Abstract
By acquiring, processing, and presenting both foreign and self-antigens, dendritic cells (DCs) initiate T cell activation that is shaped through the immunomodulatory functions of a variety of cell-membrane-bound molecules including BTLA-HVEM, CD40-CD40L, CTLA-4-CD80/CD86, CD70-CD27, ICOS-ICOS-L, OX40-OX40L, and PD-L1-PD-1, as well as several key cytokines and enzymes such as interleukin-6 (IL-6), IL-12, IL-23, IL-27, transforming growth factor-beta 1 (TGF-β1), retinaldehyde dehydrogenase (Raldh), and indoleamine 2,3-dioxygenase (IDO). Some of these distinct immunomodulatory signals are mediated by specific subsets of DCs, therefore contributing to the functional specialization of DCs in the priming and regulation of immune responses. In addition to responding to the DC-mediated signals, T cells can reciprocally modulate the immunomodulatory capacities of DCs, further refining immune responses. Here, we review recent studies, particularly in experimental mouse systems, that have delineated the integrated mechanisms of crucial immunomodulatory pathways that enable specific populations of DCs and T cells to work intimately together as single functional units that are indispensable for the maintenance of immune homeostasis.
Keywords: dendritic cell, T cell, immunoregulation, T cell differentiation, regulatory T cell, effector T cell
I. INTRODUCTION
Dendritic cells (DCs) are antigen-presenting cells (APCs) that initiate and regulate T cell responses to foreign and self-antigens. DCs patrol various tissues and internalize such antigens, which they then process and present as peptides on major histocompatibility complexes (MHCs) to T cell receptors (TCRs) that are reactive to specific peptide:MHC complexes. In addition to activating T cells to combat various pathogens, DCs have a well-established role in the induction and regulation of tolerance in the absence of pro-inflammatory stimuli, known as the steady state.1 DCs can also sense the presence of various environmental signals, including materials from dead cells, microbial products, and specific pro-inflammatory conditions, allowing them to discern what the appropriate immune response for the surrounding environment should be.2–5 Some of these environmental signals may also promote the tolerogenic functions of DCs.3,6–17 However, the molecular mechanisms by which DCs induce different immunological outcomes remain a subject of active investigation.
Depending on the cytokines secreted and the immunomodulatory molecules present on the surface of both the DCs and T cells, T cells differentiate to acquire various effector or regulatory functions. Moreover, DCs can modulate T cell functions through the production of metabolites by specific immunomodulatory enzymes. However, the interactions between DCs and T cells are not unidirectional because T cells can also influence certain aspects of DC biology. Overall, multiple immunomodulatory pathways enable DCs and T cells to work together as functional units that carry out diverse functions in order to promote an appropriate immune response (Fig. 1). Increasing our understanding of the various mechanisms utilized by different subsets of DCs to help shape T cell responses in order to maintain immune homeostasis is vital for the design of new therapies for a variety of diseases, including cancer, autoimmunity, and infections.
FIG. 1:
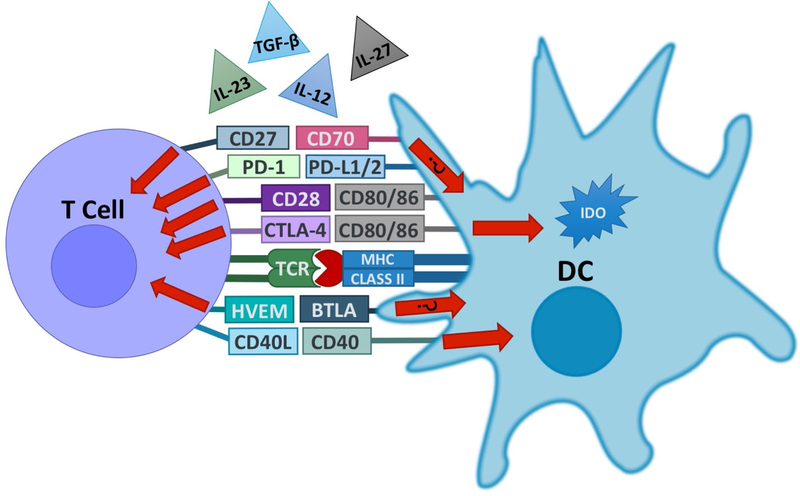
DCs and T cells form a functional partnership. DCs and T cells express a variety of surface molecules that work together as ligands and receptors in order to control various aspects of the immune response. Some of these pathways, such as BTLA–HVEM and CD27–CD70, have been shown to induce bidirectional signaling, although the impact of such bidirectional interactions between DCs and T cells remains mostly unclear at this point. In addition, DCs secrete or activate a variety of cytokines that influence T cell differentiation and proliferation. DCs upregulate expression of the CD70 and IL-12 in response to CD40 signaling induced by T cell-expressed CD40L. DCs can also produce the tolerogenic tryptophan-catabolizing enzyme IDO in response to signals mediated by CTLA-4 in T cells.
II. IMMUNOMODULATORY PATHWAYS
A. CTLA-4–CD80/86
A crucial example of an immunomodulatory molecule that facilitates a dynamic partnership between DCs and T cells in the maintenance of immune homeostasis is cytotoxic T-lymphocyte antigen 4 (CTLA-4). This molecule, with its multi-faceted tolerogenic capabilities, is a member of the CD28 family that is expressed in activated T cells, particularly regulatory T (Treg) cells, in a cell-membrane-bound form.18–22 Additionally, CTLA-4 has been shown to be expressed and secreted by DCs.23,24 In T cells, CTLA-4 competes with CD28 for binding of CD80 or CD86 expressed on DCs.25,26 Although the precise molecular signals are still unclear, signaling by membrane-bound CTLA-4 in T cells can result in tolerant CD4+ T cell responses, including clonal anergy or transforming growth factor-beta 1 (TGF-β1) production.27,28 In CD8+ T cells, CTLA-4 promotes DC-mediated T cell tolerance, although the mechanism by which this occurs is also unclear.29
In addition to T cell-intrinsic effects mediated by CTLA-4, studies by several groups have shown that Treg cells exert tolerogenic effects by modulating DC biology through several different mechanisms mediated by Treg cell-expressed CTLA-4. Foxp3+ Treg cells can inhibit autophagy in DCs by signaling with CTLA-4 to CD80 and CD86 on DCs, activating the phosphoinositide 3-kinase (PI3K)/Akt/mechanistic target of rapamycin (mTOR) pathway, which downregulates transcription of Lc3b and induces nuclear exclusion of Foxo1, thus reducing autophagy in these cells.30 Additionally, Treg cells upregulate expression of CTLA-4 following TCR engagement, which then leads to the downregulation of CD80 and CD86 on DCs through a mechanism that is at least in part mediated by the trans-endocytosis of these costimulatory molecules.31–35 Another group has shown that CD80 on DCs binding to CTLA-4 expressed by in vitro-induced Treg cells or the fusion protein (CTLA-4-Fc) leads to the phosphorylation of STAT3 and subsequent nuclear factor-kappa beta (NF-κB)-dependent downregulation of CD80 and CD86 gene transcription in DCs.36 Furthermore, human DCs can secrete CTLA-4, which binds to and downregulates CD80 and CD86 molecules on bystander DCs, resulting in decreased CD8+ T cell activity.24 This finding further illustrates the complex nature of interactions between DCs and T cells while also raising the possibility that a similar secretory mechanism may be additionally carried out by CTLA-4 expressed by Treg cells to influence DCs.
B. Indoleamine 2,3 Dioxygenase
Another important immunomodulatory mechanism facilitated by CTLA-4-CD80/86 axis is the induced expression of the tolerogenic enzyme indoleamine 2,3 dioxygenase (IDO) in DCs that is triggered through the engagement of CD80/86 by CTLA-4 expressed in Foxp3+ CD25+ Treg cells.37–39 IDO is the rate-limiting enzyme for tryptophan catabolism that is expressed by DCs and it exerts tolerogenic effects on T cell responses by producing pro-apoptotic tryptophan metabolites and depleting the tryptophan available for surrounding T cells.37,40–42 Mice with a conditional deletion of CTLA-4 in Foxp3+ cells were found to have a 50% reduction of IDO-expressing CD11c+ DCs.43 This pathway is also important in humans because CTLA-4 expressed in human Treg cells signals to human monocyte-derived DCs through CD80 and CD86, resulting in the expression of the active form of IDO.44 When CD8+ T cells are cultured with IDO+ DCs, the combination of tryptophan deprivation and the presence of tryptophan metabolite kynurenine promotes downregulation the TCR ζ-chain, leading to decreased functionality of T cells.45 This downregulation is dependent on the stress-response kinase GCN2 because this effect is not seen in CD8+ T cells from Gcn2−/− mice.45 Furthermore, IDO can lead to the direct conversion of Foxp3-expressing peripheral Treg (pTreg) cells through the production of kynurenine, which can activate the transcription factor aryl hydrocarbon receptor (AHR) and thus induce expression of Foxp3 in T cells.46 Furthermore, activation of AHR in DCs by other ligands can induce IDO expression and retinoic acid (RA) production by DCs, thus promoting their tolerogenic functions.47–49 IDO-derived tryptophan metabolites can also act on the DCs to increase their expression of the immunomodulatory molecules ILT3 (LILRB4) and ILT4 (LILRB4), which themselves play a role in the induction of Treg and IL-10-producing regulatory type 1 T (Tr1) cells.50–53 Expression of these molecules by DCs can also be increased upon interactions with CD8+ Treg cells, leading to the induction of CD4+ T cell anergy.54
C. TGF-β
There are also several secreted factors that mediate the diverse functions carried out by DCs, such as the cytokine TGF-β1, which is produced in an inactive form. Integrin αvβ8 activates TGF-β1 by binding to the propeptide of TGF-β1 (LAP-β1) and associating with membrane-type 1- matrix metalloprotease (MT1-MMP) in order to cleave the pro-peptide into its active form.55 Mice lacking expression of integrin αvβ8 in DCs develop inflammatory bowel disease and autoimmunity, further demonstrating the in vivo importance of this immunomodulatory molecule.56 In the canonical signaling pathway, binding of mature TGF-β1 to either TGF-βRIII or the heterodimeric receptor consisting of the TGF-βRI and TGF-βRII subunits results in the dimerization of SMAD2 and SMAD3, which subsequently form a complex with SMAD4 that can translocate to the nucleus and induce gene transcription.57 Non-canonical signaling is mediated by various kinase pathways, including the Jun N-terminal kinase (JNK), p38 mitogen-activated protein kinase (MAPK), and extracellular signal-regulated kinase (ERK) pathways.57 TGF-β1 signaling is critical for Treg cell differentiation due to its ability to induce Foxp3 gene expression.58,59
In addition to influencing Treg cell differentiation, TGF-β1 is also important for the development of Th17 cells in vivo. Analogous to its function of activating TGF-β1 to its active form to allow the development of Treg cells, integrin αvβ8 is also required to activate TGF-β1 to promote the development of Th17 cells. Mice lacking integrin αvβ8 expression in CD11c+ DCs do not develop experimental autoimmune encephalomyelitis (EAE), a well-established mouse model of multiple sclerosis, due to an impairment in the development of Th17 cells, which play a pathogenic role in EAE.60 Additionally, mice lacking integrin αvβ8 expression in DCs are protected against infection with the helminth Trichuris muris due to increased expression of IL-13 as a result of more efficient differentiation of Th2 cells that are protective against helminth infection.61 Mice with a DC-specific conditional knockout of the β8 integrin subunit are also unable to generate CD4+CD8αα+ intra-epithelial lymphocytes.62 These studies provide further evidence that DC-expressed integrin αvβ8 plays an important role in controlling the balance of T cell subsets by activating TGF-β1 in order to fight infections or maintain tolerance by promoting the differentiation of Th17 or Treg cells.63
D. Retinaldehyde Dehydrogenase
In addition to TGF-β1, another important soluble factor shown to modulate the differentiation of Treg cells is RA, which is generated during the metabolism of vitamin A by several related aldehyde dehydrogenase enzymes, including retinaldehyde dehydrogenase type 2 (Raldh2). In splenic DCs, TLR2 signaling can induce expression of Raldh2 and consequently the metabolism of RA through the enzyme’s actions. Together with IL-10, RA is able to promote the development of Foxp3+ Treg and Tr1 cells.13 RA can inhibit Th17 cell differentiation and also promote Treg cell differentiation in combination with TGF-β1.64 The precise mechanism by which RA enhances Foxp3 expression in differentiating T cells is still unclear, although it has been shown to be independent of IL-2, STAT3, and STAT5.65 RA also helps to promote Treg cell development by promoting Foxp3 expression that would normally be inhibited in the presence of CD28 co-stimulation from CD80/86 on DCs or an agonistic αCD28 antibody.66 RA further enhances the tolerogenic gut environment by inducing the expression of the gut-homing molecules integrin α4β7 and CCR9 on the developing Treg cells, an effect mediated by lamina propria DCs.67,68 This immunomodulatory axis demonstrates that multiple regulatory mechanisms are in place to allow DCs and T cells to maintain the appropriate level of tolerance, depending on the environmental context.
