et al. , Epidermal growth factor reduces intestinal apoptosis in an experimental model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol, 2005. 288(4):G755–62. [DOI] [PubMed] [Google Scholar]
- 18.Berseth CL. Gut motility and the pathogenesis of necrotizing enterocolitis. Clin Perinatol 1994; 21:263–70. [PubMed] [Google Scholar]
- 19.Halpern MD and Denning PW, The role of intestinal epithelial barrier function in the development of NEC. Tissue Barriers, 2015. 3(1–2):e1000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Claud EC and Walker WA, Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. FASEB J, 2001. 15(8):1398–403. [DOI] [PubMed] [Google Scholar]
- 21.Hodzic Z, Bolock AM, Good M. The Role of Mucosal Immunity in the Pathogenesis of Necrotizing Enterocolitis. Frontiers in Pediatrics. 2017; 5:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dvorak B, Halpern MD, Holubee H, et al. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol 2002;282: G156–64. [DOI] [PubMed] [Google Scholar]
- 23.Shin CE, Falcone RA Jr, Stuart L, et al. Diminished epidermal growth factor levels in infants with necrotizing enterocolitis. J Pediatr Surg 2000; 35:173–6. [DOI] [PubMed] [Google Scholar]
- 24.Warner BB, Ryan AL, Seeger KJ et al. Ontogeny of salivary epidermal growth factor and necrotizing enterocolitis. J Pediatr 2007; 150:358–63. [DOI] [PubMed] [Google Scholar]
- 25.Dvorak B, et al. , Concentrations of epidermal growth factor and transforming growth factor-alpha in preterm milk. Adv Exp Med Biol, 2004. 554:407–9. [DOI] [PubMed] [Google Scholar]
- 26.Xiao X, et al. , Epidermal growth factor concentrations in human milk, cow’s milk and cow’s milk-based infant formulas. Chin Med J (Engl), 2002. 115(3):451–4. [PubMed] [Google Scholar]
- 27.Caplan MS, Simon D, and Jilling T, The role of PAF, TLR, and the inflammatory response in neonatal necrotizing enterocolitis. Semin Pediatr Surg, 2005. 14(3):145–51. [DOI] [PubMed] [Google Scholar]
- 28.Halpern MD, et al. , Ileal cytokine dysregulation in experimental necrotizing enterocolitis is reduced by epidermal growth factor. J Pediatr Gastroenterol Nutr, 2003. 36(1):126–33. [DOI] [PubMed] [Google Scholar]
- 29.Jin K, et al. , Heparin-binding epidermal growth factor-like growth factor: hypoxia inducible expression in vitro and stimulation of neurogenesis in vitro and in vivo. J Neurosci, 2002. 22(13):5365–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishi E, et al. , N-arginine dibasic convertase is a specific receptor for heparin-binding EGF-like growth factor that mediates cell migration. EMBO J, 2001. 20(13):3342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michalsky MP, et al. , Heparin-binding EGF-like growth factor is present in human amniotic fluid and breast milk. J Pediatr Surg, 2002. 37(1):1–6. [DOI] [PubMed] [Google Scholar]
- 32.Davis-Fleischer KM and Besner GE, Structure and function of heparin-binding EGF-like growth factor (HB-EGF). Front Biosci, 1998. 3:288–99 [DOI] [PubMed] [Google Scholar]
- 33.El-Assal ON and Besner GE, HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology, 2005. 129(2):609–25. [DOI] [PubMed] [Google Scholar]
- 34.Feng J and Besner GE, Heparin-binding epidermal growth factor-like growth factor promotes enterocyte migration and proliferation in neonatal rats with necrotizing enterocolitis. J Pediatr Surg, 2007. 42(1):214–20. [DOI] [PubMed] [Google Scholar]
- 35.Su Y, Yang J, and Besner GE, HB-EGF promotes intestinal restitution by affecting integrin-extracellular matrix interactions and intercellular adhesions. Growth Factors, 2013. 31(1):39–55. [DOI] [PubMed] [Google Scholar]
