Abstract
The dorsal lateral geniculate nucleus (dLGN) of the thalamus is the principal conduit for visual information from retina to visual cortex. Viewed initially as a simple relay, recent studies in the mouse reveal far greater complexity in the way input from the retina is combined, transmitted, and processed in dLGN. Here we consider the structural and functional organization of the mouse retinogeniculate pathway by examining the patterns of retinal projections to dLGN and how they converge onto thalamocortical neurons to shape the flow of visual information to visual cortex.
Keywords: Vision, Retinogeniculate pathway, Retinal ganglion cells, Thalamocortical neurons
Introduction
The dorsal lateral geniculate nucleus of the thalamus (dLGN) connects the retina to visual cortex. Early studies suggested that the retina sends signals to dLGN through the axons of relatively few retinal ganglion cell (RGC) types that carry parallel streams of visual information (Martin, 1986). In dLGN, each thalamocortical neuron (TC) was reported to receive input from one or few RGCs (Levick et al., 1972; Chen & Regehr, 2000; Hong et al., 2014), maintaining separation of the incoming channels. As a result, dLGN was thought to function as a relatively simple relay of retinal information to visual cortex (Hubel & Wiesel, 1961; Lee et al., 1983; Tavazoie & Reid, 2000; Grubb & Thompson, 2003). Recent studies, however, have revealed far greater diversity among RGC types (Field & Chichilnisky, 2007; Baden et al., 2016), most of which send axons to dLGN (Dacey et al., 2003; Ellis et al., 2016). In addition, anatomical circuit reconstructions demonstrated that convergence of RGC axons onto TCs is higher than previously thought (Hammer et al., 2015; Morgan et al., 2016; Rompani et al., 2017); and functional recordings uncovered diverse light responses among TCs (Marshel et al., 2012; Piscopo et al., 2013; Zhao et al., 2013a). These studies have renewed interest in the functional organization of dLGN. Here, we discuss our current understanding of this organization from two sides: the projection patterns of RGC axons, and the diversity and distribution of TC neurons in dLGN. For the sake of clarity and brevity, we focus primarily on studies of mice.
The organization of RGC projections in mouse dLGN
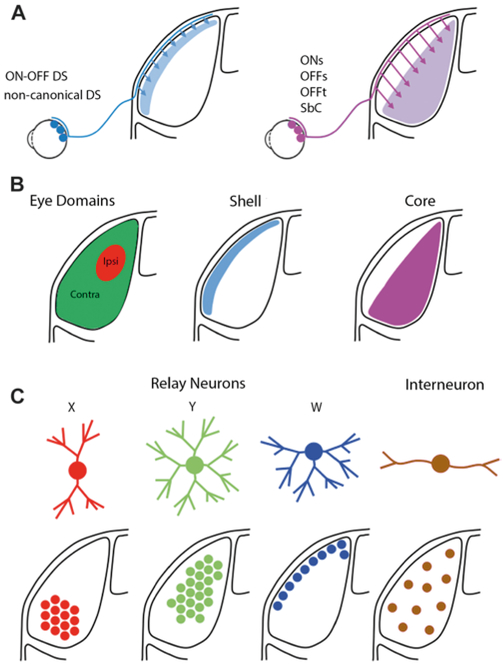
The dLGN receives information about the outside world most directly from RGC axons, the terminations of which are organized into overlapping maps according to three criteria: (i) eye of origin (i.e., eye-specific segregation), (ii) topographic position within the retina (i.e., retinotopic map), and (iii) cell type (i.e., cell-type-specific lamination) (Fig. 1).
Fig. 1.
Schematics illustrate the organization of mouse dLGN. (A) Pattern of projections for different RGC cell types. (B) Hidden lamination in mouse dLGN. Left: Eye specific patterning of retinal projections arising from the contralateral (green) and ipsilateral eye. Right: Shell (blue) and core (purple) subdivisions. The shell receives convergent input from DSGCs and the superficial layers of the superior colliculus. The core receives input largely from RGCs with a canonical center surround organization. (C) Dendritic architecture of different classes of relay neurons (X, Y, W) and interneurons along with their regional preferences within dLGN.
Eye-specific segregation of RGC axons in dLGN
In mice, as in other animals with laterally positioned eyes, the majority or RGC axons cross sides in the optic chiasm (Jaubert-Miazza et al., 2005; Petros et al., 2008; Dhande & Huberman, 2014). Tracer injections showed that axons from contra- and ipsilateral eyes occupy nonoverlapping domains of the mature dLGN (Godement et al., 1984; Reese, 1988; Muir-Robinson et al., 2002; Jaubert-Miazza et al., 2005) (Fig. 1B). The small ipsilateral projection localizes to the medial dLGN, and is topographically aligned with the contralateral projection (Reese & Jeffery, 1983; Reese, 1988). During development, eye-specific segregation emerges gradually by refinement of initially overlapping axons (Godement et al., 1984; Muir-Robinson et al., 2002; Jaubert-Miazza et al., 2005). Sparse labeling by in vivo electroporation revealed that at the level of single RGCs, refinement involves the elaboration of axon arbors prepositioned in the proper location and the elimination of inappropriately targeted sparse branches (Dhande et al., 2011). Axonal refinement is instructed by spontaneous activity patterns (i.e., retinal waves), which synchronize the firing of RGCs in the same eye (Meister et al., 1991; Ackman et al., 2012); and perturbations of retinal waves can block segregation and desegregate refined projections (Chapman, 2000; Stellwagen & Shatz, 2002; Demas et al., 2006; Koch et al., 2011; Zhang et al., 2011; Burbridge et al., 2014). The initial positioning of RGC axons in dLGN is determined by molecular gradients of Ephs and ephrins (McLaughlin & O’Leary, 2005; Huberman et al., 2008; Cang & Feldheim, 2013); and, although spontaneous activity can still drive eye-specific segregation when Eph/ephrin signaling is perturbed, ipsilateral patches are fractured and mislocalized (Huberman et al., 2005; Pfeiffenberger et al., 2005).
The small size of the ipsilateral projection (Jaubert-Miazza et al., 2005; Coleman et al., 2009) and the comparatively large size of TC dendritic arbors (Krahe et al., 2011; Morgan et al., 2016), suggest that information from both eyes may converge in dLGN. A recent trans-synaptic tracing study showed that a subset of TC neurons receive input from both eyes (Rompani et al., 2017). The extent and stimulus conditions under which binocular responses occur in dLGN are a topic of debate and ongoing investigation (Grubb et al., 2003; Ziburkus & Guido, 2006; Zhao et al., 2013b; Howarth et al., 2014) (see part II below).
Retinotopic map of RGC axons in dLGN
To preserve spatial information about the visual world, axons of neighboring RGCs project to neighboring places in dLGN, forming retinotopic maps (Reese & Jeffery, 1983; Reese, 1988; McLaughlin & O’Leary, 2005; Huberman et al., 2008). Retinotopic order is maintained beyond dLGN along the ventral and dorsal streams of the visual system (Andermann et al., 2011; Marshel et al., 2011; Wang et al., 2011; Roth et al., 2012; Wang et al., 2012). Given the convergence of multiple RGCs onto a single TC neuron (Hong et al., 2014; Hammer et al., 2015; Morgan et al., 2016), precise retinotopic mapping of RGC axons is required for contiguous high-acuity receptive fields in dLGN. In cats and ferrets, mature TC receptive fields emerge from spatially and functionally imprecise beginnings during a period of refinement (Tavazoie & Reid, 2000; Akerman et al., 2004). No data on the development of TC receptive fields in mice have been published, but anatomical studies indicate that topographic precision of RGC projections increases during the first two weeks of life (Dhande et al., 2012). Similar to eye-specific segregation, retinotopic maps of RGC axons are established and refined by the combined action of Eph/ephrin gradients and activity-dependent plasticity (McLaughlin & O’Leary, 2005; Huberman et al., 2008; Cang & Feldheim, 2013; Xu et al., 2015). When Eph/ephrin signaling is perturbed, projections from nearby RGCs are split, disrupting retinotopic order in dLGN (Pfeiffenberger et al., 2006). By contrast, termination zones of RGC axons remain appropriately localized but broaden when spontaneous activity patterns are perturbed (Grubb et al., 2003; Burbridge et al., 2014) widening TC receptive fields (Grubb et al., 2003; Cang et al., 2008).