E. BTLA–HVEM
In addition to the crucial signaling axes described above, another immunomodulatory pathway that is critical for the partnership between DCs and T cells involves the molecules B and T lymphocyte associated (BTLA) and herpesvirus entry mediatory (HVEM), which have also been shown to have bidirectional signaling capabilities. BTLA is a receptor of the immunoglobulin superfamily that was first identified as an inhibitory receptor due to its three immunoreceptor tyrosine-based inhibition motifs (ITIMs) which, when phosphorylated, can recruit Src homology domain 2 (SH2)-containing protein tyrosine phosphatases, SHP-1 and SHP-2, which generally exert inhibitory effects within the cell.69–71 BTLA was originally shown to be a negative regulator of T cell activation, but its functions have since proven to be more varied with roles in B cells and DCs.71,72 BTLA interacts with the tumor necrosis factor receptor superfamily (TNFRSF) member HVEM, which is expressed in naive T cells and downregulated following activation.73–75 HVEM has also been shown to be expressed on DCs.76,77 HVEM can additionally interact with HSV-1 glycoprotein D (gD), lymphotoxin α (LTα3), LIGHT (TNFSF14), and CD160.71,78–80 Upon binding to BTLA, HVEM induces NF-κB RelA expression via TNF receptor associated factor 2 (TRAF2) and pro-survival signals within activated T cells.73,81 The functional results of the interactions between BTLA and HVEM can be either inhibitory or activating, depending on the conditions and type of cell that expresses BTLA and HVEM. Such interactions have been shown to influence CD8+ T cell survival, memory formation, Treg cell functions, and DC homeostasis77,82–88 For example, CD8+ T cells transferred into Btla−/− mice fail to expand and survive in response to infection with either Listeria monocytogenes or vaccinia virus.82,86 The memory formation of these CD8+ T cells is dependent on HVEM signaling within the T cells that is induced by BTLA-expressing DCs.86 Conversely, BTLA signaling in CD8+ T cells, presumably induced by HVEM-expressing DCs (although this was not directly examined), reduces their ability to expand and form memory responses.85 The HVEM–BTLA pathway also regulates DC homeostasis through an unclear mechanism, but due to ability of these molecules to signal in both directions, it is possible that this effect is mediated by T cells expressing either molecule, which can bind to its partner on DCs.77
BTLA and HVEM have both been shown to play an important role in protection from autoimmunity. Mice deficient in either BTLA or HVEM are susceptible to EAE.69,89 Recently, BTLA–HVEM signaling has been shown to be critically important for pTreg cell differentiation.72 To do so, BTLA signals through HVEM in naive T cells to increase phosphorylation of mitogen-activated protein kinase (MAPK) kinase (MEK) and expression of ETS1, which in turn increases transcription of Cd5. The specific functions of CD5 in these CD5hi T cells then enable induction of Foxp3 expression and conversion into pTreg cells by opposing mTOR activation mediated by effector differentiating cytokines.72,90 Such DC-induced pTreg cells have crucial functions in mitigating autoimmunity, as shown in an EAE model.91,92 The diverse functions mediated by these molecular partners allow for fine-tuning of immune responses by DCs and T cells in multiple immune settings, something that must be appreciated in the design of immunotherapies targeting BTLA and HVEM.
F. PD-1–PD-L1/2
Other cell-membrane-bound molecules also contribute to promotion of tolerance via Treg cell induction, such as programmed death ligand-1 (PD-L1). PD-L1 is a member of the B7 family that is constitutively expressed in DCs, macrophages, mast cells, B cells, T cells, and some tumor cells that can bind to B7–1 (CD80) and programmed death-1 (PD-1) on activated T cells.93–96 PD-1 is a member of the Ig superfamily and contains both an ITIM and ITSM (immunoreceptor tyrosine-based switch motif) that contribute to its inhibitory functions by recruiting SHP-1 and SHP-2 phosphatases.95,96 PD-L1 signaling through PD-1 reduces the phosphorylation of Akt, mTOR, S6, and ERK2 while upregulating PTEN expression, the combination of which allows for efficient Treg differentiation.97 PD-L1 signaling can lead to the formation of iTreg cells in combination with TGF-β1.97,98 PD-1 signaling has been shown to be an important mechanism for pTreg cells development in vivo as well.99 To enhance this process, CD11c+ DCs can upregulate PD-1 in naive T cells during priming by signaling with PD-L1 and the related molecule PD-L2, promoting the development of Foxp3+ Treg cells that are protective against autoimmunity such as in an EAE model.100 However, the efficient induction of such anti-autoimmune pTreg cells also requires signals mediated by HVEM engaged by BTLA expressed on DCs.72 As already discussed above, the resulting increase in the functions of CD5 in T cells inhibits mTOR, thereby limiting T cell sensitivity to effector cytokines and promoting pTreg cell differentiation.90 Overall, the interactions between DCs and T cells are a dynamic process, allowing them to collaborate in order to promote tolerance.
The immunomodulatory functions of DCs can also regulate the balance of effector T cell subsets in order to refine the immune response to better protect against infections. A key example of this type of functionality is PD-L2, which binds to PD-1 with higher binding affinity than PD-L1 and has more limited expression that can be upregulated during inflammation on DCs, macrophages, mast cells, peritoneal B1 and memory B cells.101–103 In a recent study, it was observed that patients infected with malaria had a decrease in the number of PD-L2+ DCs and that patients with a higher ratio of PD-L2+ to PD-L1+ DCs had reduced parasitemia.104 Using a murine model of malaria infection, they went on to show that PD-L2 on DCs played a protective role by outcompeting PD-L1 for PD-1 expressed by T cells, thus preventing T cell exhaustion and controlling the infection. They additionally used in vitro DC: T cell co-cultures to show that PD-L2 can increase the expression of CD3 and ICOS by T cells, an opposite effect of that observed to be mediated by engagement of PD-1 through PD-L1. This combination of effects led to more effective Th1 responses, which are critical for effective immunity against malaria.104 That study highlights the idea that immunomodulatory molecules have varied context-dependent functions, some of which may still be unknown.
As its name implies, PD-L1 signaling can additionally induce programmed cell death, specifically apoptosis, of PD-1+ T cells.105 The pro-apoptotic function of DC-expressed PD-L1 is likely mediated by the pro-apoptotic molecule Bim, although this mechanism has not been directly examined for interactions between DCs and T cells.106 PD-L1–PD-1 interactions have also been shown to shorten the time of interaction between DCs and T cells, which prevents disease in a model of autoimmune diabetes.107 PD-L1+ DCs can also downregulate TCR expression in CD8+ T cells by signaling through PD-1 to upregulate Cbl-b E3 ubiquitin ligase in CD8+ T cells, preventing hyperactivation and autoimmunity.108 Furthermore, conditional deletion of PD-L1 in DCs limits EAE severity by reducing antigen-specific CD4+ T cell activation in the early stages of EAE in addition to inhibiting differentiation of T follicular helper and regulatory (TFH and TFR) cells.109
G. OX40–OX40L
An additional member of the TNFRSF with diverse immunomodulatory functions is OX40 (CD134, TNFRSF4), which is expressed in a cell membrane form in activated CD4+ and CD8+ T cells.110–112 Its ligand, OX40L (CD252, TNFSF4), is expressed in DCs and also macrophages, B cells, and endothelial cells.113–116 OX40 signaling can influence several aspects of T cell biology, including proliferation, survival, and memory, by activating the PI3K and NF-κB pathways through TRAF molecules.116 Although OX40 has been shown to skew T cells toward the Th2 phenotype by signaling through NFATc1 to upregulate expression of IL-4, its signaling can also help Th1 responses in the presence of certain cytokines.116,117 OX40 signaling has also been shown to suppress Foxp3 expression in naive T cells, preventing their conversion to pTreg cells in the presence of TGF-β1.118,119 However, OX40 signaling can also induce the proliferation of Treg cells, although the precise molecular mechanism by which this occurs still needs to be characterized.120,121
H. ICOS–ICOS-L
Similar to the OX40–OX40L axis, interactions between the cell-membrane-bound molecule inducible T cell costimulator (ICOS), which is a member of the CD28 family that is expressed in activated T cells, and its ligand, ICOS-L (B7RP-1, CD275, B7h, B7-H2), which is expressed in DCs, B cells, and macrophages, can result in functionally diverse responses.122–124 Although other signaling molecules may be involved, ICOS signals in T cells are mostly mediated by PI3K and TBK1, which is a member of the inhibitor of NF-κB kinase (IKK) family.125–127 In addition to its role in driving differentiation and cytokine production of Th1 and Th2 cells, this interaction can also promote tolerance by increasing the development of antigen-specific Treg and Tr1 cells.17,128–133 Although the majority of research investigating the functions of ICOS signaling in T cells does not specify the cell type expressing ICOS-L, it has been shown that DC-expressed ICOS-L promotes the development of effective T cell-dependent antibody responses as well as IL-10-producing Tr1 cells.134–136 ICOS–ICOS-L interactions may also be bidirectional because ICOS-L signaling induced by ICOS-Fc or ICOSIg in monocyte-derived DCs (moDCs) and bone marrow-derived dendritic cells (BMDCs) alters cytokine secretion and antigen presentation by these cells.137,138
I. CD27–CD70
Another pathway with varied functions that are crucial for the functional partnership between DCs and T cells involves CD70 and CD27. CD70, which can be expressed by DCs in response to signals from CD4+ T cells or the environment, is a cell-membrane-bound TNF superfamily (TNFSR) member that engages TNFRSF member CD27, which is expressed on T cells.139–141 CD27 signals in T cells are mediated by TRAF2 and TRAF6, which in turn activate NF-κB and JNK.142–144 Downstream, this signaling can increase expression of IL-12Rβ2 and T-bet in naive T cells and thus promote the differentiation of Th1 cells.145 CD27 signaling also represses Th17 differentiation in developing T cells by suppressing expression of IL-17 and CCR6 at the transcriptional level via the JNK pathway as well as through epigenetic modifications.146 Additionally, thymic medullary DCs that express CD70 signal to CD27+ thymocytes and promote survival of thymus-derived Treg (tTreg) cells by inhibiting the mitochondrial apoptotic pathway.147 In addition to mediating intracellular signaling upon engagement with CD70, CD27 expressed by B, T, or tumor cells has also been shown to reciprocally induce signals into CD70+ NK and B cells through the P13K/Akt and MEK pathways, raising the possibility that similar reverse signaling may occur in CD70+ DCs during their interactions with T cells.148,149 For example, CD27+ tTreg cells inhibit Th1 responses by downregulating expression of CD70 in DCs.150 The expression of CD70 by DCs can also be downregulated once it binds to CD27 through transcriptional regulation.151
In addition to its crucial roles in CD4+ T cell biology, the CD27–CD70 immunomodulatory axis is also vital for effective CD8+ T cell responses. DC-expressed CD70 has been implicated in the direct priming of CD8+ T cells as well as the expansion and survival of primed CD8+ T cells by signaling through CD27 in these T cells.152–156 Furthermore, this pathway plays a critical role in the process that allows DCs to mediate CD4+ T cell help of CD8+ T cell responses. Expression of CD70 by DCs has been shown to be necessary for CD4+ T cell-helped CD8+ responses by instilling a specific gene transcription profile in developing CD27+ CD8+ T cells.157
J. CD40–CD40L
In addition to increasing expression of CD70 by DCs, CD4+ T cells help CD8+ T cell responses via CD40–CD40L interactions to modulate other aspects of DC biology. CD40 is TNFRSF member that can be expressed on DCs, where it is engaged by TNFSF member CD40L (CD154), which is pre-dominantly expressed by activated CD4+ T cells.158–161 Like the other TNFSF members, CD40 signaling is mediated by the TRAF molecules, especially TRAF6, resulting in MAPK and JNK activation and subsequent changes in gene expression of various immunomodulatory molecules, such as CD80, CD86, and IL-12.161 CD40–CD40L interactions are also important in the thymus, where CD40L+ CD4 single-positive thymocytes interact with CD40+ thymic DCs to promote upregulation of surface molecules CD40, PD-L1, CD86, CD200, and MHCII, as well as several other genes associated with DC functions to promote the formation of tTreg cells by CD40+ DCs.162 CD40 and CD70-mediated signaling cooperate to promote clonal expansion of CD8+ T cells in response to CD40 engagement, an effect that is diminished when interaction between CD70 and CD27 are blocked.163 T cells can further refine the Th1 response by increasing expression of CD70 in DCs expression by engaging DC-expressed CD40 during a concomitant treatment with poly I:C.164,165
K. IL-12 Family Cytokines
CD40 signaling can additionally induce the production of different cytokines by DCs. An important example of such cytokines is IL-12, the namesake member of the IL-12 family of cytokines that is composed of two subunits, IL-12p35and IL-12p40, that together form IL-12p70. Upon binding to its receptor, which consists of the IL-12Rβ1 and IL-12Rβ2 subunits, TYK2 (tyrosine kinase 2) and JAK2 (Janus kinase 2) are recruited, allowing for the activation of STAT4 and subsequent induction of interferongamma (IFN-γ) expression in Th1-differentiating T cells.166 Expression of heterodimeric IL-12p70 is induced in response to cross-linking of DC-expressed CD40 in vitro, but a microbial stimulus must be administered to achieve the same effect in vivo.167
Although not necessarily induced by CD40 signaling, DCs can also express other members of the IL-12 family of cytokines that carry out important immunomodulatory functions. One of these is IL-23, which is composed of the IL-12p40 and IL-23p19 subunits. Its receptor consists of the IL-12Rβ1 and IL-23R subunits and, after recruitment of TYK2 and JAK2, its signals are mediated by STAT2 and STAT4.166 IL-23 plays an important role in Th17 cell biology by helping to maintain IL-17 expression.168–170 Another IL-12 family member is IL-27, which is composed of the IL-27p28 and EBV-induced gene 3 (Ebi3) subunits. Its receptor (IL-27R) is also composed of two subunits: gp130 and WSX-1/TCCR (IL-27Rα), which recruit JAK1, JAK2, and TYK2 in order to activate STAT1 and STAT3.166 IL-27 is produced by a variety of cells, including DCs in response to poly I:C.171 IL-27 signaling can promote Th1 cell differentiation by inducing expression of T-bet and IL-12Rβ2 in a STAT1-dependent manner.172,173 Furthermore, Foxp3+ Treg cells can induce the production of IL-27 and TGF-β1 by DCs, which synergistically promote the differentiation of Tr1 cells.174 Specifically, IL-27 signals in T cells to induce expression of c-Maf, IL-21, and ICOS in order to promote differentiation of Tr1 cells.175–177 Moreover, IL-27 signaling in DCs induces the expression of the ectonucleotidase CD39, an enzyme that degrades extracellular ATP to ADP and thus promotes a tolerogenic environment for developing T cells.178 Tumor-infiltrating DCs also express CD39, which mediates their ability to inhibit T cell activation when cultured in vitro.179 Another soluble factor that increases the tolerogenic capacity of DCs is vasoactive intestinal peptide (VIP), a neuropeptide secreted by T cells that can increase the ability of DCs to induce Treg cells.180 These pathways highlight how DCs and T cells can work together using multiple interconnected immunomodulatory pathways to maintain immune homeostasis in a tightly regulated manner.