- 36.Markel TA, et al. , Stem cells as a potential future treatment of pediatric intestinal disorders. J Pediatr Surg, 2008. 43(11):1953–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen CL, et al. , Heparin-binding EGF-like growth factor protects intestinal stem cells from injury in a rat model of necrotizing enterocolitis. Lab Invest, 2012. 92(3):331–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watkins DJ, et al. , Heparin-binding epidermal growth factor-like growth factor protects mesenchymal stem cells. J Surg Res, 2012. 177(2):359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang J, et al. , Heparin-binding epidermal growth factor-like growth factor and mesenchymal stem cells act synergistically to prevent experimental necrotizing enterocolitis. J Am Coll Surg, 2012. 215(4):534–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barlow AJ, et al. , Critical numbers of neural crest cells are required in the pathways from the neural tube to the foregut to ensure complete enteric nervous system formation. Development, 2008. 135(9):1681–91. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Y, et al. , Enteric nervous system abnormalities are present in human necrotizing enterocolitis: potential neurotransplantation therapy. Stem Cell Res Ther, 2013. 4(6): 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei J, Zhou Y, Besner GE. Heparin-Binding EGF-like growth factor and enteric neural stem cell transplantation in the prevention of experimental necrotizing enterocolitis. Pediatr Res, 2015;78(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim M, et al. , Immature oxidative stress management as a unifying principle in the pathogenesis of necrotizing enterocolitis: insights from an agent-based model. Surg Infect (Larchmt), 2012. 13(1):8–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michalsky MP, et al. , Heparin-binding EGF-like growth factor decreases apoptosis in intestinal epithelial cells in vitro. J Pediatr Surg, 2001. 36(8): 130–5. [DOI] [PubMed] [Google Scholar]
- 45.Kuhn MA, et al. , Heparin-binding EGF-like growth factor (HB-EGF) decreases oxygen free radical production in vitro and in vivo. Antioxid Redox Signal, 2002. 4(4):639–46. [DOI] [PubMed] [Google Scholar]
- 46.Feng J, El-Assal ON, and Besner GE, Heparin-binding epidermal growth factor-like growth factor reduces intestinal apoptosis in neonatal rats with necrotizing enterocolitis. J Pediatr Surg, 2006. 41(4):742–7 [DOI] [PubMed] [Google Scholar]
- 47.Rocourt DV, Mehta VB, and Besner GE, Heparin-binding EGF-like growth factor decreases inflammatory cytokine expression after intestinal ischemia/reperfusion injury. J Surg Res, 2007. 139(2): 269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia G, et al. , Heparin-binding EGF-like growth factor decreases inducible nitric oxide synthase and nitric oxide production after intestinal ischemia/reperfusion injury. Antioxid Redox Signal, 2001. 3(5): 919–30. [DOI] [PubMed] [Google Scholar]
- 49.Anand RJ, et al. , The role of the intestinal barrier in the pathogenesis of necrotizing enterocolitis. Shock, 2007. 27(2):124–33. [DOI] [PubMed] [Google Scholar]
- 50.Xia G, et al. , Heparin-binding EGF-like growth factor preserves crypt cell proliferation and decreases bacterial translocation after intestinal ischemia/reperfusion injury. J Pediatr Surg, 2002. 37(7):1081–7. [DOI] [PubMed] [Google Scholar]
- 51.Zhang HY, Radulescu A, and Besner GE, Heparin-binding epidermal growth factor-like growth factor is essential for preservation of gut barrier function after hemorrhagic shock and resuscitation in mice. Surgery, 2009. 146(2):334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng J, El-Assal ON, and Besner GE, Heparin-binding EGF-like growth factor (HB-EGF) and necrotizing enterocolitis. Semin Pediatr Surg, 2005. 14(3): 67–74. [DOI] [PubMed] [Google Scholar]
- 53.Nakame K, Kaji T, Mukai M, et al. The protective and anti-inflammatory effects of glucagon-like peptide-2 in an experimental rat model for necrotizing enterocolitis. Peptides 2016. 75:1–7. [DOI] [PubMed] [Google Scholar]
- 54.Rowland KJ and Brubaker PL, The “cryptic” mechanism of action of glucagon-like peptide-2. Am J Physiol Gastrointest Liver Physiol, 2011. 301(1):G1–8. [DOI] [PubMed] [Google Scholar]
