Abstract
PURPOSE
Multimodal therapy is a well-established approach for the treatment of sinonasal undifferentiated carcinoma (SNUC); however, the optimal sequence of the various treatments modalities is yet to be determined. This study aimed to assess the role of induction chemotherapy (IC) in guiding definitive therapy in patients with SNUC.
METHODS
Ninety-five previously untreated patients diagnosed with SNUC and treated between 2001 and 2018 at The University of Texas MD Anderson Cancer Center were included in the analysis. Patients were treated with curative intent and received IC before definitive locoregional therapy. The primary end point was disease-specific survival (DSS). Secondary end points included overall and disease-free survival, disease recurrence, and organ preservation.
RESULTS
A total of 95 treatment-naïve patients were included in the analysis. For the entire cohort, the 5-years DSS probability was 59% (95% CI, 53% to 66%). In patients who had partial or complete response to IC, the 5-year DSS probabilities were 81% (95% CI, 69% to 88%) after treatment with definitive concurrent chemoradiotherapy (CRT) after IC and 54% (95% CI, 44% to 61%) after definitive surgery and postoperative radiotherapy or CRT after IC (log-rank P = .001). In patients who did not experience at least a partial response to IC, the 5-year DSS probabilities were 0% (95% CI, 0% to 4%) in patients who were treated with concurrent CRT after IC and 39% (95% CI, 30% to 46%) in patients who were treated with surgery plus radiotherapy or CRT (adjusted hazard ratio of 5.68 [95% CI, 2.89 to 9.36]).
CONCLUSION
In patients who achieve a favorable response to IC, definitive CRT results in improved survival compared with those who undergo definitive surgery. In patients who do not achieve a favorable response to IC, surgery when feasible seems to provide a better chance of disease control and improved survival.
INTRODUCTION
Sinonasal undifferentiated carcinoma (SNUC) is a rare, high-grade carcinoma that originates from the epithelium of the nasal cavity and paranasal sinuses, displaying large, undifferentiated cells with some immunohistochemical or ultrastructural evidence of neuroendocrine differentiation.1 SNUC demonstrates characteristic hypercellular proliferation, with a trabecular growth pattern lacking squamous and glandular differentiation. The tumor cells have medium- to large-sized pleomorphic and hyperchromatic nuclei surrounded by a small amount of eosinophilic cytoplasm, resulting in a high nuclear‐to‐cytoplasmic ratio. SNUC is typically positive for pancytokeratin and negative for synaptophysin, lymphoid markers, S100, and HMB45. Because of its aggressive biologic behavior, with a propensity to early invasion (40% to 50%) of vital structures such as the orbit, skull base, and brain, in addition to its high risk of distant metastasis (20% to 30%), SNUC poses a unique therapeutic challenge to clinicians.2-6 Management decisions are further complicated by the rarity of this entity and the resulting lack of consensus regarding the optimal treatment regimen.
Most published studies report the outcomes of patients treated surgically, with or without postoperative radiotherapy (RT); some case series delineate the outcomes of patients treated with preoperative RT followed by surgery, induction chemotherapy (IC) followed by definitive RT with concurrent chemotherapy7,8, or definitive RT with concurrent chemotherapy and reserving surgery to salvage therapy.5,9,10 Most of these studies suffer from a small number of patients and inconsistent treatment strategies. Although there is agreement that multimodal therapy is needed, the optimal sequence and combination of treatment modalities is not known. The purpose of this study was to assess the role of IC in the selection of patients with SNUC who are most likely to experience a response to concurrent chemoradiotherapy (CRT) and thus guide the selection of subsequent locoregional therapy.
METHODS
Eligibility Criteria and Patient Population
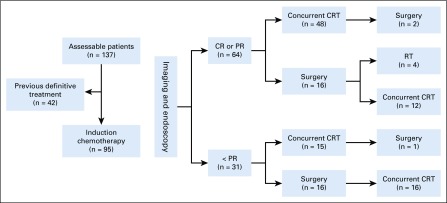
Institutional review board approval was obtained before the start of this study (protocol RCR04-0636). One hundred thirty-seven consecutive patients diagnosed with SNUC were treated between 2001 and 2018 at The University of Texas MD Anderson Cancer Center. The patient inclusion criteria were as follows: patients had to (1) be previously untreated and have histologically confirmed SNUC, (2) be candidates for treatment with curative intent, (3) have available detailed treatment and follow-up data. Patients with distant metastasis, recurrent disease after prior treatment, or a Karnofsky performance score of less than 60 were ineligible. Patient demographics (age, sex, diagnosis date, treatment date, smoking history, and alcohol intake), disease stage, tumor characteristics (epicenter, skull base, orbit, dura, and brain invasion), treatment modalities, pathologic data (neuroendocrine markers, mitotic rate, and surgical margins), and clinical outcomes were collected from patients’ records. Staging included flexible nasal endoscopy (FNE), computed tomography (CT), magnetic resonance imaging (MRI), and, when indicated, a positron emission tomography–computed tomography (PET-CT). All staging was completed according to the guidelines of the American Joint Committee on Cancer, 7th edition.11 All cases were presented at a multidisciplinary conference. After completion of IC, the tumor response was evaluated clinically and radiographically, and the patients were reintroduced in a multidisciplinary planning meeting. Treatment group schemes and responses to treatment are presented in Fig 1.
