Abstract
Purpose of Review:
The prevalence of obesity continues to rise, fueling a global public health crisis characterized by dramatic increases in type 2 diabetes, cardiovascular disease, and many cancers. In the United States, several minority populations, who bear much of the obesity burden (47% in African Americans and Hispanic/Latinos, compared to 38% in European descent groups), are particularly at risk of downstream chronic disease. Compounding these disparities, most genome-wide association studies (GWAS) – including those of obesity – have largely been conducted in populations of European or East Asian ancestry. In fact, analysis of the GWAS catalog found that while the proportion of participants of non-European or non-Asian descent had risen from 4% in 2009 to 19% in 2016, African ancestry participants are still just 3% of GWAS, Hispanic/Latinos are <0.5%, and other ancestries are <0.3% or not represented at all. This review summarizes recent developments in obesity genomics in US minority populations, with the goal of reducing obesity health disparities and improving public health programs and access to precision medicine.
Recent Findings:
GWAS of populations with the highest burden of obesity are essential to narrow candidate variants for functional follow-up, identify additional ancestry-specific variants that contribute to individual genetic susceptibility, and to advance both public health and precision medicine approaches to obesity.
Summary:
Given the global public health burden posed by obesity and downstream chronic conditions which disproportionately affect non-European populations, GWAS of obesity-related traits in diverse populations is essential to 1) locate causal variants in GWAS identified regions through fine-mapping, 2) identify variants which influence obesity across ancestries through generalization, and 3) discover novel ancestry-specific variants which may be low frequency in European populations but common in other groups. Recent efforts to expand obesity genomics studies to understudied and underserved populations, including AAAGC, PAGE, and HISLA, are working to reduce obesity health disparities, improve public health, and bring the promise of precision medicine to all.
Keywords: Obesity, GWAS, health disparities, precision medicine
Introduction
In 2016, an estimated 98 million American adults were obese,[1, 2] fueling a public health crisis with an enormous financial burden, doubling every decade to ~$900 billion by 2030.[3] Obesity has the potential to reverse gains in health and life expectancy achieved over the past century, [4, 5] with detrimental effects nearly doubling the gains attributed to public health interventions to reduce cigarette smoking.[6•] And while smoking prevalence has declined in recent years, obesity prevalence continues to climb.[2, 7] The Healthy People 2020 guidelines call for a 10% reduction in obesity in all populations to hit their target of 30.5% or less obese adults by 2020. The associated morbidity, mortality, and disability of associated chronic diseases, including type 2 diabetes, cardiovascular disease (CVD), and heart failure, among others, [4] lends urgency to obesity prevention and control research. Particularly at risk are minority populations, such as African Americans (AA) and Hispanic/Latinos (HA), who face inequitable exposures to psychosocial and behavioral risk factors for obesity[8, 9] and have an elevated obesity prevalence (47%) and associated chronic diseases compared to European descent populations (EA: 38% obesity prevalence).[10–18] There is also a difference in adult obesity prevalence by sex, with 41% of women being obese compared to 38% of men. This trend is more striking among AA, where 55% of women are obese compared to 37% of men. Unfortunately, to date the majority of genetic studies of obesity (and genetics studies in general) have been conducted in populations of European and East Asian descent. The consequences of this are two-fold: 1) It limits our potential to identify variants that may be specific to population groups, 2) It excludes those groups with the highest burden of disease from the benefits of genetic discovery and personalized medicine, and 3) It limits our potential for fine mapping to identify functional variants and causal genes, given that African descent populations have the greatest genetic diversity - and thus shorter haplotypes (genetic variants that tend to be inherited together) – providing a much narrower window of potential functional variants around GWAS tagSNPs than EA.
Genetics of Obesity
To date, genome-wide association studies (GWAS) have identified 551 genetic loci associated with measures of obesity.[19-24••] However, these loci have been identified in primarily European (EA) descent populations. In fact, as of 2016 over 80% of participants included in the EBI/NHGRI GWAS catalog as a whole are of European descent.[25•, 26•] There has been some progress in including Asian populations in GWAS studies (from 3% in 2009 to 14% in 2016, though 64% of these are East Asian) [27•]. In the past, Asian populations have been excluded from genetic studies of obesity, and obesity studies more generally, given their low obesity prevalence (just 12.7% in 2016) compared to other populations. [1] However, recent research has demonstrated that Asian descent groups have increased risk of cardiometabolic consequences associated with obesity (hyperlipidemia, T2D, CVD) at much lower BMI than other populations, and the World Health Organization and other groups now recommend lower BMI thresholds in Asian populations to capture this risk. [28] The overwhelming focus on EA in genetic studies of obesity makes it nearly impossible to identify variants that are population specific. It also presents difficulties when trying to replicate (find statistically significant genetic associations in other samples) or generalize genetic associations (i.e., identify variants with the same magnitude of association and direction of effect) to other populations, as the differing degrees of linkage disequilibrium (LD) across ancestries mean that GWAS variants identified in EA may not tag the same genetic signal in other populations. Thus, we must conduct genomic studies of obesity in diverse populations using appropriate population specific phenotype definitions of obesity, to measure the breadth of genetic variation, to aid in identifying the functional variant in a locus through fine-mapping (narrowing the window of potential candidate loci at an association signal by leveraging shorter LD blocks in non-European samples), and to identify any ancestry-specific variants to more precisely predict disease risk for specific populations and tailor public health or precision medicine interventions.