III. FUNCTIONAL SPECIALIZATION OF DC SUBSETS
Over the past few years, our knowledge about the development of various DC subsets has greatly expanded.181–183 Briefly, DCs are divided into two main groups: conventional DCs (cDCs) and plasmacytoid DCs (pDCs). The cDC population can be further delineated into the cDC1 and cDC2 subsets on the basis of the transcription factors required for their development. The cDC1 subset, which requires the transcription factors Irf8, Id2, and Batf3 for development, can be distinguished by expression of XCR1 as well as CD8α, CD103, DEC205, CD24, and DNGR-1/CLEC9A.181,182,184,185 The cDC2 lineage, which requires the transcription factor Irf4 for development, can be distinguished by expression of CD172a (SIRPα) in addition to CD11b, CD4, and DCIR2.181,182,184,185 pDCs depend on the transcription factors Irf8 and E2–2 for their development and can be distinguished by their expression of B220, Siglec-H, and Bst2.181,182,184,185 In addition to the rapid progress made in DC subset classification in recent years, our understanding of the functional importance of such delineations has also improved.
The immunomodulatory molecules discussed in section II highlight how DCs and T cells work together in a dynamic partnership, allowing them to control immune responses and maintain homeostasis. However, rather than affecting the functions of the entire population of DCs, expression of these molecules can be specific to particular DC subsets and therefore can also help to explain the functional specialization attributed to them. Specifically, cDC1s have multiple mechanisms mediating their ability to promote Th1 and Treg cell differentiation (Fig. 2), whereas cDC2s can promote Th2 and Th17 differentiation (Fig. 3).1,182 Additionally, both cDC1s and cDC2s can exert additional tolerogenic functions through multiple mechanisms (Figs. 2 and 3).1 pDCs are important for antiviral responses in addition to promoting tolerance through various mechanisms.186
FIG. 2:
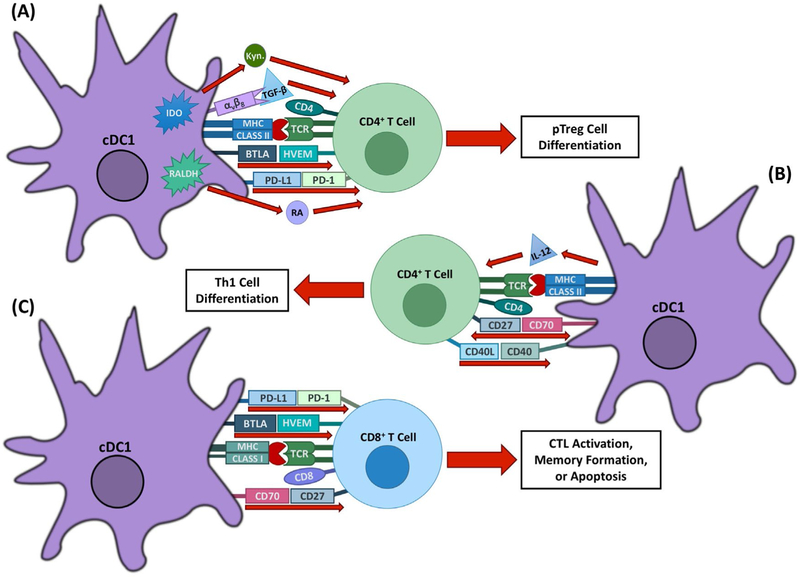
Immunomodulatory mechanisms of cDC1s. cDC1s are important for the induction of Th1 and tolerogenic T cell responses through several mechanisms. (A) cDC1s mediate T cell tolerance through several mechanisms, including pTreg induction by BTLA–HVEM, PD-L1–PD-1, RA, and TGF-β signaling. Expression of the enzyme IDO in response to CTLA-4–CD80/86 signaling also promotes tolerance through tryptophan deprivation and the production of metabolites, including kynurenine. (B) cDC1-expressed IL-12 and CD70, which are upregulated in response to CD40 signaling, work together to promote Th1 differentiation from naive CD4+ T cells. (C) cDC1s can promote CD4+ T cell-dependent expansion and effectiveness of CD8+ T cell responses through CD70–CD27 interactions. BTLA–HVEM signaling additionally plays a role in memory formation of CD8+ T cells in response to both viral and bacterial infections. cDC1s can also induce apoptosis of CD8+ T cells through PD-L1–PD-1 signaling.
FIG. 3:
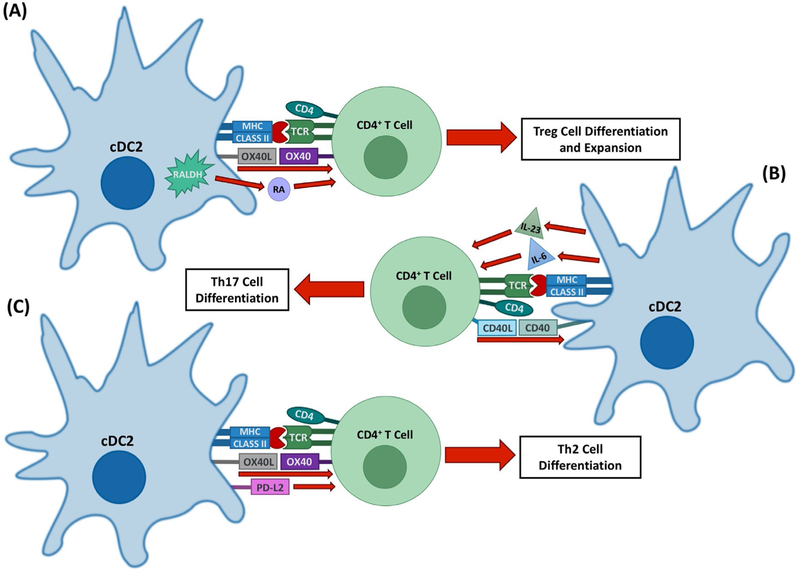
Immunomodulatory mechanisms of cDC2s. cDC2s are important for the induction of Th2 and Th17 responses as well as some aspects of tolerogenic T cell responses through several mechanisms. (A) cDC2s can promote the expansion of Treg cells, potentially through OX40 signaling. They also promote pTreg cell formation in some environments through their production of RA by Raldh2. (B) cDC2s are the main source of the Th17-polarizing cytokines IL-23 and IL-6, the expression of which can be upregulated by CD40 signaling. (C) cDC2 promote Th2 responses through OX40-L–OX40 signaling as well as through the production of Th2-polarizing cytokines. PD-L2 may also play a role in Th2 differentiation.