FIG 1.
Treatment group schemes and responses to treatment. Flowchart describing patient response to therapy and definitive treatment disposition. CR, complete response; CRT, chemoradiotherapy; PR, partial response; RT, radiotherapy.
Treatment Plan
IC.
For IC, patients received a platinum-based doublet chemotherapy regimen consisting of cisplatin (60 to 80 mg/m2 on day 1) and etoposide (100 to 120 mg/m2 [n = 74]) or docetaxel (75 mg/m2 [n = 21]) on days 1 to 3, administered intravenously every 21 days. For patients with renal insufficiency (creatinine clearance < 60 mL/min), significant hearing loss, or peripheral neuropathy (n = 20), carboplatin area under the curve 5 to 6 was used instead of cisplatin. Granulocyte colony-stimulating factor was given to the majority of patients who were older than 65 years of age. The median number of chemotherapy cycles administered in the IC setting was three (range, one to five).
Concurrent CRT.
Concurrent CRT began within 4 weeks after IC. Patients received two additional doses of platinum and etoposide chemotherapy concurrent with radiation therapy. The National Cancer Institute Common Toxicity Criteria (version 2) were used for the classification of adverse events.12 Radiation was administered once per day for 5 days each week. The dose administered to the gross disease and 1- to 2-cm margins was 66 to 70 Gy at 2 Gy/fraction, as defined by clinical examination, head and neck CT or MRI, and, when indicated, PET-CT. Tissue volumes at risk of harboring subclinical disease, including the bilateral neck, received 59 to 63 Gy at 1.7 to 1.8 Gy/fraction of intensity-modulated RT.
Surgery.
Surgical resection included open craniofacial resection, endoscopic endonasal resection, or endoscopically assisted craniofacial resection, depending on the extent and location of the disease. Neck dissections were performed in patients with clinically positive (N+) lymph node metastasis.
Tumor Assessment.
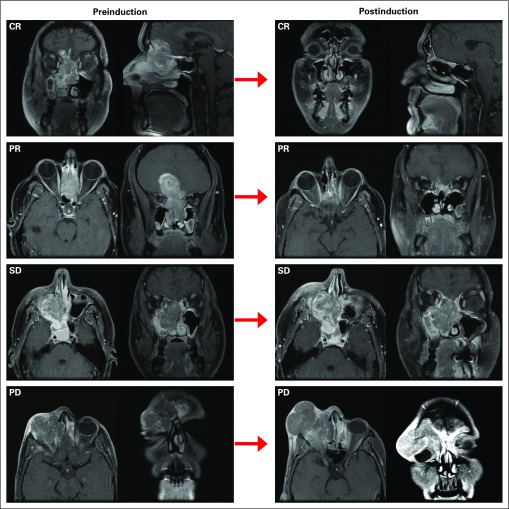
Both before treatment and at 3 weeks after IC, we performed bi-dimensional measurements of the primary tumor by FNE and cross-sectional imaging (CT or MRI) and in some cases a PET-CT (Fig 2), using the Response Evaluation Criteria in Solid Tumors Group criteria version 1.1.13
FIG 2.
Response to therapy and survival outcomes. T1 gadolinium–enhanced magnetic resonance images demonstrating complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD).
Tumor Assessment After Definitive Treatment.
Eight weeks after CRT, we performed an FNE and cross-sectional imaging (CT or MRI). Patients with residual resectable disease at the primary site underwent salvage surgical resection.
Statistical Analysis
The primary outcome measure was disease-specific survival (DSS), which was defined as the time that had elapsed between the start of treatment and SNUC-related death. Patients who were alive at last follow-up or who had died as a result of something other than SNUC were censored. Secondary outcomes were overall survival (OS), disease recurrence, and organ preservation, which was defined as conservation of intact brain tissue, orbital contents, and hard palate. The time period between the start of treatment and death or recurrence was used to calculate the OS and disease recurrence rates, respectively.
The Kaplan-Meier method and the log-rank test were used to test for differences in the survival functions between strata, as defined by clinical variables. To identify predictors of outcome, we performed a univariable analysis for each of the following variables: age, sex, T stage, N stage, site (ie, nasal, paranasal, or nasopharynx), perineural invasion, lymphovascular invasion, adjacent structure invasion (ie, skull base, orbit, dura, brain, bone, fat, muscle, and cartilage), and treatment group stratified by response to IC (ie, responders to IC treated with CRT, responders to IC treated with surgery followed by RT or CRT, nonresponders to IC treated with CRT, and nonresponders to IC treated with surgery followed by RT or CRT). We applied a process of several steps to develop a final model. The first step was to study the correlation between DSS and OS, and each covariable, via a univariable followed by a preliminary multivariable Cox proportional hazards regression model. Thus, covariates with a univariable P < .2 were included in a preliminary multivariable Cox proportional hazards regression model. Variables that remained statistically significant (P < .05) were included in the final multivariable model.