The purpose of this review is to describe, with specific examples, the current state of obesity genomics in underrepresented and underserved US minority populations; therefore, our review will focus on African and Hispanic/Latino descent populations as they display the highest prevalence of obesity. Other recent reviews describe strategies for increasing diversity in research more broadly,[25•, 27•, 29] and we recommend these important perspectives to interested readers.
Identifying novel obesity variants in ancestrally diverse populations.
As noted, the GWAS literature continues to be dominated by discoveries made in populations of European descent. Until this is remedied, we will be unable to identify associations that are uncommon in European ancestry populations but common in others, especially because of evolutionary history shaping the allele frequencies and LD patterns of global populations (see GWAS in Greenlanders,[30] and Samoans [31] as prominent examples). In fact, the gap between the amount of phenotypic variance explained by top GWAS findings and that estimated to be attributable to genetics (i.e., missing heritability) continues to be wide for most complex traits, providing a strong scientific impetus for discovery studies for complex traits across global populations. [32, 33]
There are now many examples from the literature of disease susceptibility variants that are common in an ancestrally diverse population, but rare in European descent populations. [34] For example, AA individuals are twice as likely to develop end-stage renal disease when compared to EA individuals. This observation led to the discovery of genetic variants that are common in individuals of African but not European ancestry and that contribute to explaining this heightened risk of disease.[35] Interestingly, these variants are also associated with higher rates and faster progression of kidney disease in other admixed groups with African ancestry, including HA [36] The reason the association was found in AAs is that the relevant variants had reached appreciable frequency in that ancestry, thus yielding higher power HA to map these genetic variants for a given cohort sample size. Since HA are a highly diverse group in terms of genomic ancestry, [37•] it is not surprising that the association will replicate in some (e.g., Dominicans who have a substantial proportion of African ancestry), but not others (e.g., Mexicans, who have far less).[38] These examples highlight the necessity of discovery studies in ancestrally diverse populations as well as in admixed populations for the identification of novel susceptibility variants that may be rare or absent in previous large GWAS in European descent populations. Although there is a paucity of genomic studies in ancestrally diverse populations, several notable large genomic consortia with a focus on diverse populations have been implemented, two of which are described below. These are not meant to be a comprehensive capture of the literature on ancestrally diverse genomic studies of obesity, but rather as examples that showcase the breadth of discovery that is possible in such studies.
African Ancestry Anthropometry Genetics Consortium (AAAGC)
In 2014, we assembled a consortium of African descent population studies, the African Ancestry Anthropometry Genetics Consortium (AAAGC), to identify African specific alleles for obesity-related traits and to fine-map loci first identified in EA in African ancestry participants. We conducted analyses in a three-stage design (Stage 1: discovery in AA, Stage 2: replication in AA, and Stage 3: meta-analysis of AA+EA) to evaluate associations with overall obesity (measured as BMI) and central obesity (measured as waist-hip ratio adjusted for BMI, WHRadjBMI) stratified by sex and in sexes combined.[23] The discovery stage of AAAGC for BMI included 17 GWAS of up to 42,752 AA individuals. WHRadjBMI discovery GWAS included up to 20,384 AA individuals. Variants with P < 1×10−4 in Stage 1 were carried forward for replication in Stage 2 in additional AA individuals from AAAGC (N = 10,143 for BMI, N = 2,711 for WHRadjBMI), as well as meta-analysis in Stage 3 with EA from the GIANT consortium (322,154 for BMI, 210,086 for WHRadjBMI).[19, 20]
Genotyping, imputation and quality control details from each study, measurement of obesity phenotypes, and data analysis are provided in the original publication.[23] Fine mapping was conducted among the locus-wide (defined by +/−1 cM) significant established loci and novel loci to localize putative causal variants. We constructed credible sets containing variants that jointly account for 99% posterior probability of driving the association in a locus using the corresponding sex-combined or sex-stratified meta-analysis results from AA, EA, and combined ancestry. In 58% of fine-mapped loci, we observed <20 variants in a credible set when AA results were included, which are more tractable for functional follow up (see example in Figure 1, at MAP2K5). Importantly, rs16951275 was within the credible set, and is a cis-eQTL variant regulating nearby gene expression of MAP2K5 in several tissues, including subcutaneous and visceral adipose tissue.