A. cDC1s
Various immunomodulatory molecules mediate the ability of cDC1s to promote Th1 responses. cDC1s from the spleen and lymph nodes are the predominant producers of the crucial Th1-polarizing cytokine IL-12 among DC subsets in response to microbial stimuli or to T cell-derived signals mediated through CD40.167,187–190 Despite this, IL-12 is not the only molecule mediating Th1 differentiation by cDC1s, because in vivo targeting of antigen to DEC205+ cDC1s, along with αCD40 and poly I:C, results in the induction of IFN-γ+ T cells even in IL-12-deficient mice.164 This is likely due to the signaling of CD70 and CD27 because the ability of cDC1s to induce Th1 differentiation is lost when CD70 is blocked, demonstrating the importance of this molecule in this process.164,165 Another level of regulation that further refines the Th1 response is mediated by the upregulation of CD70 expression in cDC1s in response to engagement of CD40 and a concomitant treatment with poly I:C.164,165
In addition to Th1 responses, DC-mediated CD4+ T cell help of CD8+ T cell responses has recently been shown to specifically depend on XCR1+ cDC1s because mice deficient in this subset have diminished proliferation, effector differentiation, and memory function in CD8+ T cells.191 As discussed above, CD70 is important for this process, even though cDC1s and cDC2s express similar levels of this molecule in the steady state.192 With that in mind, another aspect of CD8+ T cell responses is controlled by CD70+ cDC2s, which promote the expansion of CD8+ T cells in response to influenza infection by signaling through CD27 on the CD8+ T cells.193 Other aspects of CD8+ T cell function are mediated by cDC1s independently of CD4+ T cell help. For example, BTLA+ cDC1s promote survival and memory formation of CD8+ effector T cells in response to vaccinia virus and Listeria monocytogenes infections by signaling through HVEM in CD8+ T cells.82,86
Although it was originally thought that all DCs were able to induce pTreg cell differentiation in the steady state, increasing evidence has made it clear that cDC1s may be responsible for Treg cell differentiation in the periphery.1,98,194–196 When antigen is targeted to all CD11c+ DCs in vivo in the steady state, pTreg cells are formed at a much lower frequency than when antigen is selectively targeted to DEC205+ cDC1s, even though they comprise only a minor portion of all CD11c+ DCs.72 This finding was expanded upon to show that this function of DEC205+ cDC1s is due to the specific expression of BTLA, which signals through HVEM in naive T cells to induce pTreg cell conversion.72 CD8α+DEC205+ cDC1s also express more TGF-β1 and latent-TGF-β binding protein 2 (Ltbp2) at the transcriptional level.194 In addition to this, CD103+ and CD8α+ cDC1s are able to activate TGF-β1 to its active form through the activity of the integrin αvβ8, helping to promote the differentiation of Treg cells, and the β8 integrin subunit has shown to be specifically expressed by Irf8-dependent cDC1s in mice.62,197–199 CD103+ cDC1s from the mesenteric lymph nodes or small intestine lamina propria are able to more efficiently promote de novo differentiation of Treg cells than CD103neg cDC2s through their specific expression of Raldh2, although this subset-specific difference is lost when exogenous TGF-β1 is added to the cultures.62,67,200,201 Further, thymic medullary CD8α+ cDC1s promote survival of tTreg cells by inhibiting the mitochondrial apoptotic pathway through CD70–CD27 interactions.147
cDC1s are also the predominant source of the tolerogenic enzyme IDO, helping this subset to further promote tolerogenic responses through the multiple effects of tryptophan metabolism discussed in section II.B.39–41,202 Additionally, cDC1s are also the main source of the cytokine IL-27 in response to poly I:C, which helps them to promote Tr1 cell differentiation.171 In another tolerogenic function, Batf3-dependent cDC1s in the renal lymph node signal through PD-L1 to induce apoptosis of PD-1+ CD8+ T cells, promoting cross-tolerance to circulating antigens filtered by the kidneys.105 Expression of PD-L1 on CD103+ cDC1s is also important for successful checkpoint blockade therapy with αPD-L1 monoclonal antibodies.203 By expanding this population of DCs in the tumor with FMS-like tyrosine kinase 3 ligand (Flt3L) and poly I:C, the investigators showed that tumor-infiltrating CD8+ T cells were better able to control tumor growth upon αPD-L1 therapy, showing the importance of this pathway for anti-tumor responses.203
B. cDC2s
Irf4-dependent cDC2s have been shown to play a key role in the development of Th17 responses through their production of several different cytokines. Intestinal CD103+CD11b+ and lung CD11b+ cDC2s are crucial sources of IL-6 and IL-23p19, which promote Th17 differentiation in this environment.204–206 Additionally, cDC2s can trans-present IL-6 in complex with IL-6Rα, allowing them to signal to T cell-expressed gp130 which subsequently induces STAT3 phosphorylation and pathogenic Th17 cell differentiation, a process that is required for the development of EAE.207 This specialization is shared with other cDC2s that may frequently come in contact with the commensal and pathogenic microorganisms, such as dermal CD301b+ cDC2s that also preferentially produce IL-23.208,209 Consistently, in response to αCD40 or LPS stimulation cDC2s from the mesenteric lymph nodes also express more IL-23p19 and IL-6 at the transcriptional level.200 This is functionally important during infection because mice lacking cDC2s are unable to mount a successful Th17 response to infection with the fungus Aspergillus fumigatus.206
cDC2s have clearly been shown to play an important role in Th2 cell differentiation, although specific mechanisms are not always entirely clear. Mice lacking Irf4-dependent cDC2s fail to develop protective Th2 cell responses against infection with Nippostrongylus brasiliensis.210,211 Mice lacking expression of the transcription factor Klf4 in cDC2s fail to mount a protective Th2 cell response to the helminth Schistosoma mansoni while remaining unsusceptible to house dust mite (HDM)-induced allergic inflammation.212 HDM-induced allergic inflammation is mediated by Th2 cell differentiation by Irf4-dependent cDC2s that secrete IL-10 and IL-33.213,214 In an additional mechanism controlling Th2 cell differentiation, OX40L expression can also be induced in CD11b+ intestinal cDC2s in response to Schistosoma mansoni soluble egg antigens, helping to promote a Th2 response against the antigens.215 OX40L expression can be upregulated on lamina propria-derived CD103+ cDC1s in the mesenteric lymph nodes following administration of cholera toxin in a murine model of allergic sensitization to dietary antigens. This upregulation leads to more efficient Th2 differentiation by increased expression of Th2-differentiating cytokines, showing a compensatory redundancy in subset-specific role depending on different environmental signals.216 Further, Irf4-dependent cDC2s from skin-draining lymph nodes that are marked by expression of PD-L2 drive Th2 responses from effector and memory CD4+ T cells in vivo, although this effect does not appear to be dependent on PD-L2 functionality itself.211
Although cDC1s play a dominant role in the de novo induction of pTreg cells, some pTreg cells can be induced by cDC2s that also have a function in the expansion of existing tTreg cells.194,196,217,218 This expansion is due to cDC2-mediated proliferation of existing Treg cells through a contact-dependent mechanism that also requires IL-2 provided by CD4+ conventional T cells.194,219 The immunomodulatory molecules controlling this contact-dependent proliferation have not yet been clearly identified, although in vitro experiments with BMDCs suggest that OX40L may be required.120 In addition to expanding existing Treg cells, certain subsets of cDC2s in the skin and oral cavity can directly promote pTreg cell differentiation via RA, which is produced by these DCs through the enzymatic activity of Raldh2.220,221 Specificity of aldehyde dehydrogenase activity is not seen in lymph-borne DCs derived from the intestine because all subsets have the ability to generate RA, which subsequently induces gut-homing CCR9 expression in T cells.222 Interestingly, a role for integrin αvβ8 in the induction of Treg cells in humans has been shown, although its expression was specifically induced by pro-inflammatory signals in the human intestinal CD1c+ cDC2 counterpart, highlighting the more complex possible roles of site-specific DC activation in tolerance.223
In addition to their effects on Treg cells, cDC2s also play a tolerogenic role in non-obese diabetic (NOD) mice by increasing Zbtb32 expression in T cells upon specific delivery of antigen.218 Zbtb32 is a transcription factor that decreases proliferation and IFN-γ production when overexpressed in T cells from these mice.218 Although that study shows that cDC2s play an important role in the establishment of tolerance, the conflicting evidence on the specific roles of cDC2s in regard to pTreg cell induction warrants further investigation of these processes and the controlling mechanisms.
C. pDCs
Although they do not present antigen as efficiently as cDCs, pDCs are able to produce large amounts of type I IFNs upon recognition of viral particles by TLR7 or TLR9.224,225 This production of type I IFN supports the development of effective anti-viral CD8+ T cell responses by helping XCR1+ cDC1s to more efficiently cross-present antigens to CD8+ T cells.226 pDCs also have important roles in the regulation of tolerogenic T cell responses via multiple immunomodulatory mechanisms.186 pDCs promote Treg cell differentiation through their expression of multiple immunomodulatory molecules in the steady state or in response to various stimuli, some of which include Raldh, IDO, PD-L1, ICOS-L, and TGF-β1.17,135,202,227–233 IDO expression can be induced in pDCs following engagement of pDC-expressed CD200R by CD200, a widely expressed cell surface glycoprotein.230 Additionally, expression of PD-L1 in pDCs is induced by IL-27.234 In addition to the tolerogenic functions described in section II.B that are mediated by its enzymatic activity, IDO has also been shown to function as a signaling molecule in pDCs.229 Upon TGF-β1 signaling, ITIMs on IDO are phosphorylated by Fyn kinase, allowing the molecule to activate the non-canonical NF-κB pathway and recruit SHP proteins, leading to increased expression of IDO, TGF-β1, and type I IFNs.229 pDCs further contribute to tolerance by promoting the development of IL-10-producing CD4+ and CD8+ T cells that can suppress primary T cell responses.135,235
A recently described pathway utilized by pDCs for the maintenance of tolerance utilizes the neuronal guidance molecules Neuropilin-1 (Nrp1) and Semaphorin-4a (Sema4a).236 Engagement of Treg cell-expressed Nrp1 by Sema4a expressed on intratumoral pDCs results in a PTEN-mediated reduction of Akt phosphorylation and increased nuclear localization of the transcription factor Foxo3a, thus promoting functional stability of Treg cells.236 Induction of Nrp1 signaling in Treg cells by Sema4aIg in mice with B16 melanoma tumors promotes expression of IFN-γ and IFN-γR in Treg cells, thus reducing their suppressive function and therefore tumor size.237 Interestingly, Sema4a is also expressed by cDCs and can interact with CD72 and Tim-2 to promote T cell activation.238,239 This finding further promotes the idea that it is necessary to study the effects of these immunomodulatory pathways mediated by different DC subsets because the functional outcome may differ depending on cell type or environment.
IV. CONCLUSIONS
Our knowledge of immunomodulatory mechanisms has greatly increased in recent years, resulting in a better appreciation of the dynamic and tightly regulated functional partnership between DCs and T cells that govern effective immune responses to pathogens or tumors while also maintaining tolerance. Although the majority of research has focused on how DCs modulate T cell responses, it is clear that T cells can affect DC functionality in turn. Several of the molecules discussed here also have bidirectional signaling capacities or can be expressed on either cell type. Further investigations will be required to determine how these interactions between immunomodulatory molecules on DCs and T cells affect both types of cell in order to fully understand how these cells work together to orchestrate immune homeostasis. This will also help in the development of more precise therapies that target the immunomodulatory functions of DCs to treat cancer, autoimmunity, or infection.
ACKNOWLEDGMENTS
This work was supported in part by grants from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R01AI113903) (to D.H.). This publication is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
ABBREVIATIONS:
- APC
antigen-presenting cell
- BMDC
bone marrow-derived dendritic cell
- BTLA
B and T lymphocyte associated
- CTLA-4
cytotoxic T-lymphocyte antigen 4
- cDC
conventional dendritic cell
- DC
dendritic cell
- EAE
experimental autoimmune encephalomyelitis
- HVEM
herpesvirus entry mediatory
- ICOS
inducible T cell costimulatory
- IDO
indoleamine 2,3 dioxygenase
- ITIM
immunoreceptor tyrosine-based inhibition motif