All statistical testing was two tailed. Alpha was set at 0.05 for significance. All statistical testing was completed using SAS JMP Pro software version 12.1.0 (SAS Institute, Cary, NC) and the R language environment for statistical computing version 3.1.3 (open source).
RESULTS
Patients
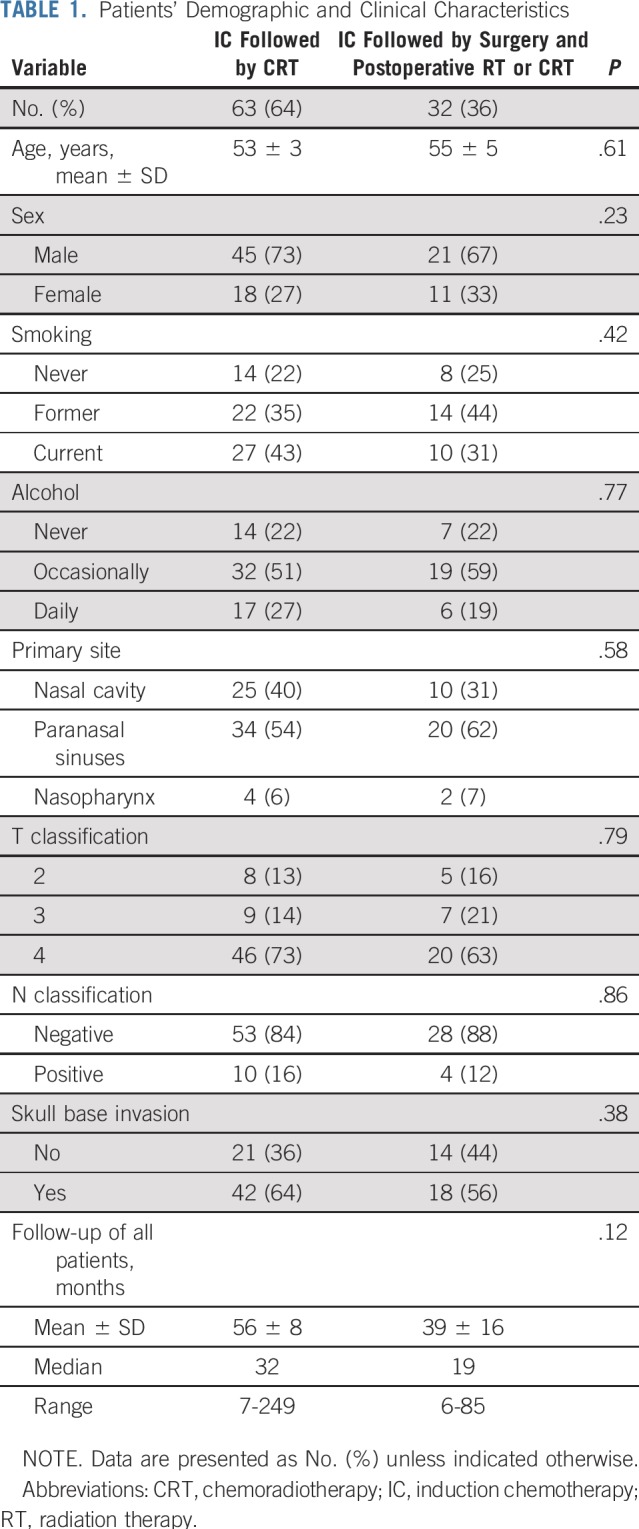
One hundred thirty-seven patients diagnosed with SNUC were treated at The University of Texas MD Anderson Cancer Center (Houston, TX) between 2001 and 2018. Of the 137 patients, 42 (31%) had undergone definitive treatment before their presentation to MD Anderson, either with surgery or with concurrent CRT, or had distant metastasis at presentation and were excluded. The baseline clinical and pathologic characteristics of the 95 patients who met the inclusion criteria and were included in this analysis are listed in Table 1. Of all the patients, 66 (69%) were classified as having T4 disease, 16 (17%) as having T3 disease, and 13 (14%) as having T2 disease. Nodal metastasis (N+) was present in 14 patients (14%). Overall stage was stage IV in 74 patients and stages III and II in 13 and eight patients, respectively.
TABLE 1.