Figure 1:
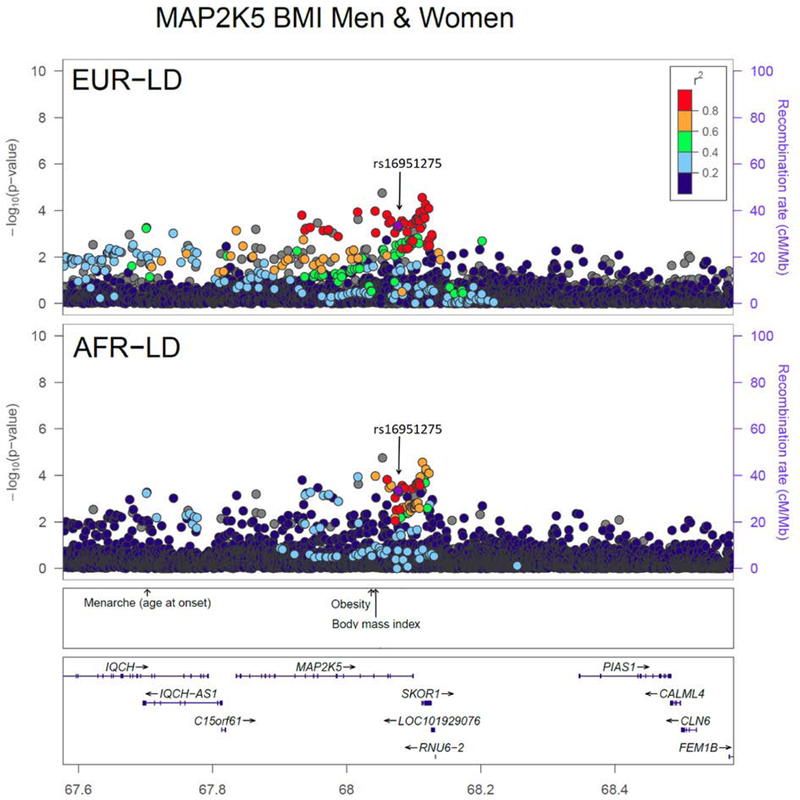
Locuszoom plots of the MAP2K5 locus using discovery results for established loci that reached genome-wide significance for BMI in men and women combined in the AAAGC [23•]. Plots use EUR and AFR LD from the 1000 Genomes phase 1 reference panel. In each plot, the most significant variant in AAAGC within a 1Mb regional locus is highlighted. P-values for all variants including the most significant variant are based on the African ancestry discovery phase only. (Reproduced from Ng MCY, Graff M, Lu Y, et al (2017) Discovery and fine-mapping of adiposity loci using high density imputation of genome-wide association studies in individuals of African ancestry: African Ancestry Anthropometry Genetics Consortium. PLoS Genet 13:e1006719. This work is made available under the Creative Commons CC0 public domain dedication) [23••].
In summary, we performed the largest meta-analyses of obesity in African ancestry populations to date.[23, 24] We identified several novel loci for BMI and WHR, and one highly suggestive locus influencing BMI. In addition, we were able to refine the window of association of some of the previously established loci, which may eventually help identify the biologically functional variant(s). Taken together, these findings demonstrate the importance of conducting genetic studies in diverse populations.
Population Architecture using Genomics and Epidemiology (PAGE)
The PAGE Study (www.pagestudy.org ) was designed to investigate the impact of ancestral diversity on genetic associations with disease. PAGE (2008–2013, NHGRI, NIMHD) originally performed large scale genotyping using the Illumina MetaboChip from over 54,000 participants of African American, Hispanic/Latino, East Asian, Native Hawaiian, and Native American descent. The MetaboChip array is a custom genotyping array designed for replication and fine-mapping of cardiometabolic traits, including 41 densely genotyped obesity-associated regions previously reported in European populations.[39] With the MetaboChip, PAGE sought to identify novel signals previously undetected in any ancestry, characterize population specific signals, identify independent signals within known cardiometabolic loci (including those for obesity), and fine-map signals previously identified in Europeans. Gong et al (2013) investigated 21 BMI loci first identified in EA, and found that eight were significant in a sample of ~30,000 AA.[40] Of these eight loci, three (SEC16B, ETV5, GNPDA2) had the same lead SNP as the EA GWAS, while the other five had different lead SNPs in AA. For MC4R and TFAB2B, the previously reported lead GWAS SNPs were not associated with BMI in AA at all, suggesting that for some loci the variant identified in EA is not tagging the same signal (or the underlying functional variant) in AA. For FTO, the lead SNP in AA (rs62048402) has a r2 of just 0.13 with the first reported EA variant (rs9939609) in this population, due the differences in linkage disequilibrium (LD) between the two groups. These differences in LD have important implications for genetic studies. While the long stretches of LD in European populations assist in identification of genomic regions associated with a particular disease, they rarely pinpoint the functional variant, or even the affected gene, making genetic studies of complex diseases in diverse populations essential.
Recently, Fernandez-Rhodes et al (2017) conducted a trans-ethnic PAGE MetaboChip analysis in >101,000 EA, AA, HA, Asian American (AS), and Native Hawaiian (NH) adults of common BMI variants previously identified in EA (164) or other populations (6), which had a MAF ≥ 0.1 in at least one non-EA PAGE population.[41•] This study expanded on Gong et al (2013), including additional ancestries to identify known BMI variants which generalize (are significant in non-EA at P<0.05 and with the same direction of effect) across populations, as well as fine-map 36 genomic regions associated with BMI. They confirmed 42 significant index GWAS variants in at least one ancestry or the combined trans-ethnic PAGE sample (18 index SNPs). In their fine-mapping analysis of 36 densely genotyped BMI loci in ~36,000 African Americans, they generalized 14, including 6 which had not been previously associated with BMI in AA (ATP2A1, COBLL1, MAP2K5, POC5, SLC22A3, TCF7L2). Variants in COBLL1 have been associated with central obesity,[20] and TCF7L2 is a known type 2 diabetes gene.[42] Similar fine-mapping in ~26,000 HL generalized 13 BMI loci (8 of which also generalized in AA, 5 of them generalizing in HA only), while fine-mapping in ~23,000 AS generalized 8 BMI loci that also generalized to AA and/or HA. In the full trans-ethnic sample of EA, AA, HA, and AS, 29 of 36 BMI MetaboChip loci were generalized trans-ethnically, suggesting that many of the currently known common obesity variants identified in EA have similar effects on obesity across these ancestries.