- iTreg
in vitro-induced (or inducible) regulatory T cell
- MHC
major histocompatibility complex
- moDC
monocyte-derived dendritic cell
- mTOR
mechanistic target of rapamycin
- PD-1
programmed death-1
- PD-L1/2
programmed death ligand-1/2
- pDC
plasmacytoid dendritic cell
- pTreg
peripheral regulatory T cell
- RA
retinoic acid
- Raldh
retinaldehyde dehydrogenase
- SHP-1/2
Src homology domain 2-containing protein tyrosine phosphatase ½
- TCR
T cell receptor
- TGF-β
transforming growth factor beta
- TNFRSF
tumor necrosis factor receptor super-family
- TNFSF
tumor necrosis factor superfamily
- Tr1
regulatory type 1 T cell
- TRAF
TNF receptor-associated factor
- Treg
regulatory T cell
- tTreg
thymus-derived regulatory T cell
REFERENCES
- 1.Iberg CA, Jones A, Hawiger D. Dendritic cells as inducers of peripheral tolerance. Trends Immunol 2017;38(11):793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006;124(4):783–801. [DOI] [PubMed] [Google Scholar]
- 3.Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu Rev Immunol 2012;30:491–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol 2015;16(4):343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu G, Wu C, Wu Y, Zhao Y. Phagocytosis of apoptotic cells and immune regulation. Scand J Immunol 2006;64(1):1–9. [DOI] [PubMed] [Google Scholar]
- 6.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med 2000;191(3):423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson TA, Herndon J, Elzey B, Griffith TS, Schoenberger S, Green DR. Uptake of apoptotic antigen-coupled cells by lymphoid dendritic cells and cross-priming of CD8+ T-cells produce active immune unresponsiveness. J Immunol 2002;168(11):5589–95. [DOI] [PubMed] [Google Scholar]
- 8.García-González P, Ubilla-Olguín G, Catalán D, Schinnerling K,Aguillón JC. Tolerogenic dendritic cells for reprogramming of lymphocyte responses in autoimmune diseases. Autoimmun Rev 2016;15(11):1071–80. [DOI] [PubMed] [Google Scholar]
- 9.Zhou F, Zhang GX, Rostami A. Apoptotic cell-treated dendritic cells induce immune tolerance by specifically inhibiting development of CD4+ effector memory T-cells. Immunol Res 2016;64(1):73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krispin A, Bledi Y, Atallah M, Trahtemberg U, Verbovetski I, Nahari E, Zelig O, Linial M, Mevorach D. Apoptotic cell thrombospondin-1 and heparin–binding domain lead to dendritic-cell phagocytic and tolerizing states. Blood 2006;108(10):3580–9. [DOI] [PubMed] [Google Scholar]
- 11.Moseman EA, Liang X, Dawson AJ, Panoskaltsis–Mortari A, Krieg AM, Liu YJ, Blazar BR, Chen W. Human plasmacytoid dendritic cells activated by cpg oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T-cells. J Immunol 2004;173(7):4433–42. [DOI] [PubMed] [Google Scholar]
- 12.Penna G, Amuchastegui S, Giarratana N, Daniel KC, Vulcano M, Sozzani S, Adorini L. 1,25-Dihydroxyvitamin D selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J Immunol 2007;178(1):145–53. [DOI] [PubMed] [Google Scholar]
- 13.Manicassamy S, Ravindran R, Deng J, Oluoch H, Denning TL, Kasturi SP, Rosenthal KM, Evavold BD, Pulendran B. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med 2009;15(4):401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manicassamy S, Pulendran B. Dendritic cell control of tolerogenic responses. Immunol Rev 2011;241(1):206–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mellman I Dendritic cells: master regulators of the immune response. Cancer Immunol Res 2013;1(3):145–9. [DOI] [PubMed] [Google Scholar]
- 16.Takenaka MC, Quintana FJ. Tolerogenic dendritic cells. Semin Immunopathol 2017;39(2):113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dasgupta S, Erturk-Hasdemir D, Ochoa-Reparaz J, Reinecker HC, Kasper Dennis L. Plasmacytoid dendritic cells mediate anti-inflammatory responses to a gut commensal molecule via both innate and adaptive mechanisms. Cell Host Microbe 2014;15(4):413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B-cell activation antigen B7. J Exp Med 1991;174(3):561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linsley PS, Bradshaw J, Greene J, Peach R, Bennett KL, Mittler RS. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity 1996;4(6):535–43. [DOI] [PubMed] [Google Scholar]
- 20.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T-cells that control autoimmune diabetes. Immunity 2000;12(4):431–40. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med 2000;192(2):303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med 2000;192(2):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laurent S, Carrega P, Saverino D, Piccioli P, Camoriano M, Morabito A, Dozin B, Fontana V, Simone R, Mortara L, Mingari MC, Ferlazzo G, Pistillo MP. CTLA-4 is expressed by human monocyte-derived dendritic cells and regulates their functions. Human Immunol 2010;71(10):934–41. [DOI] [PubMed] [Google Scholar]
- 24.Halpert MM, Konduri V, Liang D, Chen Y, Wing JB, Paust S, Levitt JM, Decker WK. Dendritic cell-secreted cytotoxic T-lymphocyte-associated protein-4 regulates the T-cell response by downmodulating bystander surface B7. Stem Cells Dev 2016;25(10):774–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L, Flies DB. Molecular mechanisms of T-cell co-stimulation and co-inhibition. Nat Rev Immunol 2013;13(4):227–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker LSK, Sansom DM. Confusing signals: Recent progress in CTLA-4 biology. Trends Immunol 2015;36(2):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity 2001;14(2):145–55. [DOI] [PubMed] [Google Scholar]
- 28.Chen W, Jin W, Wahl SM. Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor β (TGF-β) production by murine CD4+ T cells. J Exp Med 1998;188(10):1849–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T-cell tolerance through PD–1 and CTLA-4. Nat Immunol 2005;6(3):280–6. [DOI] [PubMed] [Google Scholar]
- 30.Alissafi T, Banos A, Boon L, Sparwasser T, Ghigo A, Wing K, Vassilopoulos D, Boumpas D, Chavakis T, Cadwell K, Verginis P. Tregs restrain dendritic cell autophagy to ameliorate autoimmunity. J Clin Invest 2017;127(7):2789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A 2008;105(29):10113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolton HA, Zhu E, Terry AM, Guy TV, Koh WP, Tan SY, Power CA, Bertolino P, Lahl K, Sparwasser T, Shklovskaya E, de St. Groth BF. Selective Treg reconstitution during lymphopenia normalizes DC costimulation and prevents graft-versus-host disease. J Clin Invest 2015;125(9):3627–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F. Cytotoxic T lymphocyte antigen–4–dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology 2006;118(2):240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T-cell function. Science 2008;322(5899):271–5. [DOI] [PubMed] [Google Scholar]
- 35.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, Sansom DM. Trans–endocytosis of CD80 and CD86: a molecular basis for the cell–extrinsic function of CTLA-4. Science 2011;332(6029):600–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kowalczyk A, D’Souza CA, Zhang L. Cell-extrinsic CTLA4-mediated regulation of dendritic cell maturation depends on STAT3. Eur J Immunol 2014;44(4):1143–55. [DOI] [PubMed] [Google Scholar]
- 37.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol 2003;4(12):1206–12. [DOI] [PubMed] [Google Scholar]
- 38.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. CTLA-4–Ig regulates tryptophan catabolism in vivo. Nat Immunol 2002;3(11):1097–101. [DOI] [PubMed] [Google Scholar]
- 39.Mellor AL, Chandler P, Baban B, Hansen AM, Marshall B, Pihkala J, Waldmann H, Cobbold S, Adams E, Munn DH. Specific subsets of murine dendritic cells acquire potent T-cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int Immunol 2004;16(10):1391–401. [DOI] [PubMed] [Google Scholar]
- 40.Fallarino F, Vacca C, Orabona C, Belladonna ML, Bianchi R, Marshall B, Keskin DB, Mellor AL, Fioretti MC, Grohmann U, Puccetti P. Functional expression of indoleamine 2,3-dioxygenase by murine CD8 alpha(+) dendritic cells. Int Immunol 2002;14(1):65–8. [DOI] [PubMed] [Google Scholar]
- 41.Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, Puccetti P, Chieppa M, Rescigno M. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut 2010;59(5):595–604. [DOI] [PubMed] [Google Scholar]
- 42.Harden JL, Egilmez NK. Indoleamine 2,3-dioxygenase and dendritic cell tolerogenicity. Immunol Invest 2012;41(6–7):738–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Onodera T, Jang MH, Guo Z, Yamasaki M, Hirata T, Bai Z, Tsuji NM, Nagakubo D, Yoshie O, Sakaguchi S, Takikawa O, Miyasaka M. Constitutive expression of IDO by dendritic cells of mesenteric lymph nodes: functional involvement of the CTLA-4/B7 and CCL22/CCR4 interactions. J Immunol 2009;183(9):5608–14. [DOI] [PubMed] [Google Scholar]
- 44.Munn DH, Sharma MD, Mellor AL. Ligation of B7–1/B7–2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol 2004;172(7):4100–10. [DOI] [PubMed] [Google Scholar]
- 45.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T-cell receptor zeta–chain and induce a regulatory phenotype in naive T cells. J Immunol 2006;176(11):6752–61. [DOI] [PubMed] [Google Scholar]
- 46.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol 2010;185(6):3190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogel CFA, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun 2008;375(3):331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quintana FJ, Murugaiyan G, Farez MF, Mitsdoerffer M, Tukpah AM, Burns EJ, Weiner HL. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A 2010;107(48):20768–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cella M, Colonna M. Aryl hydrocarbon receptor: Linking environment to immunity. Semin Immunol 2015;27(5):310–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, Hauben E, Roncarolo MG. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood 2010;116(6):935–44. [DOI] [PubMed] [Google Scholar]
- 51.Brenk M, Scheler M, Koch S, Neumann J, Takikawa O, Hacker G, Bieber T, von Bubnoff D. Tryptophan deprivation induces inhibitory receptors ILT3 and ILT4 on dendritic cells favoring the induction of human CD4+CD25+ Foxp3+ T regulatory cells. J Immunol 2009;183(1):145–54. [DOI] [PubMed] [Google Scholar]
- 52.Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti E, Colonna M, Adorini L. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood 2005;106(10):3490–7. [DOI] [PubMed] [Google Scholar]
- 53.Manavalan JS, Rossi PC, Vlad G, Piazza F, Yarilina A, Cortesini R, Mancini D, Suciu–Foca N. High expression of ILT3 and ILT4 is a general feature of tolerogenic dendritic cells. Transpl Immunol 2003;11(3–4):245–58. [DOI] [PubMed] [Google Scholar]
- 54.Chang CC, Ciubotariu R, Manavalan JS, Yuan J, Colovai AI, Piazza F, Lederman S, Colonna M, Cortesini R, Dalla-Favera R, Suciu-Foca N. Tolerization of dendritic cells by TS cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol 2002;3(3):237–43. [DOI] [PubMed] [Google Scholar]
- 55.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J Cell Biol 2002;157(3):493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, Bluestone JA, Sheppard D. Loss of integrin alpha(v) beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature 2007;449(7160):361–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li N, Xie C, Lu N–h. Transforming growth factor-β: an important mediator in Helicobacter pylori-associated pathogenesis. Front Cell Infect Microbiol 2015;5:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 2003;198(12):1875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity 2008;28(4):468–76. [DOI] [PubMed] [Google Scholar]
- 60.Melton AC, Bailey-Bucktrout SL, Travis MA, Fife BT, Bluestone JA, Sheppard D. Expression of alphavbeta8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. J Clin Invest 2010;120(12):4436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Worthington JJ, Klementowicz JE, Rahman S, Czajkowska BI, Smedley C, Waldmann H, Sparwasser T, Grencis RK, Travis MA. Loss of the TGFβ-activating integrin αvβ8 on dendritic cells protects mice from chronic intestinal parasitic infection via control of type 2 immunity. PLoS Pathog 2013;9(10):e1003675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luda KM, Joeris T, Persson EK, Rivollier A, Demiri M, Sitnik KM, Pool L, Holm JB, Melo-Gonzalez F, Richter L, Lambrecht BN, Kristiansen K, Travis MA, Svensson-Frej M, Kotarsky K, Agace WW. IRF8 transcription-factor-dependent classical dendritic cells are essential for intestinal T-cell homeostasis. Immunity 2016;44(4):860–74. [DOI] [PubMed] [Google Scholar]
- 63.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006;24(2):179–89. [DOI] [PubMed] [Google Scholar]
- 64.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T-cell differentiation mediated by retinoic acid. Science 2007;317(5835):256–60. [DOI] [PubMed] [Google Scholar]
- 65.Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, O’Shea JJ. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood 2008;111(3):1013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med 2007;204(8):1765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 2007;204(8):1775–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity 2004;21(4):527–38. [DOI] [PubMed] [Google Scholar]