Patients’ Demographic and Clinical Characteristics
Chemotherapy
Ninety-five treatment-naïve patients were treated with IC; of these, 62 patients (64%) completed at least two cycles of IC and 33 patients completed one cycle (range, one to five cycles; median, three cycles); the rate of adverse events possibly related to IC was 60%. The rate of grade 3 or 4 hematologic toxicity was 34% (32 of 95): neutropenia, 20%; thrombocytopenia, 11%; and febrile neutropenia, 3%. Grade 3 or 4 nonhematologic events occurred in 26% of patients and included nausea and vomiting (18%), pulmonary embolism (3%), deterioration in renal function (3%), and acute myocardial infarction (2%). Hearing impairment was documented in 24 of 70 patients (25%).
After IC, radiologic assessments indicated that 64 patients experienced a response (response rate, 67% [95% CI, 36% to 78%]), including six complete responses (6%) and 58 partial responses (61%). Stable disease was reported in 22 patients (23%), and disease progression was reported in nine patients (10%).
Surgical Procedures
After completion of IC, 63 of the 95 patients (66%) were treated with CRT, and 32 (34%) underwent surgery. Of the patients who underwent surgery (n = 32), 18 underwent craniofacial resection, 11 underwent endoscopic endonasal resection, and three underwent endoscopically assisted craniofacial resection. Complete resection with microscopically negative margins (R0) occurred in 19 of the 32 (61%); the remaining patients had either microscopically (n = 3, R1) or macroscopically (n = 6, R2) positive margins of resection; four patients had undetermined margin status. All surgically treated patients were treated with subsequent adjuvant radiation; most of them, 88% (28 of 32), received concurrent chemotherapy as well (Fig 1). Surgery resulted in organ loss in nine patients (28%): three underwent hard palatectomy; three, brain parenchyma resection; two, orbital exenteration; and one, hard palatectomy with orbital exenteration. Functionally, orbital exenteration resulted in loss of vision in the resected orbit; all patients who underwent hard palatectomy required outpatient dental, speech, and swallow rehabilitation. No cognitive deficits were recorded in the patients who underwent brain parenchyma resection; however, one patient experienced slurred speech, gait disturbances, and sensory loss. No organ loss occurred in the patients who underwent IC followed by CRT (66% [63 of 95]).
Treatment Outcomes
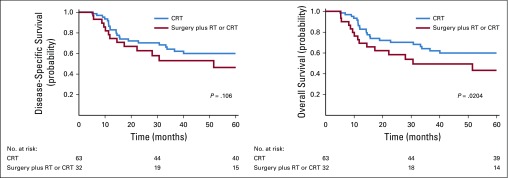
The 5-year DSS and OS probabilities for the entire cohort were 59% (95% CI, 53% to 66%), and 56% (95% CI, 51% to 61%), respectively. Survival analysis stratified by locoregional treatment after IC revealed that the 5-year DSS probability was 66% (95% CI, 57% to 72%) in patients who were treated with definitive concurrent CRT after IC compared with 50% (95% CI, 41% to 60%) in patients who were treated with definitive surgery after IC (P = .106, Fig 3). The 5-year OS probability was 66% (95% CI, 57% to 74%) in patients who were treated with concurrent CRT after IC compared with 43% (95% CI, 34% to 48%) in patients who were treated with surgery after IC (P = .0204).