Cell-type-specific lamination of RGC axons in dLGN
Morphological and functional surveys, and an increasing number of transgenic mouse lines reveal extraordinary diversity among RGCs, which comprise 30–40 distinct cell types in mice (Sun et al., 2002; Badea & Nathans, 2004; Coombs et al., 2006; Helmstaedter et al., 2013; Sumbul et al., 2014; Sanes & Masland, 2015; Baden et al., 2016). Retrograde labeling indicates that most of these RGCs project to dLGN in mice (Ellis et al., 2016), as they do in primates (Dacey et al., 2003), suggesting that a large number of parallel information streams enter dLGN. To what extent incoming streams remain separate, or how their information is combined by TCs depends in part on the cell-type-specific projection patterns of RGC axons in dLGN (Fig. 1).
In primates, cats, and ferrets, dLGN neurons are separated into distinct cellular layers that receive input from specific RGC types (Usrey & Alitto, 2015); whereas in mouse and rat, dLGN neurons show no apparent separation (Reese, 1988; Usrey & Alitto, 2015). Yet, RGC axons impose order on these comparatively unorganized targets by arborizing in cell-type-specific patterns (Fig. 1A). Early tracing studies hinted at lamination of RGC axons in rats (Reese, 1988). This organization is now being revealed in increasing detail by a growing number of transgenic mouse lines that label specific subsets or individual types of RGCs (Siegert et al., 2009; Hong et al., 2014; Dhande et al., 2015; Sanes & Masland, 2015). In addition to studies of individual mouse lines, the Allen Mouse Brain Connectivity Atlas includes adeno-associated virus (AAV) tracing studies of projections from RGCs labeled in a variety of Cre-driver lines (http://connectivity.brain-map.org/). A summary of this effort was recently published (Martersteck et al., 2017).
The mouse retina contains a large number of direction selective ganglion cell (DSGC) types (Borst & Euler, 2011; Sanes & Masland, 2015). Among these, two canonical groups are distinguished by their contrast preferences: ON-DSGCs respond to light increments and ON–OFF DSGCs respond to light increments and decrements (Borst & Euler, 2011; Sanes & Masland, 2015). ON-DSGCs prefer motion in one of three directions that are aligned with the orientation of the semicircular canals in the inner ear (Yonehara et al., 2009; Dhande et al., 2013). ON-DSGCs largely avoid dLGN, project to brainstem nuclei of the accessory optic system, and, together with the vestibular system, drive image stabilizing eye movements (Simpson, 1984; Yonehara et al., 2009; Dhande et al., 2013; Gauvain & Murphy, 2015; Osterhout et al., 2015; Sun et al., 2015). ON–OFF DSGCs prefer motion in one of four cardinal directions (nasal, temporal, dorsal, or ventral) (Borst & Euler, 2011; Sanes & Masland, 2015). More than one cell type may exist for each preferred direction (Rivlin-Etzion et al., 2011; Baden et al., 2016); and all ON–OFF DSGC types examined so far project to the ventricular margin of the dLGN, also known as the dLGN shell (Huberman et al., 2009; Kim et al., 2010; Kay et al., 2011; Rivlin-Etzion et al., 2011) (Fig. 1A and1B). Their projection patterns are not uniform, however, as axon arbors of ventral motion preferring ON–OFF DSGCs also cover an adjacent layer in the dLGN core (Kim et al., 2010; Kay et al., 2011). Interestingly, TCs in the dLGN shell and core project to different layers of visual cortex (layers 1 and 2 vs., layer 4, respectively) indicating that RGCs projecting to the respective areas participate in separate visual pathways (Grubb & Thompson, 2004; Cruz-Martin et al., 2014; Bickford et al., 2015). ON–OFF DSGCs target the dLGN shell before eye opening (Kay et al., 2011; Osterhout et al., 2014) by mechanisms that remain to be uncovered, and maintain their laminar position independent of spontaneous and sensory-evoked activity patterns (Soto et al., 2012).
Recently, three noncanonical DSGC types (J-, F-miniON-, and F-miniOFF-RGCs) were identified based on gene expression patterns, and characterized in two transgenic mouse lines (Kim et al., 2008; Joesch & Meister, 2016; Rousso et al., 2016). These noncanonical DSGCs have asymmetric dendritic arbors and uniformly prefer ventral motion (Kim et al., 2008; Rousso et al., 2016). Dendrites of noncanonical DSGCs stratify outside the ChAT (i.e., cholineacetyl-transferase) bands formed by neurites of starburst amacrine cells, which are critical for canonical direction selective responses in the retina (Borst & Euler, 2011). Although the circuit mechanisms underlying their response selectivity therefore likely differ from those of canonical DSGCs, the axons of J- and F-miniON- and F-miniOFF-RGCs similarly target the dLGN shell (Kay et al., 2011; Rousso et al., 2016) (Fig. 1A and 1B).
Patch clamp recordings from large somata in the ganglion cell layer of the retina led to the characterization of three RGC types: one responds with sustained firing to light increments (ONS-RGCs), another responds with sustained firing to light decrements (OFFS-RGC), and the third responds transiently to light decrements (OFFT-RGC) (Murphy & Rieke, 2006). Based on morphological and functional homology to RGC types in cats, these cells are also referred to as ONα (ONS), OFFδ) (OFFS), and OFFα (OFFT) (Pang et al., 2003; Park et al., 2015). OFFT-RGCs were one of the first genetically identified RGC types (CB2-EGFP mice), whose central projections were mapped (Huberman et al., 2008). Since then, different combinations ONS-, OFFS-, and OFFT-RGCs have been found to be labeled in a number of transgenic mouse lines (Ecker et al., 2010; Farrow et al., 2013; Bleckert et al., 2014; Duan et al., 2014). Results from the initial characterizations of these mice and from the Allen Brain Connectivity Atlas, suggest that ONS-, OFFS-, and OFFT-RGCs project to medial aspects of the dLGN core (Fig. 1A and 1B). This conclusion is further supported by retrograde and trans-synaptic viral labeling studies, and by the preponderance of ONS, OFFS, and OFFT responses in the core of the dLGN (Piscopo et al., 2013; Cruz-Martin et al., 2014; Ellis et al., 2016).