- 69.Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, Murphy TL, Russell JH, Allison JP, Murphy KM. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol 2003;4(7):670–9. [DOI] [PubMed] [Google Scholar]
- 70.Han P, Goularte OD, Rufner K, Wilkinson B, Kaye J. An inhibitory Ig superfamily protein expressed by lymphocytes and APCs is also an early marker of thymocyte positive selection. J Immunol 2004;172(10):5931–9. [DOI] [PubMed] [Google Scholar]
- 71.Murphy TL, Murphy KM. Slow down and survive: Enigmatic immunoregulation by BTLA and HVEM. Annu Rev Immunol 2010;28:389–411. [DOI] [PubMed] [Google Scholar]
- 72.Jones A, Bourque J, Kuehm L, Opejin A, Teague RM, Gross C, Hawiger D. Immunomodulatory functions of BTLA and HVEM govern induction of extrathymic regulatory T cells and tolerance by dendritic cells. Immunity 2016;45(5):1066–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steinberg MW, Cheung TC, Ware CF. The signaling networks of the herpesvirus entry mediator (TNFRSF14) in immune regulation. Immunol Rev 2011;244(1):169–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sedy JR, Gavrieli M, Potter KG, Hurchla MA, Lindsley RC, Hildner K, Scheu S, Pfeffer K, Ware CF, Murphy TL, Murphy KM. B and T lymphocyte attenuator regulates T-cell activation through interaction with herpesvirus entry mediator. Nat Immunol 2005;6(1):90–8. [DOI] [PubMed] [Google Scholar]
- 75.Gonzalez LC, Loyet KM, Calemine-Fenaux J, Chauhan V, Wranik B, Ouyang W, Eaton DL. A coreceptor interaction between the CD28 and TNF receptor family members B and T lymphocyte attenuator and herpesvirus entry mediator. Proc Natl Acad Sci U S A 2005;102(4):1116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morel Y, Truneh A, Sweet RW, Olive D, Costello RT. The TNF superfamily members LIGHT and CD154 (CD40 Ligand) costimulate induction of dendritic cell maturation and elicit specific CTL activity. J Immunol 2001;167(5):2479–86. [DOI] [PubMed] [Google Scholar]
- 77.De Trez C, Schneider K, Potter K, Droin N, Fulton J, Norris PS, Ha SW, Fu YX, Murphy T, Murphy KM, Pfeffer K, Benedict CA, Ware CF. The inhibitory HVEM–BTLA pathway counter regulates lymphotoxin receptor signaling to achieve homeostasis of dendritic cells. J Immunol 2008;180(1):238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Whitbeck JC, Peng C, Lou H, Xu R, Willis SH, Ponce de Leon M, Peng T, Nicola AV, Montgomery RI, Warner MS, Soulika AM, Spruce LA, Moore WT, Lambris JD, Spear PG, Cohen GH, Eisenberg RJ. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J Virol 1997;71(8):6083–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mauri DN, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu GL, Ruben S, Murphy M, Eisenberg RJ, Cohen GH, Spear PG, Ware CF. LIGHT, a new member of the TNF superfamily, and lymphotoxin α are ligands for herpesvirus entry mediator. Immunity 1998;8(1):21–30. [DOI] [PubMed] [Google Scholar]
- 80.Cai G, Anumanthan A, Brown JA, Greenfield EA, Zhu B, Freeman GJ. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat Immunol 2008;9:176–85. [DOI] [PubMed] [Google Scholar]
- 81.Cheung TC, Steinberg MW, Oborne LM, Macauley MG, Fukuyama S, Sanjo H, D’Souza C, Norris PS, Pfeffer K, Murphy KM, Kronenberg M, Spear PG, Ware CF. Unconventional ligand activation of herpesvirus entry mediator signals cell survival. Proc Natl Acad Sci U S A 2009;106(15):6244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steinberg MW, Huang Y, Wang-Zhu Y, Ware CF, Cheroutre H, Kronenberg M. BTLA interaction with HVEM expressed on CD8(+) T cells promotes survival and memory generation in response to a bacterial infection. PLoS One 2013;8(10):e77992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Soroosh P, Doherty TA, So T, Mehta AK, Khorram N, Norris PS, Scheu S, Pfeffer K, Ware C, Croft M. Herpesvirus entry mediator (TNFRSF14) regulates the persistence of T helper memory cell populations. J Exp Med 2011;208(4):797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharma S, Rajasagi NK, Veiga-Parga T, Rouse BT. Herpes virus entry mediator (HVEM) modulates proliferation and activation of regulatory T cells following HSV-1 infection. Microbes Infect 2014;16(8):648–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krieg C, Boyman O, Fu YX, Kaye J. B and T lymphocyte attenuator regulates CD8+ T-cell-intrinsic homeostasis and memory cell generation. Nat Immunol 2007;8:162–71. [DOI] [PubMed] [Google Scholar]
- 86.Flynn R, Hutchinson T, Murphy KM, Ware CF, Croft M, Salek-Ardakani S. CD8 T-cell memory to a viral pathogen requires trans cosignaling between HVEM and BTLA. PLoS One 2013;8(10):e77991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Steinberg MW, Turovskaya O, Shaikh RB, Kim G, McCole DF, Pfeffer K, Murphy KM, Ware CF, Kronenberg M. A crucial role for HVEM and BTLA in preventing intestinal inflammation. J Exp Med 2008;205(6):1463–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shui JW, Larange A, Kim G, Vela JL, Zahner S, Cheroutre H, Kronenberg M. HVEM signalling at mucosal barriers provides host defence against pathogenic bacteria. Nature 2012;488(7410):222–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Y, Subudhi SK, Anders RA, Lo J, Sun Y, Blink S, Wang Y, Wang J, Liu X, Mink K, Degrandi D, Pfeffer K, Fu YX. The role of herpesvirus entry mediator as a negative regulator of T-cell-mediated responses. J Clin Invest 2005;115(3):711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Henderson JG, Opejin A, Jones A, Gross C, Hawiger D. CD5 instructs extrathymic regulatory T-cell development in response to self and tolerizing antigens. Immunity 2015;42(3):471–83. [DOI] [PubMed] [Google Scholar]
- 91.Jones A, Opejin A, Henderson JG, Gross C, Jain R, Epstein JA, Flavell RA, Hawiger D. Peripherally induced tolerance depends on peripheral regulatory T cells that require Hopx to inhibit intrinsic IL-2 expression. J Immunol 2015;195(4):1489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jones A, Hawiger D. Peripherally induced regulatory T cells: Recruited protectors of the central nervous system against autoimmune neuroinflammation. Front Immunol 2017;8:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192(7):1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999;5:1365–9. [DOI] [PubMed] [Google Scholar]
- 95.Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity 2018;48(3):434–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol 2017;18:153–67. [DOI] [PubMed] [Google Scholar]
- 97.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009;206(13):3015–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci U S A 2008;105(27):9331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen X, Fosco D, Kline DE, Meng L, Nishi S, Savage PA, Kline J. PD-1 regulates extrathymic regulatory T-cell differentiation. Eur J Immunol 2014;44(9):2603–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yogev N, Frommer F, Lukas D, Kautz–Neu K, Karram K, Ielo D, von Stebut E, Probst HC, van den Broek M, Riethmacher D, Birnberg T, Blank T, Reizis B, Korn T, Wiendl H, Jung S, Prinz M, Kurschus FC, Waisman A. Dendritic cells ameliorate autoimmunity in the CNS by controlling the homeostasis of PD-1 receptor(+) regulatory T cells. Immunity 2012;37(2):264–75. [DOI] [PubMed] [Google Scholar]
- 101.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T-cell activation. Nat Immunol 2001;2(3):261–8. [DOI] [PubMed] [Google Scholar]
- 102.Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med 2001;193(7):839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Riella LV, Paterson AM, Sharpe AH, Chandraker A. Role of the PD-1 pathway in the immune response. Am J Trans 2012;12(10):2575–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Karunarathne DS, Horne-Debets JM, Huang JX, Faleiro R, Leow CY, Amante F, Watkins TS, Miles JJ, Dwyer PJ, Stacey KJ, Yarski M, Poh CM, Lee JS, Cooper MA, Renia L, Richard D, McCarthy JS, Sharpe AH, Wykes MN. Programmed death-1 ligand 2-mediated regulation of the PD-L1 to PD-1 axis is essential for establishing CD4(+) T-cell immunity. Immunity 2016;45(2):333–45. [DOI] [PubMed] [Google Scholar]
- 105.Gottschalk C, Damuzzo V, Gotot J, Kroczek RA, Yagita H, Murphy KM, Knolle PA, Ludwig-Portugall I, Kurts C. Batf3-dependent dendritic cells in the renal lymph node induce tolerance against circulating antigens. J Am Soc Nephrol 2013;24(4):543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Larrubia JR, Benito-Martinez S, Miquel J, Calvino M, Sanz-de-Villalobos E, Gonzalez-Praetorius A, Albertos S, Garcia-Garzon S, Lokhande M, Parra-Cid T. Bim-mediated apoptosis and PD-1/PD-L1 pathway impair reactivity of PD1(+)/CD127(−) HCV-specific CD8(+) cells targeting the virus in chronic hepatitis C virus infection. Cell Immunol 2011;269(2):104–14. [DOI] [PubMed] [Google Scholar]
- 107.Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol 2009;10(11):1185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karwacz K, Bricogne C, MacDonald D, Arce F, Bennett CL, Collins M, Escors D. PD-L1 co-stimulation contributes to ligand-induced T-cell receptor down-modulation on CD8+ T cells. EMBO Mol Med 2011;3(10):581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sage PT, Schildberg FA, Sobel RA, Kuchroo VK, Freeman GJ, Sharpe AH. Dendritic cell PD-L1 limits autoimmunity and follicular T-cell differentiation and function. J Immunol 2018;200(8):2592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Paterson DJ, Jefferies WA, Green JR, Brandon MR, Corthesy P, Puklavec M, Williams AF. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol 1987;24(12):1281–90. [DOI] [PubMed] [Google Scholar]
- 111.Calderhead DM, Buhlmann JE, van den Eertwegh AJ, Claassen E, Noelle RJ, Fell HP. Cloning of mouse Ox40: a T-cell activation marker that may mediate T-B-cell interactions. J Immunol 1993;151(10):5261. [PubMed] [Google Scholar]
- 112.Mallett S, Fossum S, Barclay AN. Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes: a molecule related to nerve growth factor receptor. EMBO J 1990;9(4):1063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stüber E, Neurath M, Calderhead D, Perry Fell H, Strober W. Cross-linking of OX40 ligand, a member of the TNF/NGF cytokine family, induces proliferation and differentiation in murine splenic B-cells. Immunity 1995;2(5):507–21. [DOI] [PubMed] [Google Scholar]
- 114.Ohshima Y, Tanaka Y, Tozawa H, Takahashi Y, Maliszewski C, Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J Immunol 1997;159(8):3838. [PubMed] [Google Scholar]
- 115.Weinberg AD, Wegmann KW, Funatake C, Whitham RH. Blocking OX-40/OX-40 ligand interaction in vitro and in vivo leads to decreased T-cell function and amelioration of experimental allergic encephalomyelitis. J Immunol 1999;162(3):1818–26. [PubMed] [Google Scholar]
- 116.Croft M, So T, Duan W, Soroosh P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev 2009;229(1):173–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.So T, Song J, Sugie K, Altman A, Croft M. Signals from OX40 regulate nuclear factor of activated T cells c1 and T-cell helper 2 lineage commitment. Proc Natl Acad Sci U S A 2006;103(10):3740–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.So T, Croft M. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J Immunol 2007;179(3):1427–30. [DOI] [PubMed] [Google Scholar]
- 119.Vu MD, Xiao X, Gao W, Degauque N, Chen M, Kroemer A, Killeen N, Ishii N, Li XC. OX40 costimulation turns off Foxp3+ Tregs. Blood 2007;110(7):2501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bhattacharya P, Gopisetty A, Ganesh BB, Sheng JR, Prabhakar BS. GM-CSF-induced, bone-marrow-derived dendritic cells can expand natural Tregs and induce adaptive Tregs by different mechanisms. J Leukoc Biol 2011;89(2):235–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kumar P, Alharshawi K, Bhattacharya P, Marinelarena A, Haddad C, Sun Z, Chiba S, Epstein AL, Prabhakar BS. Soluble OX40L and JAG1 induce selective proliferation of functional regulatory T cells independent of canonical TCR signaling. Sci Rep 2017;7:39751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T, Tafuri-Bladt A, Brankow D, Campbell P, Chang D, Chiu L, Dai T, Duncan G, Elliott GS, Hui A, McCabe SM, Scully S, Shahinian A, Shaklee CL, Van G, Mak TW, Senaldi G. T-cell co-stimulation through B7RP-1 and ICOS. Nature 1999;402(6763):827–32. [DOI] [PubMed] [Google Scholar]
- 123.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 1999;397(6716):263–6. [DOI] [PubMed] [Google Scholar]
- 124.Swallow MM, Wallin JJ, Sha WC. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFalpha. Immunity 1999;11(4):423–32. [DOI] [PubMed] [Google Scholar]
- 125.Coyle AJ, Lehar S, Lloyd C, Tian J, Delaney T, Manning S, Nguyen T, Burwell T, Schneider H, Gonzalo JA, Gosselin M, Owen LR, Rudd CE, Gutierrez-Ramos JC. The CD28-related molecule ICOS is required for effective T-cell-dependent immune responses. Immunity 2000;13(1):95–105. [DOI] [PubMed] [Google Scholar]
- 126.Fos C, Salles A, Lang V, Carrette F, Audebert S, Pastor S, Ghiotto M, Olive D, Bismuth G, Nunès JA. ICOS ligation recruits the p50α PI3K regulatory subunit to the immunological synapse. J Immunol 2008;181(3):1969–77. [DOI] [PubMed] [Google Scholar]
- 127.Wikenheiser DJ, Stumhofer JS. ICOS co-stimulation: friend or foe? Front Immunol 2016;7:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature 2001;409(6816):97–101. [DOI] [PubMed] [Google Scholar]