FIG 3.
Survival outcomes according to definitive locoregional treatment after induction chemotherapy. Disease-specific and overall survival in patients treated with concurrent chemoradiotherapy (CRT; blue) and surgery followed by radiotherapy (RT) or concurrent CRT (red).
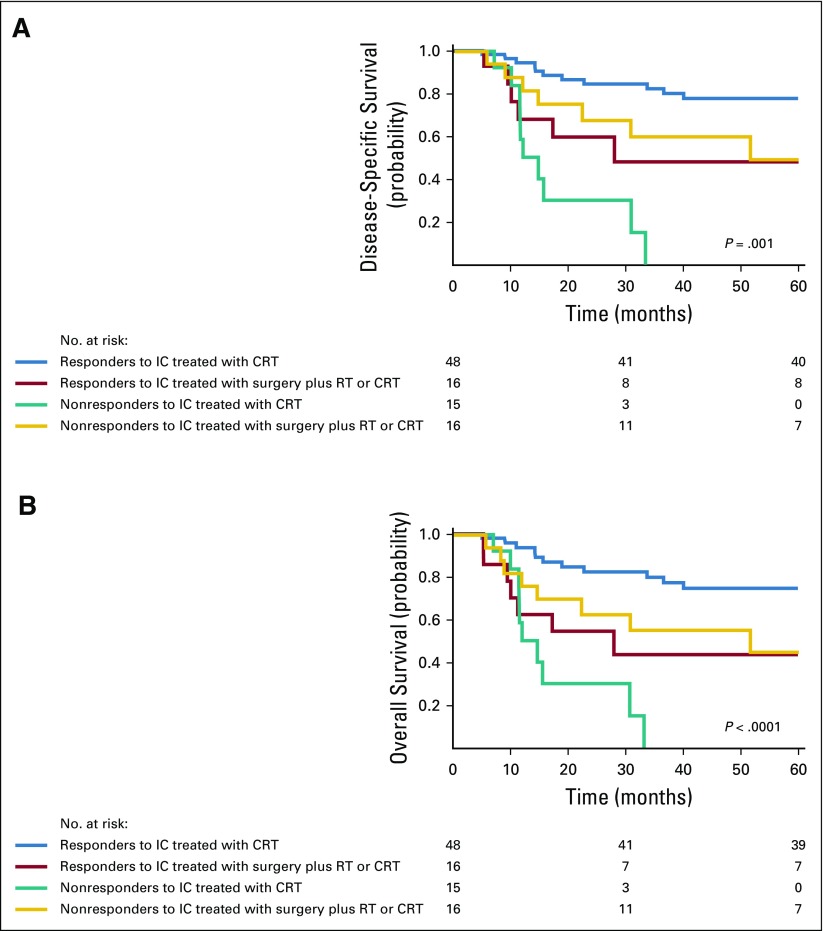
Figure 4 summarizes the DSS and OS rates in responders and nonresponders according to definitive locoregional treatment group. The 5-year DSS probabilities in patients who experienced a response to IC were 81% (95% CI, 69% to 88%) after treatment with definitive concurrent CRT after IC and 54% (95% CI, 44% to 61%) after definitive surgery and postoperative RT or CRT after IC (log-rank P = .001, Fig 4A). In patients who did not experience a response to IC, the 5-year DSS probability was 0% (95% CI, 0% to 4%) in patients who were treated with concurrent CRT after IC, compared with 39% (95% CI, 30% to 46%) in patients who were treated with surgery plus RT or CRT (Fig 4A, log-rank P = .001). The 5-year OS probabilities in patients who experienced a response to IC after definitive concurrent CRT or surgery were 81% (95% CI, 74% to 90%) and 49% (95% CI, 37% to 64%; Fig 4B, log-rank P < .0001), respectively. In patients who did not experience a response to IC, the 5-year OS probability was 0% (95% CI, 0% to 6%) in patients who were treated with concurrent CRT, compared with 39% (95% CI, 32% to 48%) in patients who were treated with surgery plus RT or CRT (log-rank P < .001).
FIG 4.
Survival outcomes according to definitive locoregional treatment by response to induction chemotherapy (IC). (A) Disease-specific and (B) overall survival in patients who responded to IC and were treated subsequently with concurrent chemoradiation (CRT; blue) or surgery followed by radiotherapy (RT) or concurrent CRT (red), and in patients who did not respond to IC and were treated subsequently with concurrent CRT (green) and surgery followed by RT or concurrent CRT (orange).
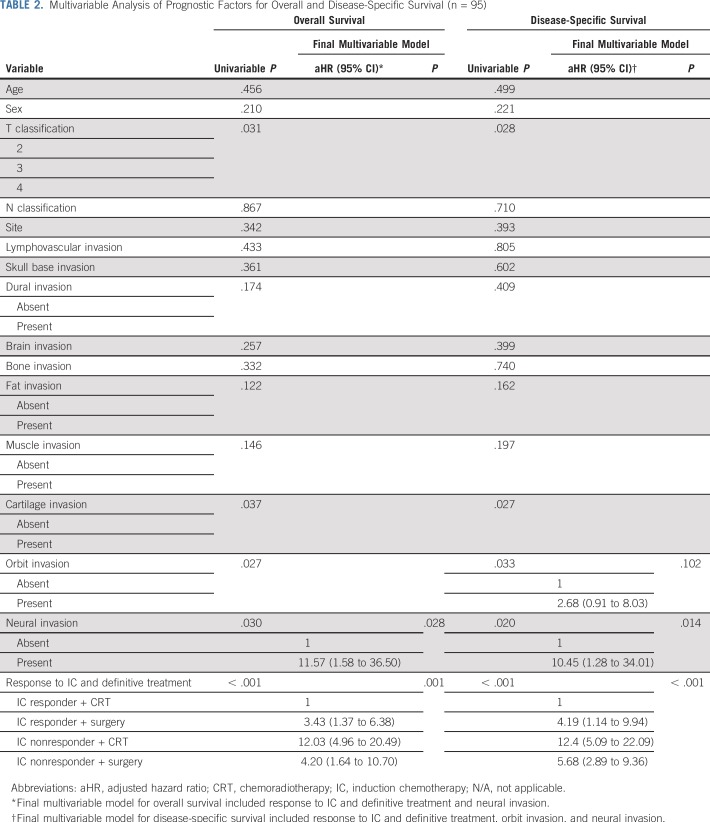
To adjust the risk associated with treatment groups, we introduced variables with prognostic potential, as indicated by univariable analyses, to a multivariable model (Table 2). The adjusted hazard ratio (HR) of 4.19 (95% CI, 1.14 to 9.94; P < .001) for DSS and 3.43 (95% CI, 1.37 to 6.38; P < .001) for OS significantly favored the use of CRT in patients who experienced a response to IC. In patients who did not experience a response to IC and were treated with CRT only, the adjusted HR of 12.4 (95% CI, 5.09 to 22.09; P < .001) for DSS and 12.03 (95% CI, 4.96 to 20.49; P < .001) for OS significantly favored the use of surgery followed by CRT (adjusted HR of 5.68 [95% CI, 2.89 to 9.36; P < .001] for DSS and 4.20 [95% CI, 1.64 to 10.70; P < .001] for OS).