PAGE MetaboChip data have also been used for an updated multi-ethnic GWAS of BMI [43], resulting in replication of 27 of 33 known BMI loci present on the MetaboChip (21 added during chip design, and 12 subsequently associated with BMI in other GWAS). This multi-ethnic GWAS also discovered two new BMI variants, rs2820436 near LYPLAL1, a variant previously associated with decreased WHRadjBMI when adjusting for physical activity[44], and rs10930502 in METAP1D. rs2820436 tags an enhancer variant in an eQTL for LYPLAL1 (lysophospholipase-like 1), also associated with lipid traits, adiposity, and T2D. In 1000 Genomes Phase 1 African reference populations, rs10930502 is in moderate LD (r2=0.48, D’=0.85), with rs34636594, located in an adipose tissue-specific transcription factor-binding region. Despite both of these variants being common in EA (risk allele frequency [RAF]=0.68 and 0.31, respectively), neither reached genome-wide significance in prior large EA GWAS. [19] This could be due to a stronger effect on BMI in non-European populations, which had the largest effect sizes in this study, or because these variants are better tagSNPs of the true causal variants in non-EA populations, which tend to have shorter LD blocks.
More recent studies in PAGE (2013–2018, NHGRI, NIMHD) have further examined how variation in allele frequency and LD across ancestry may explain differences in disease risk. To address poor coverage of non-European genetic variation in available genotyping arrays, PAGE collaborated with the Consortium on Asthma among African-ancestry Populations in the Americas (CAAPA), Illumina, and other academic partners to design the Multi-Ethnic Genotyping Array (MEGA). Using the 1000 Genomes Project Phase 3 cosmopolitan reference panel of non-European ancestries, we designed a platform with comparable imputation accuracy across all continental populations for a range of allele frequencies. A commercial version of the MEGA array is now available. [45] For the MEGA analysis, PAGE collated and harmonized data from a subset of the PAGE MetaboChip samples, including: The Coronary Artery Risk Development in Young Adults Study (CARDIA), The Hispanic Community Health Study/Study of Latinos (HCHS/SOL), The Multiethnic Cohort Study (MEC), and the Women’s Health Initiative (WHI), and added data from the Mount Sinai BioMe BioBank (BioMe)(Table 1). PAGE Investigators also developed new statistical methods to analyze genetic data from admixed populations, as well as handle cryptic relatedness and complex sampling designs. [46–48] For obesity phenotypes, in our combined African, Hispanic, Asian and Native Hawaiian sample, we identified two novel signals on chromosome 5, one for BMI in sexes combined (Beta (SE) = −0.10 (0.02), P=8.2×10−9), and one for waist-hip ratio adjusted for BMI (WHRadjBMI) in women only Beta (SE) = 0.065(0.01), P=1.0×10−9). The BMI variant (rs76493495) is intronic to SLIT3, with a combined effect allele frequency (EAF) of 0.04 (Figure 2). This variant was most common in AA (EAF=0.08) and HL (EAF=0.01), rare in Native Hawaiians (EAF=0.001), and absent in both Asians in PAGE and the 1000 Genomes European reference population. SLIT3 codes for a slit guidance ligand involved in cell migration and is most highly expressed in human subcutaneous adipose and arterial tissues.
Table 1.
Studies included in AAAGC, PAGE, and HISLA Consortia
| Obesity Genetic Studies in Diverse Populations | AAAGC | PAGE Metabochip | PAGE MEGA | HISLA |
|---|---|---|---|---|
| 1982 Pelotas (Brazil) Birth Cohort Study (1982 PELOTAS) | ||||
| African American Breast Cancer Consortium (AABC) | ||||
| African American Prostate Cancer Consortium (AAPC) | ||||
| Atherosclerosis Risk in Communities Study (ARIC) | ||||
| Baependi Heart Study (BHS) | ||||
| Bone Mineral Density in Childhood Study (BMDCS) | ||||
| Cancer de Mama Study (CAMA) | ||||
| Cardiovascular Health Study (CHS) | ||||
| Children’s Hospital of Philadelphia’s Center for Applied Genomics (CHOP/CAG) | ||||
| Cleveland Family Study (CFS) | ||||
| Consortium for the Analysis of the Diversity and Evolution of Latin America (CANDELA) | ||||
| Coronary Artery Risk Development in Young Adults (CARDIA) | ||||
| Family Heart Study (FamHS) | ||||
| Family Investigation of Nepropathy and Diabetes (FIND) | ||||
| Genetic Relationships between NIDDM and atherosclerosis (NIDDM-Athero) | ||||
| Genetic Study of Atherosclerosis Risk (GeneSTAR) | ||||
| Genetics of Hypertension in Blacks (Maywood) | ||||
| Genetics of Hypertension in Blacks (Nigeria) | ||||