- 129.Kopf M, Coyle AJ, Schmitz N, Barner M, Oxenius A, Gallimore A, Gutierrez-Ramos JC, Bachmann MF. Inducible costimulator protein (ICOS) controls T helper cell subset polarization after virus and parasite infection. J Exp Med 2000;192(1):53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeham A, Boucher LM, Bouchard D, Chan VS, Duncan G, Odermatt B, Ho A, Itie A, Horan T, Whoriskey JS, Pawson T, Penninger JM, Ohashi PS, Mak TW. ICOS is essential for effective T-helper-cell responses. Nature 2001;409(6816):105–9. [DOI] [PubMed] [Google Scholar]
- 131.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med 2002;8(9):1024–32. [DOI] [PubMed] [Google Scholar]
- 132.Hawiger D, Tran E, Du W, Booth CJ, Wen L, Dong C, Flavell RA. ICOS mediates the development of insulin–dependent diabetes mellitus in nonobese diabetic mice. J Immunol 2008;180(5):3140–7. [DOI] [PubMed] [Google Scholar]
- 133.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annual Rev Immunol 2005;23:515–48. [DOI] [PubMed] [Google Scholar]
- 134.Larimore K, Liang L, Bakkour S, Sha WC. B7h-expressing dendritic cells and plasma B-cells mediate distinct outcomes of ICOS costimulation in T-cell-dependent antibody responses. BMC Immunol 2012;13(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, Qin XF, Liu YJ, Gilliet M. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med 2007;204(1):105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Faget J, Bendriss-Vermare N, Gobert M, Durand I, Olive D, Biota C, Bachelot T, Treilleux I, Goddard-Leon S, Lavergne E, Chabaud S, Blay JY, Caux C, Ménétrier-Caux C. ICOS-ligand expression on plasmacytoid dendritic cells supports breast cancer progression by promoting the accumulation of immunosuppressive CD4+ T cells. Cancer Res 2012;72(23):6130–41. [DOI] [PubMed] [Google Scholar]
- 137.Tang G, Qin Q, Zhang P, Wang G, Liu M, Ding Q, Qin Y, Shen Q. Reverse signaling using an inducible costimulator to enhance immunogenic function of dendritic cells. Cell Mol Life Sci 2009;66(18):3067–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Occhipinti S, Dianzani C, Chiocchetti A, Boggio E, Clemente N, Gigliotti CL, Soluri MF, Minelli R, Fantozzi R, Yagi J, Rojo JM, Sblattero D, Giovarelli M, Dianzani U. Triggering of B7h by the ICOS modulates maturation and migration of monocyte-derived dendritic cells. J Immunol 2013;190(3):1125–34. [DOI] [PubMed] [Google Scholar]
- 139.Goodwin RG, Alderson MR, Smith CA, Armitage RJ, VandenBos T, Jerzy R, Tough TW, Schoenborn MA, Davis–Smith T, Hennen K, Falk B, Cosman D, Baker E, Sutherland GR, Grabstein KH, Farrah T, Giri JG, Beckmann MP. Molecular and biological characterization of a ligand for CD27 defines a new family of cytokines with homology to tumor necrosis factor. Cell 1993;73(3):447–56. [DOI] [PubMed] [Google Scholar]
- 140.Nolte MA, Van Olffen RW, Van Gisbergen KPJM, Van Lier RAW. Timing and tuning of CD27–CD70 interactions: the impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunol Rev 2009;229(1):216–31. [DOI] [PubMed] [Google Scholar]
- 141.Denoeud J, Moser M. Role of CD27/CD70 pathway of activation in immunity and tolerance. J Leuk Biol 2011;89(2):195–203. [DOI] [PubMed] [Google Scholar]
- 142.Van Deusen KE, Rajapakse R, Bullock TNJ. CD70 expression by dendritic cells plays a critical role in the immunogenicity of CD40-independent, CD4+ T-cell-dependent, licensed CD8+ T-cell responses. J Leuk Biol 2010;87(3):477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yamamoto H, Kishimoto T, Minamoto S. NF-κB activation in CD27 signaling: involvement of TNF receptor-associated factors in its signaling and identification of functional region of CD27. J Immunol 1998;161(9):4753–9. [PubMed] [Google Scholar]
- 144.Akiba H, Nakano H, Nishinaka S, Shindo M, Kobata T, Atsuta M, Morimoto C, Ware CF, Malinin NL, Wallach D, Yagita H, Okumura K. CD27, a member of the tumor necrosis factor receptor superfamily, activates NF-κB and stress–activated protein kinase/c–Jun N–terminal kinase via TRAF2, TRAF5, and NF-κB-inducing kinase. J Biol Chem 1998;273(21):13353–8. [DOI] [PubMed] [Google Scholar]
- 145.van Oosterwijk MF, Juwana H, Arens R, Tesselaar K, van Oers MHJ, Eldering E, van Lier RAW. CD27– CD70 interactions sensitise naive CD4+ T cells for IL-12-induced Th1 cell development. Int Immunol 2007;19(6):713–8. [DOI] [PubMed] [Google Scholar]
- 146.Coquet JM, Middendorp S, van der Horst G, Kind J, Veraar EA, Xiao Y, Jacobs H, Borst J. The CD27 and CD70 costimulatory pathway inhibits effector function of T helper 17 cells and attenuates associated autoimmunity. Immunity 2013;38(1):53–65. [DOI] [PubMed] [Google Scholar]
- 147.Coquet JM, Ribot JC, Babala N, Middendorp S, van der Horst G, Xiao Y, Neves JF, Fonseca-Pereira D, Jacobs H, Pennington DJ, Silva-Santos B, Borst J. Epithelial and dendritic cells in the thymic medulla promote CD4+Foxp3+ regulatory T-cell development via the CD27-CD70 pathway. J Exp Med 2013;210(4):715–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Arens R, Nolte MA, Tesselaar K, Heemskerk B, Reedquist KA, van Lier RAW, van Oers MHJ. Signaling through CD70 regulates B-cell activation and IgG production. J Immunol 2004;173(6):3901–8. [DOI] [PubMed] [Google Scholar]
- 149.Al Sayed MF, Ruckstuhl CA, Hilmenyuk T, Claus C, Bourquin JP, Bornhauser BC, Radpour R, Riether C, Ochsenbein AF. CD70 reverse signaling enhances NK cell function and immunosurveillance in CD27-expressing B-cell malignancies. Blood 2017;130(3):297–309. [DOI] [PubMed] [Google Scholar]
- 150.Dhainaut M, Coquerelle C, Uzureau S, Denoeud J, Acolty V, Oldenhove G, Galuppo A, Sparwasser T, Thielemans K, Pays E, Yagita H, Borst J, Moser M. Thymus-derived regulatory T cells restrain pro-inflammatory Th1 responses by downregulating CD70 on dendritic cells. EMBO J 2015;34(10):1336–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kuka M, Munitic I, Giardino Torchia ML, Ashwell JD. CD70 is downregulated by interaction with CD27. J Immunol 2013;191(5):2282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Taraban VY, Rowley TF, Tough DF, Al–Shamkhani A. Requirement for CD70 in CD4+ Th cell-dependent and innate receptor-mediated CD8+ T-cell priming. J Immunol 2006;177(5):2969–75. [DOI] [PubMed] [Google Scholar]
- 153.Schildknecht A, Miescher I, Yagita H, Broek Mvd. Priming of CD8+ T-cell responses by pathogens typically depends on CD70-mediated interactions with dendritic cells. Eur J Immunol 2007;37(3):716–28. [DOI] [PubMed] [Google Scholar]
- 154.Laouar A, Haridas V, Vargas D, Zhinan X, Chaplin D, van Lier RAW, Manjunath N. CD70+ antigen-presenting cells control the proliferation and differentiation of T cells in the intestinal mucosa. Nat Immunol 2005;6:698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rowley TF, Al–Shamkhani A. Stimulation by soluble CD70 promotes strong primary and secondary CD8+ cytotoxic T-cell responses in vivo. J Immunol 2004;172(10):6039–46. [DOI] [PubMed] [Google Scholar]
- 156.Feau S, Garcia Z, Arens R, Yagita H, Borst J, Schoenberger SP. The CD4(+) T-cell help signal is transmitted from APC to CD8(+) T cells via CD27-CD70 interactions. Nat Commun 2012;3:948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ahrends T, Spanjaard A, Pilzecker B, Babala N, Bovens A, Xiao Y, Jacobs H, Borst J. CD4(+) T-cell help confers a cytotoxic T-cell effector program including coinhibitory receptor downregulation and increased tissue invasiveness. Immunity 2017;47(5):848–61.e5. [DOI] [PubMed] [Google Scholar]
- 158.Clark EA, Ledbetter JA. Activation of human B-cells mediated through two distinct cell surface differentiation antigens, Bp35 and Bp50. Proc Natl Acad Sci 1986;83(12):4494–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Armitage RJ, Fanslow WC, Strockbine L, Sato TA, Clifford KN, Macduff BM, Anderson DM, Gimpel SD, Davis-Smith T, Maliszewski CR, Clark EA, Smith CA, Grabstein KH, Cosman D, Spriggs MK. Molecular and biological characterization of a murine ligand for CD40. Nature 1992;357:80. [DOI] [PubMed] [Google Scholar]
- 160.Noelle RJ, Roy M, Shepherd DM, Stamenkovic I, Ledbetter JA, Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B-cells. Proc Natl Acad Sci 1992;89(14):6550–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ma DY, Clark EA. The role of CD40 and CD154/CD40L in dendritic cells. Semin Immunol 2009;21(5):265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Oh J, Wu N, Barczak AJ, Barbeau R, Erle DJ, Shin JS. CD40 Mediates maturation of thymic dendritic cells driven by self-reactive CD4(+) thymocytes and supports development of natural regulatory T cells. J Immunol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Taraban VY, Rowley TF, Al–Shamkhani A. Cutting edge: a critical role for CD70 in CD8 T-cell priming by CD40-licensed APCs. J Immunol 2004;173(11):6542–6. [DOI] [PubMed] [Google Scholar]
- 164.Soares H, Waechter H, Glaichenhaus N, Mougneau E, Yagita H, Mizenina O, Dudziak D, Nussenzweig MC, Steinman RM. A subset of dendritic cells induces CD4+ T cells to produce IFN-gamma by an IL-12-independent but CD70-dependent mechanism in vivo. J Exp Med 2007;204(5):1095–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kurche JS, Burchill MA, Sanchez PJ, Haluszczak C, Kedl RM. Comparison of OX40 ligand and CD70 in the promotion of CD4+ T-cell responses. J Immunol 2010;185(4):2106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sun L, He C, Nair L, Yeung J, Egwuagu CE. Interleukin 12 (IL-12) family cytokines: Role in immune pathogenesis and treatment of CNS autoimmune disease. Cytokine 2015;75(2):249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schulz O, Edwards DA, Schito M, Aliberti J, Manickasingham S, Sher A, Reis e Sousa C. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity 2000;13(4):453–62. [DOI] [PubMed] [Google Scholar]
- 168.Stritesky GL, Yeh N, Kaplan MH. IL-23 promotes maintenance but not commitment to the Th17 lineage. J Immunol 2008;181(9):5948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 2013;496(7446):513–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wu C, Chen Z, Xiao S, Thalhamer T, Madi A, Han T, Kuchroo V. SGK1 governs the reciprocal development of Th17 and regulatory T cells. Cell Rep 2018;22(3):653–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kilgore AM, Welsh S, Cheney EE, Chitrakar A, Blain TJ, Kedl BJ, Hunter CA, Pennock ND, Kedl RM. IL–27p28 production by XCR1+ dendritic cells and monocytes effectively predicts adjuvant-elicited CD8+ T-cell responses. ImmunoHorizons 2018;2(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U S A 2003;100(25):15047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hibbert L, Pflanz S, de Waal Malefyt R, Kastelein RA. IL-27 and IFN-α signal via Stat1 and Stat3 and induce T-Bet and IL-12Rβ2 in naive T cells. J Interferon Cytokine Res 2003;23(9):513–22. [DOI] [PubMed] [Google Scholar]
- 174.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol 2007;8(12):1380–9. [DOI] [PubMed] [Google Scholar]
- 175.Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, Sharpe AH, Karp CL, Miaw SC, Ho IC, Kuchroo VK. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol 2009;183(2):797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, Burns EJ, Sherr DH, Weiner HL, Kuchroo VK. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol 2010;11:854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gandhi R, Kumar D, Burns EJ, Nadeau M, Dake B, Laroni A, Kozoriz D, Weiner HL, Quintana FJ. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T-cell-like and Foxp3+ regulatory T cells. Nat Immunol 2010;11:846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mascanfroni ID, Yeste A, Vieira SM, Burns EJ, Patel B, Sloma I, Wu Y, Mayo L, Ben-Hamo R, Efroni S, Kuchroo VK, Robson SC, Quintana FJ. IL-27 acts on DCs to suppress the T-cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat Immunol 2013;14(10):1054–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Trad M, Gautheron A, Fraszczak J, Alizadeh D, Larmonier C, LaCasse CJ, Centuori S, Audia S, Samson M, Ciudad M, Bonnefoy F, Lemaire-Ewing S, Katsanis E, Perruche S, Saas P, Bonnotte B. T lymphocyte inhibition by tumor-infiltrating dendritic cells involves ectonucleotidase CD39 but not arginase-1. Biomed Res Int 2015;2015:891236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ganea D, Hooper KM, Kong W. The neuropeptide vasoactive intestinal peptide: direct effects on immune cells and involvement in inflammatory and autoimmune diseases. Acta Physiol (Oxf) 2014;213(2):442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol 2013;31:563–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Durai V, Murphy KM. Functions of murine dendritic cells. Immunity 2016;45(4):719–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity 2014;40(5):642–56. [DOI] [PubMed] [Google Scholar]