TABLE 2.
Multivariable Analysis of Prognostic Factors for Overall and Disease-Specific Survival (n = 95)
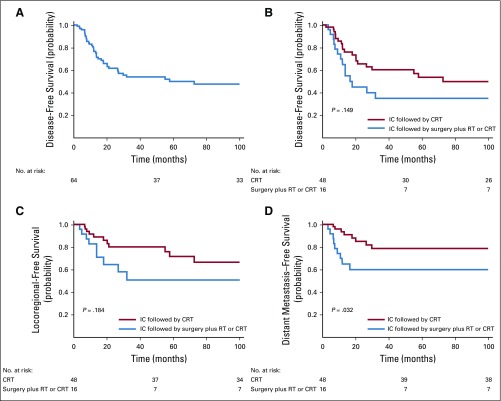
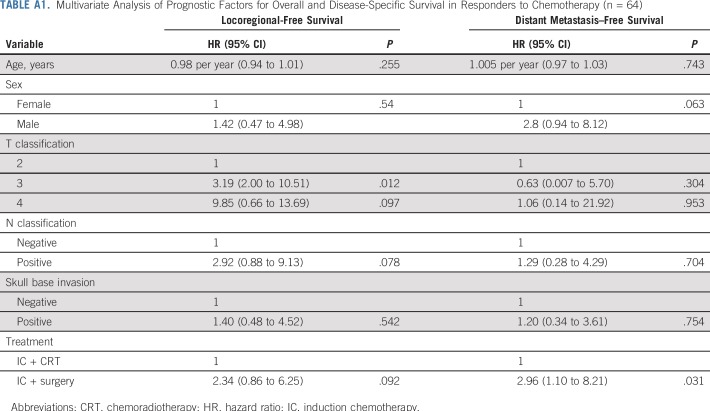
Finally, we analyzed the patterns of treatment failure in patients who experienced a response to IC. The 5-year disease-free survival of this patient group was 53% (Appendix Fig A1A, online only). In patients who experienced a response to IC, the 5-year disease-free survival rate was 57% after treatment with concurrent CRT and 38% after surgery plus RT or CRT (log-rank P = .149; Appendix Fig A1B). The 5-year locoregional recurrence-free survival rate was 74% after treatment with concurrent CRT after IC and 55% after surgery plus RT or CRT after IC (log-rank P = .184; Appendix Fig A1C). The 5-year distant metastasis-free survival rate was 83% after treatment with concurrent CRT after IC and 59% after surgery plus RT or CRT after IC (log-rank P = .032; Appendix Fig A1D). A multivariable analysis revealed that the adjusted HR for distant metastasis was 2.96 (95% CI, 1.1 to 8.21; P = .031; Appendix Table A1, online only) in patients who experienced a response to IC and were treated subsequently with surgery, compared with patients treated with concurrent CRT after IC.
DISCUSSION
The treatment of patients with SNUC is challenging because of its aggressive biologic behavior, the advanced stage at presentation, the early invasion of critical structures such as the eye and the brain, and its propensity to distant metastasis. To date, most published data support the multimodality approach, including surgery and RT, with or without chemotherapy.5,10,14 Although there seems to be general consensus regarding the need for multimodality therapy, there is significant variation in the choice and sequence of such treatment modalities.15 Primary surgical resection is advocated by many on the basis of results of limited case series or systematic reviews of such studies.10,15-18 However, the process by which patients are selected for primary surgery is not well defined in these reports, and selection is inherently biased by surgical resectability favoring earlier-stage disease, which is uncommon.19 Even with radical surgery, the achievement of tumor-free margins is difficult, locoregional recurrence is common, and the reported 5-year DSS in most reports and systematic reviews for surgically treated patients is less than 50%.10,15,20 Other studies favored the use of primary radiation with, in most cases, concurrent chemotherapy, and surgery is reserved for salvage in patients with persistent disease.5,13,21 One disadvantage to this approach is that surgical salvage of patients not responding to high-dose definitive concurrent chemoradiation is rarely successful and is associated with increased surgical complications. A recent review demonstrated a significant role for such a combined modality treatment, with improved local control rates; however, the authors documented a high rate of severe late toxicity and permanent vision loss.10 In addition, it was not clear if the improvement in local control translated into a DSS benefit.12,22
For many years, our approach to patients with SNUC has been to incorporate IC before definitive locoregional therapy. The rationale for this approach is the relatively high risk of distant metastasis and the known value of IC in reducing such risk.2-6 Another advantage to IC is its potential role in predicting radiation sensitivity when choosing a primary treatment modality.23 At MD Anderson, treatment recommendations are made by our multidisciplinary team, and final treatment plans are decided by the treating physician. In the current study, most of the patients who had a favorable response to IC (either partial response or complete response) were treated with CRT. The rationale for this is the known sensitivity to concurrent CRT in patients who respond to IC.23,24 A minority of patients who had a favorable response to IC were treated surgically. This may have been because of the limited nature of the disease, or because of the preference of the treating clinician. In contrast, in patients with less than a partial response to IC who had resectable disease, a surgical approach aiming to achieve gross total resection followed by concurrent CRT was favored. Patients with unresectable disease were treated with CRT because surgery was not an option.