| Genetics of Latino Diabetic Retinopathy Study (GOLDR) | ||||
| Genome-Wide Association Study of Breast Cancer in the African Diaspora (ROOT) | ||||
| Health and Retirement Study (HRS) | ||||
| Health, Aging, and Body Composition Study (Health ABC) | ||||
| Healthy Aging in Neighborhoods of Diversity across the Life Span Study (HANDLS) | ||||
| Hispanic Community Health Study/ Study of Latinos (HCHS/SOL) | ||||
| Howard University Family Study (HUFS) | ||||
| Hypertension Genetic Epidemiology Network (HyperGEN) | ||||
| Insulin Resistance Atherosclerosis Family Study (IRASFS) | ||||
| Insulin Resistance Atherosclerosis Study (IRAS) | ||||
| Jackson Heart Study (JHS) | ||||
| Los Angeles Latino Eye Study (LALES) | ||||
| Mapping the genes for atherosclerosis and insulin resistance (MACAD) | ||||
| Mapping the genes for hypertension, insulin resistance, and salt sensitivity (HTN-IR) | ||||
| Mexican Hypertriglyceride Study (MHS) | ||||
| Mexico City Study (MCS) | ||||
| Mount Sinai BioMe BioBank (BioMe) | ||||
| Multi-Center Genetic Study of Hypertension (GenNet) | ||||
| Multi-Ethnic Study of Atherosclerosis (MESA) | ||||
| Multiethnic Cohort Study – Slim Initiative in Genomic Medicine for the Americas Type 2 Diabetes Consortium (MEC-SIGMA) | ||||
| Multiethnic Cohort Study (MEC) | ||||
| Pharmacogenetics of hypertriglyceridemia in Hispanics (Hyper-TG) | ||||
| San Antonio Mexican American Family Study (SAMAFS) | ||||
| San Francisco Bay Area Breast Cancer Study (SFBCS) | ||||
| Sea Islands Genetics Network – Reasons for Geographic and Racial Differences in Stroke (SIGNET-REGARDS) | ||||
| Starr County Health Studies (STARR COUNTY) | ||||
| Strong Heart Study (SHS) | ||||
| Study of Asthma Phenotypes and Pharmacogenomic Interactions by Race-ethnicity (SAPPHIRE) | ||||
| Taiwan Metabochip Study for Cardiovascular Disease (TaiChi) | ||||
| Vanderbilt School of Medicine Biobank (BioVu) | ||||
| Wake Forest School of Medicine Study (WFSM) | ||||
| Women’s Health Initiative (WHI) |
Blue = African/ African Americans, Green = Hispanic/Latino, Orange = Multiethnic
Figure 2:
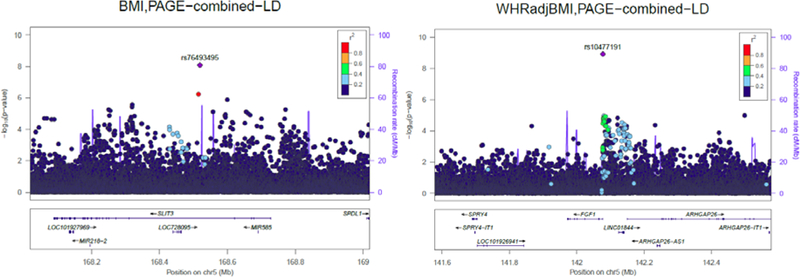
Locus zoom plots of chromosome 5 for body mass index (sexes combined) and WHRadjBMI (women only) in the pooled PAGE II study.[57•] Left panel: Lead BMI SNP rs76493495 and 1 MB surrounding region, body mass index was adjusted for age, sex, PC1–10, study, study center, and ancestry. Right panel: Lead WHRadjBMI SNP rs10477191 and 1 MB surrounding region, waist-hip ratio was first adjusted for BMI, and then the residuals were adjusted for age, PC-10, study, study center, and ancestry. (Reproduced from Wojcik G, Graff M, Nishimura KK, et al (2017) Genetic diversity turns a new PAGE in our understanding of complex traits. bioRxiv doi:10.1101/188094. This work is made available under the creativecommons.org/licenses/by-nd/4.0) [57••].
The WHRadjBMI variant (rs10477191) significant in women only is 97kb upstream of FGF1, with an ancestry combined EAF in PAGE of 0.30. This variant is most common in AA (EAF=0.53) and AS (EAF=0.26), and also more common in Native Hawaiians (EAF=0.17) and HL (EAF=0.15) than in the 1000 Genomes European reference panel (EAF=0.07). FGF1 codes for Fibroblast Growth Factor 1, a cell cycle regulator that promotes differentiation of human preadipocytes into mature adipocytes through regulation by PPARγ.[49] Variants in LD (r2>0.4) with rs10477191 have been previously associated with plasma phospholipid trans fatty acid levels.[50] FGF-1 knockout mice fed a high fat diet have upregulated FGF-1 expression in white adipose tissue,[51] and both mice and humans administered FGF-1 show improved insulin sensitivity.[52]
HISpanic/Latino Anthropometry (HISLA) Consortium
To address the lack of genomic studies of obesity in Hispanic/Latino populations, we have also formed the HISpanic/Latino Anthropometry (HISLA) Consortium, including over 23 individual studies as well as the Slim Initiative in Genomic Medicine for the Americas (SIGMA) Type 2 Diabetes Consortium[53] and the Consortium for the Analysis of the Diversity and Evolution of Latin America. [54] Together, HISLA includes over 56,000 Native American, Brazilian, or Hispanic/Latino participants living in the United States, Chile, Colombia, Mexico or Peru. These samples have been imputed to the 1000 Genomes Phase 1 cosmopolitan reference panel to search for novel loci associated with overall and central obesity, as well as fine-map established obesity loci. Preliminary analyses have been completed, and manuscripts detailing these results are in preparation.