- 55.Lee YC, Brubaker PL, and Drucker DJ, Developmental and tissue-specific regulation of proglucagon gene expression. Endocrinology, 1990. 127(5):2217–22. [DOI] [PubMed] [Google Scholar]
- 56.Benight NM, Stoll B, Olutoye OO, Holst JJ, Burrin DG. GLP-2 Delays but Does Not Prevent the Onset of Necrotizing Enterocolitis in Preterm Pigs. Journal of pediatric gastroenterology and nutrition. 2013;56(6):623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alavi K, Schwartz MZ, Palazzo JP, et al. Treatment of inflammatory bowel disease in a rodent model with the intestinal growth factor glucagon-like peptide-2. J Pediatr Surg. 2000; 35:847–51. [DOI] [PubMed] [Google Scholar]
- 58.Yazbeck R, Sulda ML, Howarth GS, et al. Dipeptidyl peptidase expression during experimental colitis in mice. Inflamm Bowel Dis. 2010; 16:1340–51. [DOI] [PubMed] [Google Scholar]
- 59.Xie S, et al. , GLP-2 Suppresses LPS-Induced Inflammation in Macrophages by Inhibiting ERK Phosphorylation and NF-kappaB Activation. Cell Physiol Biochem, 2014. 34(2):590–602. [DOI] [PubMed] [Google Scholar]
- 60.Sigalet DL, et al. , A pilot study examining the relationship among Crohn disease activity, glucagon-like peptide-2 signalling and intestinal function in pediatric patients. Can J Gastroenterol, 2013. 27(10):587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blonski W, et al. , Teduglutide in Crohn’s disease. Expert Opin Biol Ther, 2013. 13(8): 1207–14. [DOI] [PubMed] [Google Scholar]
- 62.Drucker DJ, et al. Human [Gly2]GLP-2 reduces the severity of colonic injury in a murine model of experimental colitis. Am J Physiol, 1999. 276(1 Pt 1):G79–91. [DOI] [PubMed] [Google Scholar]
- 63.Latres E, et al. , Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J Biol Chem, 2005. 280(4):2737–44. [DOI] [PubMed] [Google Scholar]
- 64.Freier S, et al. , Relative expression and localization of the insulin-like growth factor system components in the fetal, child and adult intestine. J Pediatr Gastroenterol Nutr, 2005. 40(2): 202–9. [DOI] [PubMed] [Google Scholar]
- 65.Tian F, Liu GR, Li N, Yuan G. Insulin-like growth factor I reduces the occurrence of necrotizing enterocolitis by reducing inflammatory response and protecting intestinal mucosal barrier in neonatal rat model. Eur Rev Med Pharmacol Sci, 2017. 21(20):4711–4719. [PubMed] [Google Scholar]
- 66.Wilkins HR, et al. , Reduction of spontaneous and irzadiation-induced apoptosis in small intestine of IGF-I transgenic mice. Am J Physiol Gastrointest Liver Physiol, 2002. 283(2):G457–64. [DOI] [PubMed] [Google Scholar]
- 67.Ni F, Sun R, Fu B, et al. IGF-1 promotes the development and cytotoix activity of human NK cells. Natu Commun, 2013. 4:1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jeschke MG, et al. , Gut mucosal homeostasis and cellular mediators after severe thermal trauma and the effect of insulin-like growth factor-I in combination with insulin-like growth factor binding protein-3. Endocrinology, 2007. 148(1): 354–62. [DOI] [PubMed] [Google Scholar]
- 69.Ozen S, et al. , Insulin-like growth factor attenuates apoptosis and mucosal damage in hypoxia/reoxygenation-induced intestinal injury. Biol Neonate, 2005. 87(2):91–6. [DOI] [PubMed] [Google Scholar]
- 70.Lorenzo-Zuniga V, et al. , Insulin-like growth factor I improves intestinal barrier function in cirrhotic rats. Gut, 2006. 55(9):1306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hunninghake GW, et al. , Insulin-like growth factor-1 levels contribute to the development of bacterial translocation in sepsis. Am J Respir Crit Care Med, 2010. 182(4): 517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang W, et al. , Insulin-like growth factor-I improves mucosal structure and function in transplanted rat small intestine. Transplantation, 1995. 59(5):755–61 [DOI] [PubMed] [Google Scholar]
- 73.Corpeleijn WE, et al. , Effect of enteral IGF-1 supplementation on feeding tolerance, growth, and gut permeability in enterally fed premature neonates. J Pediatr Gastroenterol Nutr, 2008. 46(2):184–90. [DOI] [PubMed] [Google Scholar]