In reviewing the results of our study of this treatment approach, several observations can be made. First, patients with a favorable response to IC have improved DSS and OS compared with those without a favorable response to IC. This is consistent with other studies of IC in sinonasal cancers.17,25,26 Second, in patients with a favorable response, CRT seems to provide better survival outcomes and organ preservation than does surgery. A possible explanation for such an improvement may be that patients with an inherent sensitivity to radiation-based treatment were selected. It is possible that in such patients, surgery may delay definitive treatment with radiation. Another possible explanation is the reduced dose of radiation typically given in the postoperative adjuvant setting compared with primary definitive radiation. In contrast, patients who fail to achieve a favorable response to chemotherapy seem to have better survival if they are treated with surgery and postoperative radiation with or without chemotherapy, compared with those who are treated with CRT. A possible explanation is that these potentially resistant tumors are not likely to be responsive to radiation-based treatments and are best treated with surgical resection. In fact, in our study, none of the patients who did not respond to IC and were treated with primary CRT survived their disease. Taken together, these observations suggest that IC may help guide the selection of definitive locoregional therapy and optimize the overall treatment strategy.
Despite the limitations inherent to a single-institution retrospective design, the strength of our study is that, to our knowledge, it represents the largest cohort of patients with SNUC treated with a uniform and consistent treatment strategy using IC. Our findings must be further validated in a prospective study that will likely need multi-institutional participation because of the rarity of this disease. There is also a critical need to identify molecular markers of response to treatment to further guide the selection of therapy and perhaps provide targets for novel therapies for patients with SNUC.
Appendix
FIG A1.
(A) Disease-free survival of patients who responded to induction chemotherapy (IC), and (B) disease-free survival, (C) locoregional-free survival, and (D) distant metastasis–free survival according to the definitive locoregional treatment in patients who responded to IC. CRT, chemoradiotherapy; RT, radiotherapy.
TABLE A1.
Multivariate Analysis of Prognostic Factors for Overall and Disease-Specific Survival in Responders to Chemotherapy (n = 64)
Footnotes
Supported in part by the National Institutes of Health through Cancer Center Support Grant P30 CA016672.
AUTHOR CONTRIBUTIONS
Conception and design: Moran Amit, Michael E. Kupferman, Ehab Y. Hanna
Financial support: Ehab Y. Hanna
Provision of study material or patients: Bonnie Glisson, Franco DeMonte, Ehab Y. Hanna
Collection and assembly of data: Moran Amit, Ahmed S, Abdelmeguid, Teemaranawich Watcherporn, Samantha Tam, Renata Ferrarotto, Dianna B. Roberts, Shirley Y. Su, Shaan M. Raza, Ehab Y. Hanna
Data analysis and interpretation: Moran Amit, Hideaki Takahashi, Diana Bell, Renata Ferrarotto, Bonnie Glisson, Michael E. Kupferman, Dianna B. Roberts, Franco DeMonte, Ehab Y. Hanna
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Induction Chemotherapy Response as a Guide for Treatment Optimization in Sinonasal Undifferentiated Carcinoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Renata Ferrarotto
Consulting or Advisory Role: Ayala Pharmaceuticals
Research Funding: OncoMed Pharmaceuticals (Inst), G1 Therapeutics (Inst), AstraZeneca-MedImmune (Inst), EMD Serono (Inst), Genentech-Roche (Inst), Merck Serono (Inst)
Bonnie Glisson
Research Funding: Pfizer (Inst), Medimmune (Inst), Bristol-Myers Squibb (Inst), ISA Pharmaceuticals (Inst), AbbVie-Stemcentrx (Inst), Turnstone Bio (Inst)
Travel, Accommodations, Expenses: Abbvie-Stemcentrx, ISA Pharmaceuticals
No other potential conflicts of interest were reported.