Future directions
One of the long-term goals of obesity genomics is to make accurate health predictions for the development of obesity, weight loss, and weight gain, based on genetic information. Many strategies are being developed to harness information gained from GWAS, particularly identification of thousands of SNPs that can be used in aggregate to predict individual risk of (or protection from) disease, in the form of Polygenic Risk Scores (PRS). However, recent studies have highlighted the pitfalls of relying on the results from genetic databases of predominantly European populations to generate polygenic risk scores (PRS) to in other ancestries.[55, 56] The results indicate that PRS generated from European GWAS are unpredictably biased when applied to non-European populations, due to poor coverage of global genetic variation in early GWAS chips designed to capture variation in European populations, differences in linkage disequilibrium between ancestries, and genetic drift. These findings underscore the need to include diverse populations in primary genetic analyses as well as to develop risk-prediction methods that provide the flexibility needed to account for local genetic ancestry. As high-dimensional prediction scores begin to make their way into clinical practice [Framingham/Q-risk score], we are likely to see increasing health disparities when underlying data driving the scores are not well calibrated to most global populations.
Conclusion
As evidenced by our work with AAAGC, PAGE, and HISLA, trans-ethnic GWAS of common, complex diseases like obesity can identify additional, population specific signals in established loci. In fact, using the multi-ethnic PAGE MEGA excluding Europeans, we tested the hypothesis that effect size heterogeneity among populations may exist for many SNP associations in the largely European GWAS catalog. While we were able to replicate (P<5×10−8) a total of 574 lead SNPs in 261 distinct genomic regions across 26 traits, 132 lead SNPs (23.0%) showed significant evidence of effect heterogeneity by genetic ancestry (SNPxPC P<8.71×10−5). [57•] In addition, for 77% of the 261 regions that were replicated, the strongest signal was not the previously reported lead SNP from the GWAS Catalog but a different tag SNP. These results have important implications for precision medicine, as risk prediction models based on heterogeneous lead SNPs from the GWAS Catalog will likely have poor accuracy in non-European ancestries.
However, the number of non-European GWAS remains small, hampering replication efforts of population-specific signals, as consortia efforts aiming to achieve the largest possible sample sizes to maximize discovery may find few if any studies available for replication. The genetic diversity of many non-European groups also presents challenges, as traditional race/ethnic categorizations mask the underlying genetic diversity of these groups, such as Hispanic/Latinos, who, depending on their country of origin, may have a high proportion of African ancestry (if they are of Caribbean descent) or none at all (if they are from mainland Central or South America). While efforts such as PAGE MEGA, designed to capture global genetic variation, make discovery of population-specific susceptibility variants more likely, what’s needed are genetic studies across continental populations to replicate and confirm such findings.Given the increased burden of obesity disability, morbidity, and mortality in minority populations that have been underservered in clinical settings and underrepresented in research to date, our responsibility as researchers must be to address existing health disparities and prevent the deluge of big data for precision medicine from exacerbating them. Our work with the AAAGC, PAGE, and HISLA demonstrates that the samples and data are available, and coordination and harmonization are possible. To truly realize the potential of public health and precision medicine for all and to confront the global obesity epidemic with all the tools at our disposal, we must work together to truly engage with the populations who are most at risk and can most benefit from our findings.
Footnotes
Conflict of Interest
Kristin L. Young, Mariaelisa Graff, Lindsay Fernandez-Rhodes, and Kari E. North declare that they have no conflict of interest.
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
SpringerLink Header: Obesity (J McCaffery, Section Editor)
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Hales CM, Carroll MD, Fryar CD, Ogden CL (2017) Prevalence of obesity among adults and youth: United States, 2015–2016 NCHS Data Brief, no 288. Hyattsville, MD: National Center for Health Statistics. [PubMed] [Google Scholar]
- 2.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL (2018) Trends in obesity and severe obesity prevalence in US Youth and Adults by Sex and Age, 2007–2008 to 2015–2016. JAMA 319:1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zamosky L (2013) The obesity epidemic. While America swallows $147 billion in obesity-related healthcare costs, physicians called on to confront the crisis. Med Econ 90:14–17. [PubMed] [Google Scholar]
- 4.Global BMI Mortality Collaboration, Di Angelantonio E, Bhupathiraju S, et al. (2016) Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 388:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao B (2016) Future healthy life expectancy among older adults in the US: a forecast based on cohort smoking and obesity history. Popul Health Metr 14:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Preston SH, Vierboom YC, Stokes A (2018) The role of obesity in exceptionally slow US mortality improvement. PNAS 115:957–961. • Analysis of NHANES data describing the impact of increasing BMI on the rate of US mortality improvement.• Analysis of NHANES data describing the impact of increasing BMI on the rate of US mortality improvement.