Among the transgenic mice that label ONS-RGCs is Opn4-Cre, a line in which Cre recombinase is expressed from the Opn4 (i.e., melanopsin) locus (Ecker et al., 2010; Schmidt et al., 2014). Melanopsin mediates light responses in a subset of RGCs, referred to collectively as intrinsically photosensitive RGCs (ipRGCs) (Provencio et al., 2000; Berson et al., 2002; Hattar et al., 2002). A number of different ipRGC types have been distinguished (M1–M4) (Tu et al., 2005; Ecker et al., 2010; Schmidt et al., 2011; Estevez et al., 2012). All ipRGCs receive synaptic input from the retinal circuitry in addition to their intrinsic responses. The strengths of synaptic and intrinsic inputs appear to be inversely proportional and vary between ipRGC types, with M1 ipRGCs showing the strongest intrinsic responses and ONS-RGCs (i.e., M4 ipRGCs) showing the weakest intrinsic responses (Wong et al., 2007; Schmidt & Kofuji, 2009; Estevez et al., 2012; Schmidt et al., 2014). M1–M3 ipRGCs project to numerous subcortical visual areas, but avoid dLGN (Hattar et al., 2006), whereas ONS-RGCs (i.e., M4 ipRGCs) project to the dLGN core (Ecker et al., 2010) (Fig. 1A and 1B). In addition to this direct pathway, melanopsin-mediated light responses regulate visual signals in dLGN through intraretinal influences of ipRGCs (Zhang et al., 2008; Brown et al., 2010; Allen et al., 2014; Schmidt et al., 2014; Reifler et al., 2015; Prigge et al., 2016).
RGCs are often broadly divided into ON, OFF, and ON–OFF groups, based on whether their firing rate increases in response to light increments, decrements, or both. However, one (or several) RGC type(s) does not fit into this classification scheme, and instead exhibits high baseline firing rates that are suppressed by ON and OFF stimuli. These cells are conserved from rodents to primates and are referred to as Suppressed-by-Contrast (SbC-) RGCs or uniformity detectors (Levick, 1967; Rodieck, 1967; de Monasterio, 1978; Sivyer et al., 2010; Tien et al., 2015). With the help of transgenic mice, the circuit mechanisms underlying the suppressive responses of SbC-RGCs are being worked out (Jacoby et al., 2015; Tien et al., 2015; Lee et al., 2016; Tien et al., 2016). Unfortunately, no line so far labels SbC-RGCs exclusively, and their central projection patterns therefore remain somewhat uncertain. Nonetheless, two transgenic mouse lines that cover SbC-RGCs show strong projections to the dLGN core (Ivanova et al., 2013; Zhu et al., 2014), and SbC responses have been recorded in dLGN and V1 (Niell & Stryker, 2010; Piscopo et al., 2013) (Fig. 1A and 1B). Together these findings suggest that signals from SbC-RGCs may propagate along a dedicated retino-geniculo-cortical pathway. Alternatively, SbC signals could be generated by different mechanisms at subsequent stages of the visual system, similar to orientation selective (OS) responses (Niell, 2013).
In spite of the recent progress, the projection patterns of many RGC types are still unknown. In addition to providing a more comprehensive picture of cell-type-specific lamination, future work will further elucidate what retinal information is excluded from dLGN. In addition to ON-DSGCs and M1–M3 ipRGCs, a recent study comparing functional properties of RGCs retrogradely labeled from dLGN and superior colliculus (SC), indicates that, although a majority of cells project to both targets, several RGC types that respond transiently and selectively to small stimuli avoid dLGN (Ellis et al., 2016).
The organization of mouse dLGN
In mouse, the dLGN is a bean-shaped nucleus that resides in the dorsal lateral aspect of thalamus. In Nissl stained material, it is a homogenous structure with cytoarchitectural boundaries that separate it from the ventral basal complex, the intrageniculate leaflet, and ventral geniculate nuclei. As discussed above, “hidden laminae” exist in the form of eye specific retinal terminal domains, and as a shell and core region (Fig. 1B). The shell occupies a small strip of dLGN parallel to and just beneath the optic tract that receives input exclusively from the contralateral eye. The much larger core division lies beneath the shell, and receives input from both eyes, with those from the ipsilateral eye forming a small nonoverlapping, patchy cylinder that courses through the antero-medial region of the core. As discussed above, the shell and core receive input from distinct classes of RGCs (Fig. 1A and 1B). The shell is the primary recipient domain for many types of DSGCs, while the core harbors a diverse group of RGC input that in the aggregate appear to mediate canonical aspects of spatial vision (Dhande & Huberman, 2014) (Fig. 1A and 1B). Additionally, the shell receives strong, excitatory input from superficial layers of SC, and together with input from DSGCs is believed to form a highly specialized visual channel that conveys information about stimulus motion and eye position to the superficial layers of visual cortex (Cruz-Martin et al., 2014; Bickford et al., 2015). Indeed, the shell of mouse dLGN shares many of the same features noted in the C-laminae of carnivores and the koniocellular division of some primates (Demeulemeester et al., 1991; Harting et al., 1991).
Neuronal cell types of dLGN
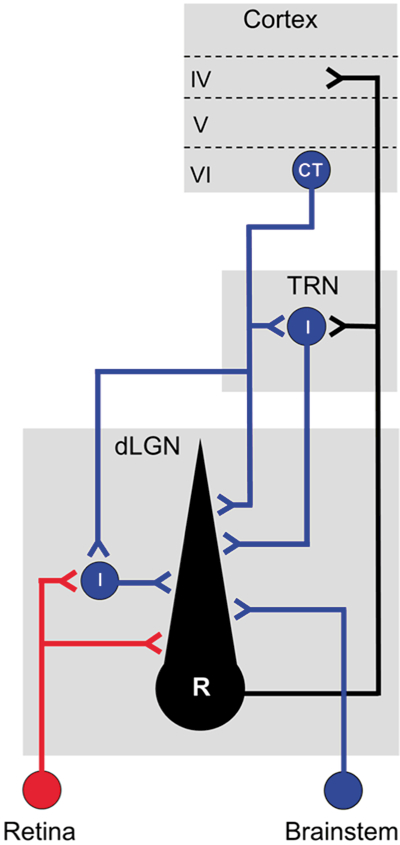
The neuronal composition of mouse dLGN is similar to that of other mammals (Parnavelas et al., 1977; Sherman & Guillery, 2002). There are two principal cell types, thalamocortical relay cells (TC) and interneurons (Fig. 1C). In rodents, roughly 90% of all cells in dLGN are TC neurons, and the remainder interneurons (Arcelli et al., 1997). Both cell types receive retinal input, but only TC neurons have axons that exit the dLGN and project to the visual areas of cortex (Fig. 2). Ascending axons of TC neurons also have collaterals that terminate in the thalamic reticular nucleus, a shell-like structure comprised of GABAergic inhibitory neurons that surrounds the dorsal thalamus (Pinault, 2004). TC neurons make excitatory connections with TRN neurons, which in turn provide feedback inhibition onto TC neurons. Intrinsic interneurons have processes that are restricted to dLGN and form feedforward inhibitory connections with TC neurons (Fig. 2). A more detailed explanation of these inhibitory circuits and underlying synaptic arrangements can be found in accompanying review by Cox.
Fig. 2.
Circuit diagram that depicts retinal (red) and nonretinal (blue) connections of intrinsic interneurons and thalamocortical relay neurons of mouse dLGN.