- 184.Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, Tussiwand R, Yona S. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 2014;14(8):571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Guilliams M, Dutertre CA, Scott CL, McGovern N, Sichien D, Chakarov S, Van Gassen S, Chen J, Poidinger M, De Prijck S, Tavernier SJ, Low I, Irac SE, Mattar CN, Sumatoh HR, Low GH, Chung TJ, Chan DK, Tan KK, Hon TL, Fossum E, Bogen B, Choolani M, Chan JK, Larbi A, Luche H, Henri S, Saeys Y, Newell EW, Lambrecht BN, Malissen B, Ginhoux F. Unsupervised high-dimensional analysis aligns dendritic cells across tissues and species. Immunity 2016;45(3):669–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol 2015;15(8):471–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Reis e Sousa C, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T-cell areas. J Exp Med 1997;186(11):1819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O’Keeffe M. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J Immunol 2001;166(9):5448–55. [DOI] [PubMed] [Google Scholar]
- 189.Everts B, Tussiwand R, Dreesen L, Fairfax KC, Huang SC, Smith AM, O’Neill CM, Lam WY, Edelson BT, Urban JF, Murphy KM, Pearce EJ. Migratory CD103+ dendritic cells suppress helminth–driven type 2 immunity through constitutive expression of IL-12. J Exp Med 2016;213(1):35–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mashayekhi M, Sandau MM, Dunay IR, Frickel EM, Khan A, Goldszmid RS, Sher A, Ploegh HL, Murphy TL, Sibley LD, Murphy KM. CD8alpha(+) dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity 2011;35(2):249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Eickhoff S, Brewitz A, Gerner MY, Klauschen F, Komander K, Hemmi H, Garbi N, Kaisho T, Germain RN, Kastenmuller W. Robust Anti-viral immunity requires multiple distinct T-cell-dendritic cell interactions. Cell 2015;162(6):1322–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Keller AM, Schildknecht A, Xiao Y, van den Broek M, Borst J. Expression of costimulatory ligand CD70 on steady-state dendritic cells breaks CD8+ T-cell tolerance and permits effective immunity. Immunity 2008;29(6):934–46. [DOI] [PubMed] [Google Scholar]
- 193.Ballesteros-Tato A, Leon B, Lund FE, Randall TD. Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8(+) T-cell responses to influenza. Nat Immunol 2010;11(3):216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, Nussenzweig MC, Steinman RM. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol 2008;181(10):6923–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T-cell populations by foreign antigen. Nat Immunol 2005;6(12):1219–27. [DOI] [PubMed] [Google Scholar]
- 196.Fukaya T, Murakami R, Takagi H, Sato K, Sato Y, Otsuka H, Ohno M, Hijikata A, Ohara O, Hikida M, Malissen B, Sato K. Conditional ablation of CD205+ conventional dendritic cells impacts the regulation of T-cell immunity and homeostasis in vivo. Proc Natl Acad Sci U S A 2012;109(28):11288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Paidassi H, Acharya M, Zhang A, Mukhopadhyay S, Kwon M, Chow C, Stuart LM, Savill J, Lacy-Hulbert A. Preferential expression of integrin alphavbeta8 promotes generation of regulatory T cells by mouse CD103+ dendritic cells. Gastroenterology 2011;141(5):1813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Worthington JJ, Czajkowska BI, Melton AC, Travis MA. Intestinal dendritic cells specialize to activate transforming growth factor-beta and induce Foxp3+ regulatory T cells via integrin alphavbeta8. Gastroenterology 2011;141(5):1802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Boucard-Jourdin M, Kugler D, Endale Ahanda ML, This S, De Calisto J, Zhang A, Mora JR, Stuart LM, Savill J, Lacy-Hulbert A, Paidassi H. beta8 integrin expression and activation of TGF-beta by intestinal dendritic cells are determined by both tissue microenvironment and cell lineage. J Immunol 2016;197(5):1968–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 2007;204(8):1757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, Berg PL, Davidsson T, Powrie F, Johansson-Lindbom B, Agace WW. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med 2008;205(9):2139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mellor AL, Baban B, Chandler P, Marshall B, Jhaver K, Hansen A, Koni PA, Iwashima M, Munn DH. Cutting edge: induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T-cell clonal expansion. J Immunol 2003;171(4):1652–5. [DOI] [PubMed] [Google Scholar]
- 203.Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S, Casanova-Acebes M, Khudoynazarova M, Agudo J, Tung N, Chakarov S, Rivera C, Hogstad B, Bosenberg M, Hashimoto D, Gnjatic S, Bhardwaj N, Palucka AK, Brown BD, Brody J, Ginhoux F, Merad M. Expansion and activation of CD103(+) dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity 2016;44(4):924–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Persson EK, Uronen-Hansson H, Semmrich M, Rivollier A, Hagerbrand K, Marsal J, Gudjonsson S, Hakansson U, Reizis B, Kotarsky K, Agace WW. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity 2013;38(5):958–69. [DOI] [PubMed] [Google Scholar]
- 205.Satpathy AT, Briseno CG, Lee JS, Ng D, Manieri NA, Kc W, Wu X, Thomas SR, Lee WL, Turkoz M, McDonald KG, Meredith MM, Song C, Guidos CJ, Newberry RD, Ouyang W, Murphy TL, Stappenbeck TS, Gommerman JL, Nussenzweig MC, Colonna M, Kopan R, Murphy KM. Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nat Immunol 2013;14(9):937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, Ho AW, See P, Shin A, Wasan PS, Hoeffel G, Malleret B, Heiseke A, Chew S, Jardine L, Purvis HA, Hilkens CM, Tam J, Poidinger M, Stanley ER, Krug AB, Renia L, Sivasankar B, Ng LG, Collin M, Ricciardi-Castagnoli P, Honda K, Haniffa M, Ginhoux F. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 2013;38(5):970–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Heink S, Yogev N, Garbers C, Herwerth M, Aly L, Gasperi C, Husterer V, Croxford AL, Möller-Hackbarth K, Bartsch HS, Sotlar K, Krebs S, Regen T, Blum H, Hemmer B, Misgeld T, Wunderlich TF, Hidalgo J, Oukka M, Rose-John S, Schmidt-Supprian M, Waisman A, Korn T. Trans-presentation of IL-6 by dendritic cells is required for the priming of pathogenic TH17 cells. Nat Immunol 2016;18:74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kashem SW, Riedl MS, Yao C, Honda CN, Vulchanova L, Kaplan DH. Nociceptive sensory fibers drive interleukin-23 production from CD301b+ dermal dendritic cells and drive protective cutaneous immunity. Immunity 2015;43(3):515–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kim TG, Kim SH, Park J, Choi W, Sohn M, Na HY, Lee M, Lee JW, Kim SM, Kim DY, Kim HP, Choi JH, Park CG, Lee MG. Skin-specific CD301b(+) dermal dendritic cells drive IL-17-mediated psoriasis-like immune response in mice. J Invest Dermatol 2018;138(4):844–53. [DOI] [PubMed] [Google Scholar]
- 210.Kumamoto Y, Linehan M, Weinstein JS, Laidlaw BJ, Craft JE, Iwasaki A. CD301b(+) dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity 2013;39(4):733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gao Y, Nish SA, Jiang R, Hou L, Licona-Limon P, Weinstein JS, Zhao H, Medzhitov R. Control of T helper 2 responses by transcription factor IRF4-dependent dendritic cells. Immunity 2013;39(4):722–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Tussiwand R, Everts B, Grajales–Reyes GE, Kretzer NM, Iwata A, Bagaitkar J, Wu X, Wong R, Anderson DA, Murphy TL, Pearce EJ, Murphy KM. Klf4 expression in conventional dendritic cells is required for T helper 2 cell responses. Immunity 2015;42(5):916–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, Vanhoutte L, Neyt K, Killeen N, Malissen B, Hammad H, Lambrecht Bart N. conventional and monocyte-derived CD11b+ dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 2013;38(2):322–35. [DOI] [PubMed] [Google Scholar]
- 214.Williams JW, Tjota MY, Clay BS, Vander Lugt B, Bandukwala HS, Hrusch CL, Decker DC, Blaine KM, Fixsen BR, Singh H, Sciammas R, Sperling AI. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat Commun 2013;4:2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mayer JU, Demiri M, Agace WW, MacDonald AS, Svensson-Frej M, Milling SW. Different populations of CD11b(+) dendritic cells drive Th2 responses in the small intestine and colon. Nat Commun 2017;8:15820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Blazquez AB, Berin MC. Gastrointestinal dendritic cells promote Th2 skewing via OX40L. J Immunol 2008;180(7):4441–50. [DOI] [PubMed] [Google Scholar]
- 217.Nutsch K, Chai JN, Ai TL, Russler-Germain E, Feehley T, Nagler CR, Hsieh CS. Rapid and efficient generation of regulatory T cells to commensal antigens in the periphery. Cell Rep 2016;17(1):206–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Price JD, Hotta-Iwamura C, Zhao Y, Beauchamp NM, Tarbell KV. DCIR2+ cDC2 DCs and Zbtb32 restore CD4+ T-cell tolerance and inhibit diabetes. Diabetes 2015;64(10):3521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zou T, Caton AJ, Koretzky GA, Kambayashi T. Dendritic cells induce regulatory T-cell proliferation through antigen-dependent and -independent interactions. J Immunol 2010;185(5):2790–9. [DOI] [PubMed] [Google Scholar]
- 220.Guilliams M, Crozat K, Henri S, Tamoutounour S, Grenot P, Devilard E, de Bovis B, Alexopoulou L, Dalod M, Malissen B. Skin-draining lymph nodes contain dermis-derived CD103(−) dendritic cells that constitutively produce retinoic acid and induce Foxp3(+) regulatory T cells. Blood 2010;115(10):1958–68. [DOI] [PubMed] [Google Scholar]
- 221.Tanaka Y, Nagashima H, Bando K, Lu L, Ozaki A, Morita Y, Fukumoto S, Ishii N, Sugawara S. Oral CD103(−) CD11b(+) classical dendritic cells present sublingual antigen and induce Foxp3(+) regulatory T cells in draining lymph nodes. Mucosal Immunol 2017;10(1):79–90. [DOI] [PubMed] [Google Scholar]
- 222.Cerovic V, Houston SA, Scott CL, Aumeunier A, Yrlid U, Mowat AM, Milling SW. Intestinal CD103(−) dendritic cells migrate in lymph and prime effector T cells. Mucosal Immunol 2013;6(1):104–13. [DOI] [PubMed] [Google Scholar]
- 223.Fenton TM, Kelly A, Shuttleworth EE, Smedley C, Atakilit A, Powrie F, Campbell S, Nishimura SL, Sheppard D, Levison S, Worthington JJ, Lehtinen MJ, Travis MA. Inflammatory cues enhance TGFbeta activation by distinct subsets of human intestinal dendritic cells via integrin alphavbeta8. Mucosal Immunol 2017;10(3):624–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity 2008;29(3):352–61. [DOI] [PubMed] [Google Scholar]
- 225.Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annual Rev Immunol 2011;29(1):163–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Brewitz A, Eickhoff S, Dähling S, Quast T, Bedoui S, Kroczek RA, Kurts C, Garbi N, Barchet W, Iannacone M, Klauschen F, Kolanus W, Kaisho T, Colonna M, Germain RN, Kastenmüller W. CD8+ T cells orchestrate pDC-XCR1+ dendritic cell spatial and functional cooperativity to optimize priming. Immunity 2017;46(2):205–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lombardi V, Speak AO, Kerzerho J, Szely N, Akbari O. CD8α+β− and CD8α+β+ plasmacytoid dendritic cells induce Foxp3+ regulatory T cells and prevent the induction of airway hyper-reactivity. Mucosal Immunol 2012;5:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol 2008;181(8):5396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M, Bicciato S, Mazza EMC, Boon L, Grassi F, Fioretti MC, Fallarino F, Puccetti P, Grohmann U. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol 2011;12:870–8. [DOI] [PubMed] [Google Scholar]
- 230.Fallarino F, Asselin-Paturel C, Vacca C, Bianchi R, Gizzi S, Fioretti MC, Trinchieri G, Grohmann U, Puccetti P. Murine plasmacytoid dendritic cells initiate the immunosuppressive pathway of tryptophan catabolism in response to CD200 receptor engagement. J Immunol 2004;173(6):3748–54. [DOI] [PubMed] [Google Scholar]
- 231.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest 2004;114(2):280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Boasso A, Herbeuval J–P, Hardy AW, Anderson SA, Dolan MJ, Fuchs D, Shearer GM. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood 2007;109(8):3351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Diana J, Brezar V, Beaudoin L, Dalod M, Mellor A, Tafuri A, von Herrath M, Boitard C, Mallone R, Lehuen A. Viral infection prevents diabetes by inducing regulatory T cells through NKT-cell-plasmacytoid dendritic cell interplay. J Exper Med 2011;208(4):729–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Matta BM, Raimondi G, Rosborough BR, Sumpter TL, Thomson AW. IL-27 production and STAT3-dependent upregulation of B7-H1 mediate immune regulatory functions of liver plasmacytoid dendritic cells. J Immunol 2012;188(11):5227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med 2002;195(6):695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, Workman CJ, Vignali DA. Stability and function of regulatory Tcells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 2013;501(7466):252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Overacre-Delgoffe AE, Chikina M, Dadey RE, Yano H, Brunazzi EA, Shayan G, Horne W, Moskovitz JM, Kolls JK, Sander C, Shuai Y, Normolle DP, Kirkwood JM, Ferris RL, Delgoff GM, Bruno TC, Workman CJ, Vignali DAA. Interferon-γ drives Treg fragility to promote anti-tumor immunity. Cell 2017;169(6):1130–41.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kumanogoh A, Marukawa S, Suzuki K, Takegahara N, Watanabe C, Ch’ng E, Ishida I, Fujimura H, Sakoda S, Yoshida K, Kikutani H. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature 2002;419:629–33. [DOI] [PubMed] [Google Scholar]
- 239.Kumanogoh A, Kikutani H. The CD100–CD72 interaction: a novel mechanism of immune regulation. Trends Immunol 2001;22(12):670–6. [DOI] [PubMed] [Google Scholar]