- 7.Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM (2016) Current cigarette smoking among adults - United States, 2005–2015. MMWR Morb Mortal Wkly Rep 65:1205–1211. [DOI] [PubMed] [Google Scholar]
- 8.McCurley JL, Penedo F, Roesch SC, et al. (2017) Psychosocial Factors in the Relationship between Socioeconomic status and cardiometabolic risk: the HCHS/SOL sociocultural ancillary study. Ann Behav Med 51:477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stepanikova I, Baker EH, Simoni ZR, Zhu A, Rutland SB, Sims M, Wilkinson LL (2017) The role of perceived discrimination in obesity among African Americans. Amer J Prev Med 52:S77–S85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Lancet Neurology (2013) Disparities in stroke: not just black and white. The Lancet Neurology 12:623. [DOI] [PubMed] [Google Scholar]
- 11.Sturtz LA, Melley J, Mamula K, Shriver CD, Ellsworth RE (2014) Outcome disparities in African American women with triple negative breast cancer: a comparison of epidemiological and molecular factors between African American and Caucasian women with triple negative breast cancer. BMC Cancer 14:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grubbs SS, Polite BN, Carney J, Bowser W, Rogers J, Katurakes N, Hess P, Paskett ED (2013) Eliminating racial disparities in colorectal cancer in the real world: it took a village. J Clin Oncol 31:1928–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin DN, Starks AM, Ambs S (2013) Biological determinants of health disparities in prostate cancer. Curr Opin Oncol 25:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma A, Colvin-Adams M, Yancy CW (2014) Heart failure in African Americans: Disparities can be overcome. Cleve Clin J Med 81:301–311. [DOI] [PubMed] [Google Scholar]
- 15.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL (2016) Trends in obesity among adults in the United States, 2005 to 2014. JAMA 315:2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ortega FB, Lavie CJ, Blair SN (2016) Obesity and cardiovascular disease. Circ Res 118:1752–1770. [DOI] [PubMed] [Google Scholar]
- 17.Bastien M, Poirier P, Lemieux I, Després J-P (2014) Overview of epidemiology and contribution of obesity to cardiovascular disease. Progress in Cardiovascular Diseases 56:369–381. [DOI] [PubMed] [Google Scholar]
- 18.Kallwitz ER, Daviglus ML, Allison MA, et al. (2015) Prevalence of suspected nonalcoholic fatty liver disease in Hispanic/Latino individuals differs by heritage. Clin Gastroenterol Hepatol 13:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Locke AE, Kahali B, Berndt SI, et al. (2015) Genetic studies of body mass index yield new insights for obesity biology. 518:197–206.•• Most recent GIANT BMI GWAS of >339,000 individuals, identifying 97 BMI loci.
- 20.Shungin D, Winkler TW, Croteau-Chonka DC, et al. (2015) New genetic loci link adipose and insulin biology to body fat distribution. 518:187–196.•• Most recent GIANT central adiposity GWAS of >220,000 individuals, identifying 49 central adiposity loci.
- 21.Turcot V, Turcot V, Lu Y, et al. (2018) Protein-altering variants associated with body mass index implicate pathways that control energy intake and expenditure in obesity. Nat Genet 50:26–41.•• GIANT BMI GWAS of low frequency and rare (MAF <5%) coding variants in >700,000 individuals, identifying 14 coding variants associated with BMI, several with effect sizes ~10x larger than that of common variants.
- 22.Willer CJ, Schmidt EM, Sengupta S, et al. (2013) Discovery and refinement of loci associated with lipid levels. Nat Genet 45:1274–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng MCY, Graff M, Lu Y, et al. (2017) Discovery and fine-mapping of adiposity loci using high density imputation of genome-wide association studies in individuals of African ancestry: African Ancestry Anthropometry Genetics Consortium. PLoS Genet 13:e1006719.•• GWAS of and fine-mapping obesity traits in African Americans, identifying three novel and replicating seven established loci for BMI, and three novel and replicating one established locus for WHRadjBMI, as well as reducing credible SNP sets in established loci.
- 24.Monda KL, Chen GK, Taylor KC, et al. (2013) A meta-analysis identifies new loci associated with body mass index in individuals of African ancestry. Nat Genet 45:690–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popejoy AB, Fullerton SM (2016) Genomics is failing on diversity. Nature 538:161–164.• Commentary on the continuing lack of diversity in genomic studies.
- 26.Bentley AR, Callier S, Rotimi CN (2017) Diversity and inclusion in genomic research: Why the uneven progress? J Community Genet 8:255–266.• Commentary on the factors contributing to limited diversity in genomic studies.
- 27.Morales J, Welter D, Bowler EH, et al. (2018) A standardized framework for representation of ancestry data in genomics studies, with application to the NHGRI-EBI GWAS Catalog. Genome Biol 19:1–10.• Framework for consistent reporting of ancestry in genomic studies.