The morphology of neurons in the rodent LGN has been examined in Golgi impregnated material (Rafols & Valverde, 1973; Parnavelas et al., 1977), and more recently in mouse from single cell intracellular fills performed during in vitro recording experiments (Jaubert-Miazza et al., 2005; Krahe et al., 2011; Seabrook et al., 2013; El-Danaf et al., 2015). Overall, TC neurons have a thick unbranched axon, large round soma, and complex multipolar dendritic arbors, whereas interneurons have a fusiform shaped soma and just a few sinuous dendritic processes. 3-D reconstructions of the dendritic architecture of TC neurons show they can be grouped into three distinct morphological classes that bear a striking resemblance to X (bi-conical), Y (symmetrical), and W (hemispheric) cells of the cat (Friedlander et al., 1981; Stanford et al., 1981, 1983; Krahe et al., 2011) (Fig. 1C). Additionally, each class exhibits a regional preference within dLGN (Krahe et al., 2011). X cells are confined to the monocularly innervated, ventral region of dLGN. Y cells are found in the binocularly innervated central core region, and in some instances exhibit dendritic fields that extend into areas innervated by the contralateral and ipsilateral eye. W cells reside along the outer perimeter, and exclusively in the shell (Bickford et al., 2015). These regional preferences are consistent with earlier studies in the rat, suggesting dLGN is organized into three separate retino-recipient domains; a central core that receives input from large, fast-conducting RGCs, an outer dorsal shell that receives input from small, slowly conducting RGCs and a ventral region for subset of smaller type RGCs (Martin, 1986; Reese, 1988). How these regional preferences and receptive field properties of X, Y, and W correspond to the projection streams of identified RCC cell types remains unclear (see below).
Similar 3-D reconstructions of interneurons do not reveal any subclass distinctions, although two classes may exist based on differences in their intrinsic membrane properties (Leist et al., 2016). Unlike TC neurons, interneurons are evenly dispersed throughout dLGN and have dendrites that readily cross eye-specific domains (Seabrook et al., 2013) (Fig. 1C).
The degree and nature of retinal convergence onto TC neurons has been a topic of intense investigation. Studies in different species including mouse, reveal that retinal input onto dLGN neurons comprise about 10% of the total number of synapses in dLGN, with roughly 90% arising from a variety of nonretinal sources including layer V1 of visual cortex, brainstem cholinergic nuclei, and the thalamic reticular nucleus (Sherman & Guillery, 2002; Sherman, 2004; Bickford et al., 2010) (Fig. 2). Despite this disparity, retinal terminals provide the primary excitatory drive for TC neurons, forming multiple contacts on proximal regions of TC dendrites (Hamos et al., 1987). In mouse, estimates of retinal convergence derived from in vitro slice recordings reveal that at early postnatal ages developing TC neurons receive relatively weak synaptic input from several RGCs, and during the first few weeks of postnatal life then undergo a substantial pruning to ultimately receive strong input from just a few (Guido, 2008; Hong & Chen, 2011). By contrast, interneurons do not go through a pruning period, but instead retain a relatively high level of retinal convergence into adulthood (Seabrook et al., 2013), a feature that is consistent with their unique electronic structure and the synaptic arrangements they have with TC neurons (Sherman, 2004) (see accompanying review by Cox).
The degree of retinal convergence onto mouse TC neurons has been challenged by recent ultrastructural and trans-synaptic tracing studies, suggesting that an individual TC neuron can receive far more inputs than estimated using electrophysiological criteria (Hammer et al., 2015; Morgan et al., 2016; Rompani et al., 2017) (see accompanying review by Morgan). Using innovative trans-synaptic tracing techniques, Rompani et al. (2017) analyzed the number and type of RGCs innervating individual TC neurons. Among the 25 TC neurons analyzed, three modes of convergence were found; a relay mode where a given TC neuron receives monocular input from 1–5 RGCs of the same type, a combination mode where a TC neuron receives monocular input from 6–36 RGCs of different types, and a binocular mode where up 90 inputs of many different types from both eyes converge onto a single TC neuron. How these diverse patterns of convergence relate to receptive field properties of TC neurons and the nature of information transfer to visual cortex remains an open question. While these anatomical and physiological approaches provide somewhat discrepant results, they raise interesting questions about the relationship between form (ultrastructural) and function. One intriguing possibility is that only a few retinal inputs provide the excitatory drive for a TC neuron, while many others remain nascent, perhaps fluctuating in synaptic strength based on postnatal age or the quality of visual experience (Chen et al., 2016). As discussed below, whether TC neurons receive input from just a few or many RGCs, like carnivores and primates, their receptive field properties in many instances appear driven by a single RGC type.
Receptive field properties of dLGN neurons
Generally speaking, most dLGN neurons in mouse have large receptive fields (center diameter of 10–20 deg), summate information in a linear manner, and have a center-surround organization with an RF center that responds either in a sustained or transient manner to stimulus onset (ON) or offset (OFF) (Grubb & Thompson, 2003; Piscopo et al., 2013; Denman & Contreras, 2016; Durand et al., 2016; Suresh et al., 2016; Tang et al., 2016). Sustained ON and OFF responses are encountered more frequently than transient ones, with the latter restricted to OFF responses (Piscopo et al., 2013; Tang et al., 2016). In mouse, dLGN neurons have poor spatial resolution (0.01–0.05 c/d), and respond optimally to relatively low temporal frequencies (1–4 Hz) (Grubb & Thompson, 2003; Piscopo et al., 2013; Durand et al., 2016; Tang et al., 2016). In addition to these somewhat classical dLGN response properties, mouse dLGN neurons display a rather rich and diverse repertoire of unconventional properties. Most notable is the prevalence of responses that show a strong selectivity for one direction (direction selectivity, DS) or to two opposing directions (orientation selective, OS) of a moving stimulus (Marshel et al., 2012; Piscopo et al., 2013; Scholl et al., 2013; Zhao et al., 2013a). These DS/OS responses have broad tuning profiles along the four cardinal axes, remain unaffected by the removal of corticofugal input, and tend to cluster in the dorsal shell, the target recipient zone for many ON–OFF DSGCs (Fig. 1A and 1B). Another unusual property of some dLGN neurons is their ability to signal the absence of contrast in a visual scene (Piscopo et al., 2013; Suresh et al., 2016; Piscopo et al., 2013; Suresh et al., 2016). Such a response profile is similar to that of suppressed by contrast RGCs, showing a decreased firing to either the onset or offset of a visual stimulus (Tien et al., 2015).
Arguably, one of the unique properties of mouse dLGN neurons reported falls outside the realm of image encoding. Using chromatic visual stimuli to activate RGCs that contain the photopigment melanopsin (ipRGCs), it was shown that up to 40% of dLGN neurons respond to whole-field ambient light steps, thereby acting as irradiance detectors (Brown et al., 2010). Irradiant responses in dLGN could possibly originate from core projecting, intrinsically photo-sensitive ON alpha RGCs (i.e., M4 ipRGCs) (Brown et al., 2010; Ecker et al., 2010; Schmidt et al., 2014) but a direct link between this RGC cell type and melanopsin signaling in dLGN is lacking (Fig. 1A and 1B).
There is a consensus that in rodents, dLGN neurons are monocularly driven largely through the contralateral eye (Reese, 1988; Grubb & Thompson, 2003). However of notable exception is one report that provides evidence for a high incidence of binocular responses among mouse dLGN neurons (Howarth et al., 2014). These authors found little evidence to support monocular responses driven through the ipsilateral eye, but instead encountered many neurons with a response profile modulated by bright visual stimuli presented to the ipsilateral eye. A recent trans-synaptic labeling study provides additional support, suggesting that dLGN neurons residing in the binocular segment receive multiple inputs from both eyes (Rompani et al., 2017). The robust binocular responses recorded in mouse dLGN are in stark contrast to the weak polysynaptic, non-dominate eye influences reported in cat and primates (Marrocco & McClurkin, 1979; Guido et al., 1989), and perhaps represent an emergent property unique to the rodent (Grieve, 2005; Zhao et al., 2013b). Certainly, the small ipsilateral terminal domain and large dendritic arbor of Y cells located in the core provide a potential substrate for direct monosynaptic convergence (Fig. 1), but the full extent and the stimulus conditions that underlie binocular responsiveness wait further testing.