- 74.Baregamian N, et al. , Phosphatidylinositol 3-kinase pathway regulates hypoxia inducible factor-1 to protect from intestinal injury during necrotizing enterocolitis. Surgery, 2007. 142(2): 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Juul SE, et al. , Origin and fate of erythropoietin in human milk. Pediatr Res, 2000. 48(5):660–7. [DOI] [PubMed] [Google Scholar]
- 76.Yu Y, Shiou S-R, Guo Y, et al. Erythropoietin Protects Epithelial Cells from Excessive Autophagy and Apoptosis in Experimental Neonatal Necrotizing Enterocolitis. Denning PW, ed. PLoS ONE. 2013;8(7):e69620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guneli E, et al. , Erythropoietin protects the intestine against ischemia/reperfusion injury in rats. Mol Med, 2007. 13(9–10):509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumral A, et al. , Erythropoietin protects against necrotizing enterocolitis of newborn rats by the inhibiting nitric oxide formation. Biol Neonate, 2003. 84(4):325–9. [DOI] [PubMed] [Google Scholar]
- 79.Shiou SR, et al. , Erythropoietin protects intestinal epithelial barrier function and lowers the incidence of experimental neonatal necrotizing enterocolitis. J Biol Chem, 2011. 286(14):12123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ledbetter DJ and Juul SE, Erythropoietin and the incidence of necrotizing enterocolitis in infants with very low birth weight. J Pediatr Surg, 2000. 35(2):178–81. [DOI] [PubMed] [Google Scholar]
- 81.El-Ganzoury MM, et al. , Enteral Granulocyte-Colony Stimulating Factor and Erythropoietin Early in Life Improves Feeding Tolerance in Preterm Infants: A Randomized Controlled Trial. J Pediatr, 2014. [DOI] [PubMed] [Google Scholar]
- 82.Rosenfeld RG and Hwa V, The growth hormone cascade and its role in mammalian growth. Horm Res, 2009. 71 Suppl 2:36–40. [DOI] [PubMed] [Google Scholar]
- 83.Tei TM, et al. , Growth hormone treatment increases transmural colonic growth in GH-deficient dwarf rats. Growth Horm IGF Res, 2000. 10(2): 85–92. [DOI] [PubMed] [Google Scholar]
- 84.Ido A, et al. , Mucosal repair and growth factors: recombinant human hepatocyte growth factor as an innovative therapy for inflammatory bowel disease. J Gastroenterol, 2005. 40(10): 925–31. [DOI] [PubMed] [Google Scholar]
- 85.Zarnegar R and Michalopoulos GK, The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J Cell Biol, 1995. 129(5): 1177–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Uehara Y, et al. , Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature, 1995. 373(6516): p. 702–5. [DOI] [PubMed] [Google Scholar]
- 87.Kuenzler KA, Pearson PY, and Schwartz MZ, Hepatocyte growth factor pretreatment reduces apoptosis and mucosal damage after intestinal ischemia reperfusion. J Pediatr Surg, 2002. 37(7): 1093–7. [DOI] [PubMed] [Google Scholar]
- 88.Itoh H, et al. , Regeneration of injured intestinal mucosa is impaired in hepatocyte growth factor activator-deficient mice. Gastroenterology, 2004. 127(5):1423–35. [DOI] [PubMed] [Google Scholar]
- 89.Jain SK, et al. , Amniotic fluid-borne hepatocyte growth factor protects rat pups against experimental necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol, 2014. 306(5):G361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cai Y, et al. , Keratinocyte growth factor pretreatment prevents radiation-induced intestinal damage in a mouse model. Scand J Gastroenterol, 2013. 48(4):419–26. [DOI] [PubMed] [Google Scholar]
- 91.Taupin DR, Kinoshita K, and Podolsky DK, Intestinal trefoil factor confers colonic epithelial resistance to apoptosis. Proc Natl Acad Sci U S A, 2000. 97(2):799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.MohanKumar K, et al. , Gut mucosal injury in neonates is marked by macrophage infiltration in contrast to pleomorphic infiltrates in adult: evidence from an animal model. Am J Physiol Gastrointest Liver Physiol, 2012. 303(1):G93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]