- 28.Jih J, Mukherjea A, Vittinghoff E, Nguyen TT, Tsoh JY, Fukuoka Y, Bender MS, Tseng W, Kanaya AM (2014) Using appropriate body mass index cut points for overweight and obesity among Asian Americans. Prev Med 65:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rotimi CN, Bentley AR, Doumatey AP, Chen G, Shriner D, Adeyemo A (2017) The genomic landscape of African populations in health and disease. Hum Mol Genet 26:R225–R236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moltke I, Grarup N, Jørgensen ME, et al. (2014) A common Greenlandic TBC1D4 variant confers muscle insulin resistance and type 2 diabetes. Nature 512:190–193. [DOI] [PubMed] [Google Scholar]
- 31.Minster RL, Hawley NL, Su C-T, et al. (2016) A thrifty variant in CREBRF strongly influences body mass index in Samoans. Nat Genet 48:1049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.López-Cortegano E, Caballero A (2018) Inferring the nature of missing heritability in human traits. bioRxiv doi: 10.1101/373290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J, Bakshi A, Zhu Z, et al. (2015) Genetic variance estimation with imputed variants finds negligible missing heritability for human height and body mass index. Nat Genet 47:1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujikura K (2016) Global carrier rates of rare inherited disorders using population exome sequences. PLoS ONE 11:e0155552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer ND, Ng MCY, Hicks PJ, Mudgal P, Langefeld CD, Freedman BI, Bowden DW (2014) Evaluation of candidate nephropathy susceptibility genes in a genome-wide association study of African American diabetic kidney disease. PLoS ONE 9:e88273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parsa A, Kao WHL, Xie D, et al. (2013) APOL1 risk variants, race, and progression of chronic kidney disease. NEJM 369:2183–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conomos MP, Laurie CA, Stilp AM, et al. (2016) Genetic diversity and association studies in US Hispanic/Latino Populations: Applications in the Hispanic Community Health Study/Study of Latinos. AJHG 98:165–184.• Describes method to account for diversity beyond PCs in highly admixed samples
- 38.Behar DM, Rosset S, Tzur S, et al. (2010) African ancestry allelic variation at the MYH9 gene contributes to increased susceptibility to non-diabetic end-stage kidney disease in Hispanic Americans. Hum Mol Genet 19:1816–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voight BF, Kang HM, Ding J, et al. (2012) The MetaboChip, a custom genotyping array for genetic studies of metabolic, cardiovascular, and anthropometric traits. PLoS Genet 8:e1002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gong J, Schumacher F, Lim U, et al. (2013) Fine mapping and identification of BMI loci in African Americans. AJHG 93:661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandez-Rhodes L, Gong J, Haessler J, et al. (2017) Trans-ethnic fine-mapping of genetic loci for body mass index in the diverse ancestral populations of the Population Architecture using Genomics and Epidemiology (PAGE) Study reveals evidence for multiple signals at established loci. Hum Genet 136:771–800.• Trans-ethnic fine-mapping MetaboChip study of 36 BMI loci which identifies multiple independent signals at nine loci, and novel independent signals at 7 loci.
- 42.Fuchsberger C, Flannick J, Teslovich TM, et al. (2016) The genetic architecture of type 2 diabetes. Nature 536:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gong J, Nishimura KK, Fernández-Rhodes L, et al. (2017) Trans-ethnic analysis of MetaboChip data identifies two new loci associated with BMI. Int J Obes 4:579–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graff M, Scott RA, Justice AE, et al. (2017) Genome-wide physical activity interactions in adiposity - A meta-analysis of 200,452 adults. PLoS Genet 13:e1006528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bien SA, Wojcik GL, Zubair N, et al. (2016) Strategies for Enriching Variant Coverage in Candidate Disease Loci on a Multiethnic Genotyping Array. PLoS ONE 11:e0167758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin D-Y, Tao R, Kalsbeek WD, Zeng D, Gonzalez FII, Fernandez-Rhodes L, Graff M, Koch GG, North KE, Heiss G (2014) Genetic association analysis under complex survey sampling: The Hispanic Community Health Study/Study of Latinos. AJHG 95:675–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conomos MP, Reiner AP, Weir BS, Thornton TA (2016) Model-free estimation of recent genetic relatedness. AJHG 98:127–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conomos MP, Miller MB, Thornton TA (2015) Robust inference of population structure for ancestry prediction and correction of stratification in the presence of relatedness. Genet Epidemiol 39:276–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He J, Chen DL, Samocha-Bonet D, Gillinder KR, Barclay JL, Magor GW, Perkins AC, Greenfield JR, Yang G, Whitehead JP (2016) Fibroblast growth factor-1 (FGF-1) promotes adipogenesis by downregulation of carboxypeptidase A4 (CPA4) - a negative regulator of adipogenesis implicated in the modulation of local and systemic insulin sensitivity. Growth Factors 34:210–216. [DOI] [PubMed] [Google Scholar]
- 50.Mozaffarian D, Kabagambe EK, Johnson CO, et al. (2015) Genetic loci associated with circulating phospholipid trans fatty acids: A meta-analysis of genome-wide association studies from the CHARGE Consortium. AJCN 101:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jonker JW, Suh JM, Atkins AR, et al. (2012) A PPARγ-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature 485:391–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gasser E, Moutos CP, Downes M, Evans RM (2017) FGF1 - A new weapon to control type 2 diabetes mellitus. Nat Rev Endocrinol 13:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.The SIGMA Type 3 Diabetes Consortium (2014) Sequence variants in SLC16A11 are a common risk factor for type 2 diabetes in Mexico. Nature Comm 506:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruiz-Linares A, Adhikari K, Acuña-Alonzo V, et al. (2014) Admixture in Latin America: Geographic structure, phenotypic diversity and self-perception of ancestry based on 7,342 individuals. PLoS Genet 10:e1004572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium, Asian Genetic Epidemiology Network Type 2 Diabetes (AGEN-T2D) Consortium, South Asian Type 2 Diabetes (SAT2D) Consortium, et al. (2014) Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet 46:234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mahajan A, Wessel J, Willems SM, et al. (2018) Refining the accuracy of validated target identification through coding variant fine-mapping in type 2 diabetes. Nat Genet 50:559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wojcik G, Graff M, Nishimura KK, et al. (2017) Genetic diversity turns a new PAGE in our understanding of complex traits. bioRxiv doi: 10.1101/188094.•• First GWAS of 26 phenotypes (including BMI) using the MEGA array in diverse populations.