Acknowledgments
We thank T. Krahe for his assistance in the design of Fig. 1. This work was supported by NIH EY023341 (DK), EY026978 (DK), EY027411 (DK), and EY 012716 (WG).
References
- Ackman JB, Burbridge TJ & Crair MC (2012). Retinal waves coordinate patterned activity throughout the developing visual system. Nature 490, 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerman CJ, Grubb MS & Thompson ID (2004). Spatial and temporal properties of visual responses in the thalamus of the developing ferret. Journal of Neuroscience 24, 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AE, Storchi R, Martial FP, Petersen RS, Montemurro MA, Brown TM & Lucas RJ (2014). Melanopsin-driven light adaptation in mouse vision. Current Biology 24, 2481–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andermann ML, Kerlin AM, Roumis DK, Glickfeld LL & Reid RC (2011). Functional specialization of mouse higher visual cortical areas. Neuron 72, 1025–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcelli P, Frassoni C, Regondi MC, De Biasi S & Spreafico R (1997). GABAergic neurons in mammalian thalamus: A marker of thalamic complexity? Brain Research Bulletin 42, 27–37. [DOI] [PubMed] [Google Scholar]
- Badea TC & Nathans J (2004). Quantitative analysis of neuronal morphologies in the mouse retina visualized by using a genetically directed reporter. Journal of Comparative Neurology 480, 331–351. [DOI] [PubMed] [Google Scholar]
- Baden T, Berens P, Franke K, Roman Roson M, Bethge M & Euler T (2016). The functional diversity of retinal ganglion cells in the mouse. Nature 529, 345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, Dunn FA & Takao M (2002). Phototransduction by retinal ganglion cells that set the circadian clock. Science 295, 1070–1073. [DOI] [PubMed] [Google Scholar]
- Bickford ME, Slusarczyk A, Dilger EK, Krahe TE, Kucuk C & Guido W (2010). Synaptic development of the mouse dorsal lateral geniculate nucleus. Journal of Comparative Neurology 518, 622–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford ME, Zhou N, Krahe TE, Govindaiah G & Guido W (2015). Retinal and tectal “driver-like” inputs converge in the shell of the mouse dorsal lateral geniculate nucleus. Journal of Neuroscience 35, 10523–10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleckert A, Schwartz GW, Turner MH, Rieke F & Wong RO (2014). Visual space is represented by nonmatching topographies of distinct mouse retinal ganglion cell types. Current Biology 24, 310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst A & Euler T (2011). Seeing things in motion: Models, circuits, and mechanisms. Neuron 71, 974–994. [DOI] [PubMed] [Google Scholar]
- Brown TM, Gias C, Hatori M, Keding SR, Semo M, Coffey PJ, Gigg J, Piggins HD, Panda S & Lucas RJ (2010). Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biology 8, e1000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbridge TJ, Xu HP, Ackman JB, Ge X, Zhang Y, Ye MJ, Zhou ZJ, Xu J, Contractor A & Crair MC (2014). Visual circuit development requires patterned activity mediated by retinal acetylcholine receptors. Neuron 84, 1049–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J & Feldheim DA (2013). Developmental mechanisms of topographic map formation and alignment. Annual Review of Neuroscience 36, 51–77. [DOI] [PubMed] [Google Scholar]
- Cang J, Niell CM, Liu X, Pfeiffenberger C, Feldheim DA & Stryker MP (2008). Selective disruption of one Cartesian axis of cortical maps and receptive fields by deficiency in ephrin-As and structured activity. Neuron 57, 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B (2000). Necessity for afferent activity to maintain eye-specific segregation in ferret lateral geniculate nucleus. Science 287, 2479–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Bickford ME & Hirsch JA (2016). Untangling the web between eye and brain. Cell 165, 20–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C & Regehr WG (2000). Developmental remodeling of the retinogeniculate synapse. Neuron 28, 955–966. [DOI] [PubMed] [Google Scholar]
- Coleman JE, Law K & Bear MF (2009). Anatomical origins of ocular dominance in mouse primary visual cortex. Neuroscience 161, 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs J, van der List D, Wang GY & Chalupa LM (2006). Morphological properties of mouse retinal ganglion cells. Neuroscience 140, 123–136. [DOI] [PubMed] [Google Scholar]
- Cruz-Martin A, El-Danaf RN, Osakada F, Sriram B, Dhande OS, Nguyen PL, Callaway EM, Ghosh A & Huberman AD (2014). A dedicated circuit links direction-selective retinal ganglion cells to the primary visual cortex. Nature 507, 358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM, Peterson BB, Robinson FR & Gamlin PD (2003). Fireworks in the primate retina: In vitro photodynamics reveals diverse LGN-projecting ganglion cell types. Neuron 37, 15–27. [DOI] [PubMed] [Google Scholar]
- de Monasterio FM (1978). Properties of ganglion cells with atypical receptive-field organization in retina of macaques. Journal of Neurophysiology 41, 1435–1449. [DOI] [PubMed] [Google Scholar]
- Demas J, Sagdullaev BT, Green E, Jaubert-Miazza L, McCall MA, Gregg RG, Wong RO & Guido W (2006). Failure to maintain eye-specific segregation in nob, a mutant with abnormally patterned retinal activity. Neuron 50, 247–259. [DOI] [PubMed] [Google Scholar]
- Demeulemeester H, Arckens L, Vandesande F, Orban GA, Heizmann CW & Pochet R (1991). Calcium binding proteins as molecular markers for cat geniculate neurons. Experimental Brain Research 83, 513–520. [DOI] [PubMed] [Google Scholar]
- Denman DJ & Contreras D (2016). On parallel streams through the mouse dorsal lateral geniculate nucleus. Frontiers in Neural Circuits 10, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhande OS, Bhatt S, Anishchenko A, Elstrott J, Iwasato T, Swindell EC, Xu HP, Jamrich M, Itohara S, Feller MB & Crair MC (2012). Role of adenylate cyclase 1 in retinofugal map development. Journal of Comparative Neurology 520, 1562–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhande OS, Estevez ME, Quattrochi LE, El-Danaf RN, Nguyen PL, Berson DM & Huberman AD (2013). Genetic dissection of retinal inputs to brainstem nuclei controlling image stabilization. Journal of Neuroscience 33, 17797–17813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhande OS, Hua EW, Guh E, Yeh J, Bhatt S, Zhang Y, Ruthazer ES, Feller MB & Crair MC (2011). Development of single retinofugal axon arbors in normal and beta2 knock-out mice. Journal of Neuroscience 31, 3384–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhande OS & Huberman AD (2014). Retinal ganglion cell maps in the brain: Implications for visual processing. Current Opinion in Neurobiology 24, 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhande OS, Stafford BK, Lim J-HA & Huberman AD (2015). Contributions of retinal ganglion cells to subcortical visual processing and behaviors. Annual Review of Vision Science 1, 291–328. [DOI] [PubMed] [Google Scholar]
- Duan X, Krishnaswamy A, De la Huerta I & Sanes JR (2014). Type II cadherins guide assembly of a direction-selective retinal circuit. Cell 158, 793–807. [DOI] [PubMed] [Google Scholar]
- Durand S, Iyer R, Mizuseki K, de Vries S, Mihalas S & Reid RC (2016). A comparison of visual response properties in the lateral geniculate nucleus and primary visual cortex of awake and anesthetized mice. Journal of Neuroscience 36, 12144–12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM & Hattar S (2010). Melanopsin-expressing retinal ganglion-cell photoreceptors: Cellular diversity and role in pattern vision. Neuron 67, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Danaf RN, Krahe TE, Dilger EK, Bickford ME, Fox MA & Guido W (2015). Developmental remodeling of relay cells in the dorsal lateral geniculate nucleus in the absence of retinal input. Neural Development 10, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis EM, Gauvain G, Sivyer B & Murphy GJ (2016). Shared and distinct retinal input to the mouse superior colliculus and dorsal lateral geniculate nucleus. Journal of Neurophysiology 116, 602–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez ME, Fogerson PM, Ilardi MC, Borghuis BG, Chan E, Weng S, Auferkorte ON, Demb JB & Berson DM (2012). Form and function of the M4 cell, an intrinsically photosensitive retinal ganglion cell type contributing to geniculocortical vision. Journal of Neuroscience 32, 13608–13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow K, Teixeira M, Szikra T, Viney TJ, Balint K, Yonehara K & Roska B (2013). Ambient illumination toggles a neuronal circuit switch in the retina and visual perception at cone threshold. Neuron 78, 325–338. [DOI] [PubMed] [Google Scholar]
- Field GD & Chichilnisky EJ (2007). Information processing in the primate retina: Circuitry and coding. Annual Review of Neuroscience 30, 1–30. [DOI] [PubMed] [Google Scholar]
- Friedlander MJ, Lin CS, Stanford LR & Sherman SM (1981). Morphology of functionally identified neurons in lateral geniculate nucleus of the cat. Journal of Neurophysiology 46, 80–129. [DOI] [PubMed] [Google Scholar]
- Gauvain G & Murphy GJ (2015). Projection-specific characteristics of retinal input to the brain. Journal of Neuroscience 35, 6575–6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godement P, Salaun J & Imbert M (1984). Prenatal and postnatal development of retinogeniculate and retinocollicular projections in the mouse. Journal of Comparative Neurology 230, 552–575. [DOI] [PubMed] [Google Scholar]
- Grieve KL (2005). Binocular visual responses in cells of the rat dLGN. The Journal of Physiology 566, 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MS, Rossi FM, Changeux JP & Thompson ID (2003). Abnormal functional organization in the dorsal lateral geniculate nucleus of mice lacking the beta 2 subunit of the nicotinic acetylcholine receptor. Neuron 40, 1161–1172. [DOI] [PubMed] [Google Scholar]
- Grubb MS & Thompson ID (2003). Quantitative characterization of visual response properties in the mouse dorsal lateral geniculate nucleus. Journal of Neurophysiology 90, 3594–3607. [DOI] [PubMed] [Google Scholar]
- Grubb MS & Thompson ID (2004). Biochemical and anatomical subdivision of the dorsal lateral geniculate nucleus in normal mice and in mice lacking the beta2 subunit of the nicotinic acetylcholine receptor. Vision Research 44, 3365–3376. [DOI] [PubMed] [Google Scholar]
- Guido W (2008). Refinement of the retinogeniculate pathway. The Journal of Physiology 586, 4357–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido W, Tumosa N & Spear PD (1989). Binocular interactions in the cat’s dorsal lateral geniculate nucleus. I. Spatial-frequency analysis of responses of X, Y, and W cells to nondominant-eye stimulation. Journal of Neurophysiology 62, 526–543. [DOI] [PubMed] [Google Scholar]
- Hammer S, Monavarfeshani A, Lemon T, Su J & Fox MA (2015). Multiple retinal axons converge onto relay cells in the adult mouse thalamus. Cell Reports 12, 1575–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamos JE, Van Horn SC, Raczkowski D & Sherman SM (1987). Synaptic circuits involving an individual retinogeniculate axon in the cat. Journal of Comparative Neurology 259, 165–192. [DOI] [PubMed] [Google Scholar]
- Harting JK, Huerta MF, Hashikawa T & van Lieshout DP (1991). Projection of the mammalian superior colliculus upon the dorsal lateral geniculate nucleus: Organization of tectogeniculate pathways in nineteen species. Journal of Comparative Neurology 304, 275–306. [DOI] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW & Berson DM (2006). Central projections of melanopsin-expressing retinal ganglion cells in the mouse. Journal of Comparative Neurology 497, 326–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM & Yau KW (2002). Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science 295, 1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter M, Briggman KL, Turaga SC, Jain V, Seung HS & Denk W (2013). Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature 500, 168–174. [DOI] [PubMed] [Google Scholar]
- Hong YK & Chen C (2011). Wiring and rewiring of the retinogeniculate synapse. Current Opinion in Neurobiology 21, 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YK, Park S, Litvina EY, Morales J, Sanes JR & Chen C (2014). Refinement of the retinogeniculate synapse by bouton clustering. Neuron 84, 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth M, Walmsley L & Brown TM (2014). Binocular integration in the mouse lateral geniculate nuclei. Current Biology 24, 1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH & Wiesel TN (1961). Integrative action in the cat’s lateral geniculate body. The Journal of Physiology 155, 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Manu M, Koch SM, Susman MW, Lutz AB, Ullian EM, Baccus SA & Barres BA (2008). Architecture and activity-mediated refinement of axonal projections from a mosaic of genetically identified retinal ganglion cells. Neuron 59, 425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Murray KD, Warland DK, Feldheim DA & Chapman B (2005). Ephrin-As mediate targeting of eye-specific projections to the lateral geniculate nucleus. Nature Neuroscience 8, 1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Wei W, Elstrott J, Stafford BK, Feller MB & Barres BA (2009). Genetic identification of an ON–OFF direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron 62, 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E, Lee P & Pan ZH (2013). Characterization of multiple bistratified retinal ganglion cells in a purkinje cell protein 2-Cre transgenic mouse line. Journal of Comparative Neurology 521, 2165–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby J, Zhu Y, DeVries SH & Schwartz GW (2015). An amacrine cell circuit for signaling steady illumination in the retina. Cell Reports 13, 2663–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaubert-Miazza L, Green E, Lo FS, Bui K, Mills J & Guido W (2005). Structural and functional composition of the developing retino-geniculate pathway in the mouse. Visual Neuroscience 22, 661–676. [DOI] [PubMed] [Google Scholar]
- Joesch M & Meister M (2016). A neuronal circuit for colour vision based on rod-cone opponency. Nature 532, 236–239. [DOI] [PubMed] [Google Scholar]
- Kay JN, De la Huerta I, Kim IJ, Zhang Y, Yamagata M, Chu MW, Meister M & Sanes JR (2011). Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. Journal of Neuroscience 31, 7753–7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IJ, Zhang Y, Meister M & Sanes JR (2010). Laminar restriction of retinal ganglion cell dendrites and axons: Subtype-specific developmental patterns revealed with transgenic markers. Journal of Neuroscience 30, 1452–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IJ, Zhang Y, Yamagata M, Meister M & Sanes JR (2008). Molecular identification of a retinal cell type that responds to upward motion. Nature 452, 478–482. [DOI] [PubMed] [Google Scholar]
- Koch SM, Dela Cruz CG, Hnasko TS, Edwards RH, Huberman AD & Ullian EM (2011). Pathway-specific genetic attenuation of glutamate release alters select features of competition-based visual circuit refinement. Neuron 71, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahe TE, El-Danaf RN, Dilger EK, Henderson SC & Guido W (2011). Morphologically distinct classes of relay cells exhibit regional preferences in the dorsal lateral geniculate nucleus of the mouse. Journal of Neuroscience 31, 17437–17448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Virsu V & Creutzfeldt OD (1983). Linear signal transmission from prepotentials to cells in the macaque lateral geniculate nucleus. Experimental Brain Research 52, 50–56. [DOI] [PubMed] [Google Scholar]
- Lee S, Zhang Y, Chen M & Zhou ZJ (2016). Segregated glycine–glutamate Co-transmission from vGluT3 amacrine cells to contrast-suppressed and contrast-enhanced retinal circuits. Neuron 90, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist M, Datunashvilli M, Kanyshkova T, Zobeiri M, Aissaoui A, Cerina M, Romanelli MN, Pape HC, & Budde T (2016). Two types of interneurons in the mouse lateral geniculate nucleus are characterized by different h-current density. Scientific Reports 6, 24904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick WR (1967). Receptive fields and trigger features of ganglion cells in the visual streak of the rabbits retina. The Journal of Physiology 188, 285–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick WR, Cleland BG & Dubin MW (1972). Lateral geniculate neurons of cat: Retinal inputs and physiology. Investigative Ophthalmology 11, 302–311. [PubMed] [Google Scholar]
- Marrocco RT & McClurkin JW (1979). Binocular interaction in the lateral geniculate nucleus of the monkey. Brain Research 168, 633–637. [DOI] [PubMed] [Google Scholar]
- Marshel JH, Garrett ME, Nauhaus I & Callaway EM (2011). Functional specialization of seven mouse visual cortical areas. Neuron 72, 1040–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshel JH, Kaye AP, Nauhaus I & Callaway EM (2012). Anterior–posterior direction opponency in the superficial mouse lateral geniculate nucleus. Neuron 76, 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martersteck EM, Hirokawa KE, Evarts M, Bernard A, Duan X, Li Y, Ng L, Oh SW, Ouellette B, Royall JJ, Stoecklin M, Wang Q, Zeng H, Sanes JR & Harris JA (2017). Diverse central projection patterns of retinal ganglion cells. Cell Reports 18, 2058–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PR (1986). The projection of different retinal ganglion cell classes to the dorsal lateral geniculate nucleus in the hooded rat. Experimental Brain Research 62, 77–88. [DOI] [PubMed] [Google Scholar]
- McLaughlin T & O’Leary DD (2005). Molecular gradients and development of retinotopic maps. Annual Review of Neuroscience 28, 327–355. [DOI] [PubMed] [Google Scholar]
- Meister M, Wong RO, Baylor DA & Shatz CJ (1991). Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science 252, 939–943. [DOI] [PubMed] [Google Scholar]
- Morgan JL, Berger DR, Wetzel AW & Lichtman JW (2016). The fuzzy logic of network connectivity in mouse visual thalamus. Cell 165, 192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir-Robinson G, Hwang BJ & Feller MB (2002). Retinogeniculate axons undergo eye-specific segregation in the absence of eye-specific layers. Journal of Neuroscience 22, 5259–5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GJ & Rieke F (2006). Network variability limits stimulusevoked spike timing precision in retinal ganglion cells. Neuron 52, 511–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM (2013). Vision: More than expected in the early visual system. Current Biology 23, R681–684. [DOI] [PubMed] [Google Scholar]
- Niell CM & Stryker MP (2010). Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhout JA, El-Danaf RN, Nguyen PL & Huberman AD(2014).Birthdate and outgrowth timing predict cellular mechanisms of axon target matching in the developing visual pathway. Cell Reports 8, 1006–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhout JA, Stafford BK, Nguyen PL, Yoshihara Y & Huberman AD (2015). Contactin-4 mediates axon-target specificity and functional development of the accessory optic system. Neuron 86, 985–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F & Wu SM (2003). Light-evoked excitatory and inhibitory synaptic inputs to ON and OFF alpha ganglion cells in the mouse retina. Journal of Neuroscience 23, 6063–6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Borghuis BG, Rahmani P, Zeng Q, Kim IJ & Demb JB(2015).Function and circuitry of VIP+ interneurons in the mouse retina. Journal of Neuroscience 35, 10685–10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnavelas JG, Mounty EJ, Bradford R & Lieberman AR (1977). The postnatal development of neurons in the dorsal lateral geniculate nucleus of the rat: A Golgi study. Journal of Comparative Neurology 171, 481–499. [DOI] [PubMed] [Google Scholar]
- Petros TJ, Rebsam A & Mason CA (2008). Retinal axon growth at the optic chiasm: To cross or not to cross. Annual Review of Neuroscience 31, 295–315. [DOI] [PubMed] [Google Scholar]
- Pfeiffenberger C, Cutforth T, Woods G, Yamada J, Renteria RC, Copenhagen DR, Flanagan JG & Feldheim DA (2005). Ephrin-As and neural activity are required for eye-specific patterning during retinogeniculate mapping. Nature Neuroscience 8, 1022–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffenberger C, Yamada J & Feldheim DA (2006). Ephrin-As and patterned retinal activity act together in the development of topographic maps in the primary visual system. Journal of Neuroscience 26, 12873–12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D (2004). The thalamic reticular nucleus: Structure, function and concept. Brain Research Reviews 46, 1–31. [DOI] [PubMed] [Google Scholar]
- Piscopo DM, El-Danaf RN, Huberman AD & Niell CM (2013). Diverse visual features encoded in mouse lateral geniculate nucleus. Journal of Neuroscience 33, 4642–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge CL, Yeh PT, Liou NF, Lee CC, You SF, Liu LL, McNeill DS, Chew KS, Hattar S, Chen SK & Zhang DQ (2016). M1 ipRGCs influence visual function through retrograde signaling in the retina. Journal of Neuroscience 36, 7184–7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF & Rollag MD (2000). A novel human opsin in the inner retina. Journal of Neuroscience 20, 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafols JA & Valverde F (1973). The structure of the dorsal lateral geniculate nucleus in the mouse. A Golgi and electron microscopic study. Journal of Comparative Neurology 150, 303–332. [DOI] [PubMed] [Google Scholar]
- Reese BE (1988). ‘Hidden lamination’ in the dorsal lateral geniculate nucleus: The functional organization of this thalamic region in the rat. Brain Research 472, 119–137. [DOI] [PubMed] [Google Scholar]
- Reese BE & Jeffery G (1983). Crossed and uncrossed visual topography in dorsal lateral geniculate nucleus of the pigmented rat. Journal of Neurophysiology 49, 877–885. [DOI] [PubMed] [Google Scholar]
- Reifler AN, Chervenak AP, Dolikian ME, Benenati BA, Li BY, Wachter RD, Lynch AM, Demertzis ZD, Meyers BS, Abufarha FS, Jaeckel ER, Flannery MP & Wong KY (2015). All spiking, sustained ON displaced amacrine cells receive gap-junction input from melanopsin ganglion cells. Current Biology 25, 2763–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Zhou K, Wei W, Elstrott J, Nguyen PL, Barres BA, Huberman AD & Feller MB (2011). Transgenic mice reveal unexpected diversity of on-off direction-selective retinal ganglion cell subtypes and brain structures involved in motion processing. Journal of Neuroscience 31, 8760–8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck RW (1967). Receptive fields in the cat retina: A new type. Science 157, 90–92. [DOI] [PubMed] [Google Scholar]
- Rompani SB, Mullner FE, Wanner A, Zhang C, Roth CN, Yonehara K & Roska B (2017). Different modes of visual integration in the lateral geniculate nucleus revealed by single-cell-initiated transsynaptic tracing. Neuron 93, 767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MM, Helmchen F & Kampa BM (2012). Distinct functional properties of primary and posteromedial visual area of mouse neocortex. Journal of Neuroscience 32, 9716–9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousso DL, Qiao M, Kagan RD, Yamagata M, Palmiter RD & Sanes JR (2016). Two pairs of ON and OFF retinal ganglion cells are defined by intersectional patterns of transcription factor expression. Cell Reports 15, 1930–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR & Masland RH (2015). The types of retinal ganglion cells: Current status and implications for neuronal classification. Annual Review of Neuroscience 38, 221–246. [DOI] [PubMed] [Google Scholar]
- Schmidt TM, Alam NM, Chen S, Kofuji P, Li W, Prusky GT & Hattar S (2014). A role for melanopsin in alpha retinal ganglion cells and contrast detection. Neuron 82, 781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Chen SK & Hattar S (2011). Intrinsically photosensitive retinal ganglion cells: Many subtypes, diverse functions. Trends in Neurosciences 34, 572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM & Kofuji P (2009). Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. Journal of Neuroscience 29, 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl B, Tan AY, Corey J & Priebe NJ (2013). Emergence of orientation selectivity in the mammalian visual pathway. Journal of Neuroscience 33, 10616–10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook TA, Krahe TE, Govindaiah G & Guido W (2013). Interneurons in the mouse visual thalamus maintain a high degree of retinal convergence throughout postnatal development. Neural Development 8, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM (2004). Interneurons and triadic circuitry of the thalamus. Trends in Neurosciences 27, 670–675. [DOI] [PubMed] [Google Scholar]
- Sherman SM & Guillery RW (2002). The role of the thalamus in the flow of information to the cortex. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences 357, 1695–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert S, Scherf BG, Del Punta K, Didkovsky N, Heintz N & Roska B (2009). Genetic address book for retinal cell types. Nature Neuroscience 12, 1197–1204. [DOI] [PubMed] [Google Scholar]
- Simpson JI (1984). The accessory optic system. Annual Review of Neuroscience 7, 13–41. [DOI] [PubMed] [Google Scholar]
- Sivyer B, Taylor WR & Vaney DI (2010). Uniformity detector retinal ganglion cells fire complex spikes and receive only light-evoked inhibition. Proceedings of the National Academy of Sciences of the United States of America 107, 5628–5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F, Ma X, Cecil JL, Vo BQ, Culican SM & Kerschensteiner D (2012). Spontaneous activity promotes synapse formation in a cell-type-dependent manner in the developing retina. Journal of Neuroscience 32, 5426–5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford LR, Friedlander MJ & Sherman SM (1981). Morphology of physiologically identified W-cells in the C laminae of the cat’s lateral geniculate nucleus. Journal of Neuroscience 1, 578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford LR, Friedlander MJ & Sherman SM (1983). Morphological and physiological properties of geniculate W-cells of the cat: A comparison with X- and Y-cells. Journal of Neurophysiology 50, 582–608. [DOI] [PubMed] [Google Scholar]
- Stellwagen D & Shatz CJ (2002). An instructive role for retinal waves in the development of retinogeniculate connectivity. Neuron 33, 357–367. [DOI] [PubMed] [Google Scholar]
- Sumbul U, Song S, McCulloch K, Becker M, Lin B, Sanes JR, Masland RH & Seung HS (2014). A genetic and computational approach to structurally classify neuronal types. Nature Communications 5, 3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LO, Brady CM, Cahill H, Al-Khindi T, Sakuta H, Dhande OS, Noda M, Huberman AD, Nathans J & Kolodkin AL (2015). Functional assembly of accessory optic system circuitry critical for compensatory eye movements. Neuron 86, 971–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Li N & He S (2002). Large-scale morphological survey of mouse retinal ganglion cells. Journal of Comparative Neurology 451, 115–126. [DOI] [PubMed] [Google Scholar]
- Suresh V, Ciftcioglu UM, Wang X, Lala BM, Ding KR, Smith WA, Sommer FT & Hirsch JA (2016). Synaptic contributions to receptive field structure and response properties in the rodent lateral geniculate nucleus of the thalamus. Journal of Neuroscience 36, 10949–10963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Ardila Jimenez SC, Chakraborty S & Schultz SR (2016). Visual receptive field properties of neurons in the mouse lateral geniculate nucleus. PLoS One 11, e0146017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie SF & Reid RC (2000). Diverse receptive fields in the lateral geniculate nucleus during thalamocortical development. Nature Neuroscience 3, 608–616. [DOI] [PubMed] [Google Scholar]
- Tien NW, Kim T & Kerschensteiner D (2016). Target-specific glycinergic transmission from VGluT3-expressing amacrine cells shapes suppressive contrast responses in the retina. Cell Reports 15, 1369–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien NW, Pearson JT, Heller CR, Demas J & Kerschensteiner D (2015). Genetically identified suppressed-by-contrast retinal ganglion cells reliably signal self-generated visual stimuli. Journal of Neuroscience 35, 10815–10820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu DC, Zhang D, Demas J, Slutsky EB, Provencio I, Holy TE & Van Gelder RN (2005). Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron 48, 987–999. [DOI] [PubMed] [Google Scholar]
- Usrey WM & Alitto HJ (2015). Visual functions of the thalamus. Annual Review of Vision Science 1, 351–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Gao E & Burkhalter A (2011). Gateways of ventral and dorsal streams in mouse visual cortex. Journal of Neuroscience 31, 1905–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Sporns O & Burkhalter A (2012). Network analysis of corticocortical connections reveals ventral and dorsal processing streams in mouse visual cortex. Journal of Neuroscience 32, 4386–4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KY, Dunn FA, Graham DM & Berson DM (2007). Synaptic influences on rat ganglion-cell photoreceptors. The Journal of Physiology 582, 279–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HP, Burbridge TJ, Chen MG, Ge X, Zhang Y, Zhou ZJ & Crair MC (2015). Spatial pattern of spontaneous retinal waves instructs retinotopic map refinement more than activity frequency. Developmental Neurobiology 75, 621–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonehara K, Ishikane H, Sakuta H, Shintani T, Nakamura-Yonehara K, Kamiji NL, Usui S & Noda M (2009). Identification of retinal ganglion cells and their projections involved in central transmission of information about upward and downward image motion. PLoS One 4, e4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE & McMahon DG (2008). Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proceedings of the National Academy of Sciences of the United States of America 105, 14181–14186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ackman JB, Xu HP & Crair MC (2011). Visual map development depends on the temporal pattern of binocular activity in mice. Nature Neuroscience 15, 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Chen H, Liu X & Cang J (2013. a). Orientation-selective responses in the mouse lateral geniculate nucleus. Journal of Neuroscience 33, 12751–12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Liu M & Cang J (2013b). Sublinear binocular integration preserves orientation selectivity in mouse visual cortex. Nature Communications 4, 2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Xu J, Hauswirth WW & DeVries SH (2014). Genetically targeted binary labeling of retinal neurons. Journal of Neuroscience 34, 7845–7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziburkus J & Guido W (2006). Loss of binocular responses and reduced retinal convergence during the period of retinogeniculate axon segregation. Journal of Neurophysiology 96, 2775–2784. [DOI] [PubMed] [Google Scholar]