REFERENCES
- 1.Thompson LDR BD, Bishop JA.Neuroendocrine carcinomasinEl-Naggar AK, Chan JKC, Grandis JR, et al.(eds)WHO Classification of Head and Neck Tumors ed 4Lyon, France: IARC Press; 2017. pp21–23. [Google Scholar]
- 2.Zielinski V, Laban S, Tribius S, et al. Management of sinonasal undifferentiated carcinoma with intracerebral invasion: Clinical experience at a single institution and review of the literature. Ear Nose Throat J. 2016;95:23–28. [PubMed] [Google Scholar]
- 3.Lopez F, Suárez V, Vivanco B, et al. Current management of sinonasal undifferentiated carcinoma. Rhinology. 2015;53:212–220. doi: 10.4193/Rhino14.054. [DOI] [PubMed] [Google Scholar]
- 4.Tanzler ED, Morris CG, Orlando CA, et al. Management of sinonasal undifferentiated carcinoma. Head Neck. 2008;30:595–599. doi: 10.1002/hed.20748. [DOI] [PubMed] [Google Scholar]
- 5.Chen AM, Daly ME, El-Sayed I, et al. Patterns of failure after combined-modality approaches incorporating radiotherapy for sinonasal undifferentiated carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2008;70:338–343. doi: 10.1016/j.ijrobp.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 6.Lin EM, Sparano A, Spalding A, et al. Sinonasal undifferentiated carcinoma: A 13-year experience at a single institution. Skull Base. 2010;20:61–67. doi: 10.1055/s-0029-1236165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ansari M, Guo S, Fakhri S, et al. Sinonasal undifferentiated carcinoma (SNUC): Morphoproteomic-guided treatment paradigm with clinical efficacy. Ann Clin Lab Sci. 2013;43:45–53. [PubMed] [Google Scholar]
- 8.Rischin D, Porceddu S, Peters L, et al. Promising results with chemoradiation in patients with sinonasal undifferentiated carcinoma. Head Neck. 2004;26:435–441. doi: 10.1002/hed.10396. [DOI] [PubMed] [Google Scholar]
- 9.Levine PA, Frierson HF, Jr, Stewart FM, et al. Sinonasal undifferentiated carcinoma: A distinctive and highly aggressive neoplasm. Laryngoscope. 1987;97:905–908. [PubMed] [Google Scholar]
- 10.Al-Mamgani A, van Rooij P, Mehilal R, et al. Combined-modality treatment improved outcome in sinonasal undifferentiated carcinoma: Single-institutional experience of 21 patients and review of the literature. Eur Arch Otorhinolaryngol. 2013;270:293–299. doi: 10.1007/s00405-012-2008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edge S, Byrd DR, Compton CC, et al: AJCC Cancer Staging Manual (ed 7). New York, NY, Springer, 2010. [Google Scholar]
- 12. National Cancer Institute: Investigator’s handbook: A manual for participants in clinical trials of investigational agents sponsored by division of cancer treatment and diagnosis. Bethesda, MD, National Cancer Institute, 1993. [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Mourad WF, Hauerstock D, Shourbaji RA, et al. Trimodality management of sinonasal undifferentiated carcinoma and review of the literature. Am J Clin Oncol. 2013;36:584–588. doi: 10.1097/COC.0b013e31825eb3a5. [DOI] [PubMed] [Google Scholar]
- 15.Morand GB, Anderegg N, Vital D, et al. Outcome by treatment modality in sinonasal undifferentiated carcinoma (SNUC): A case-series, systematic review and meta-analysis. Oral Oncol. 2017;75:28–34. doi: 10.1016/j.oraloncology.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Reiersen DA, Pahilan ME, Devaiah AK. Meta-analysis of treatment outcomes for sinonasal undifferentiated carcinoma. Otolaryngol Head Neck Surg. 2012;147:7–14. doi: 10.1177/0194599812440932. [DOI] [PubMed] [Google Scholar]
- 17.Kuo P, Manes RP, Schwam ZG, et al. Survival outcomes for combined modality therapy for sinonasal undifferentiated carcinoma. Otolaryngol Head Neck Surg. 2017;156:132–136. doi: 10.1177/0194599816670146. [DOI] [PubMed] [Google Scholar]
- 18.Bhasker S, Mallick S, Benson R, et al. A multimodality approach to sinonasal undifferentiated carcinoma: A single institute experience. J Laryngol Otol. 2017;131:19–25. doi: 10.1017/S0022215116009543. [DOI] [PubMed] [Google Scholar]
- 19.Kuan EC, Arshi A, Mallen-St Clair J, et al. Significance of tumor stage in sinonasal undifferentiated carcinoma survival: A population-based analysis. Otolaryngol Head Neck Surg. 2016;154:667–673. doi: 10.1177/0194599816629649. [DOI] [PubMed] [Google Scholar]
- 20.Khan MN, Konuthula N, Parasher A, et al. Treatment modalities in sinonasal undifferentiated carcinoma: An analysis from the national cancer database. Int Forum Allergy Rhinol. 2017;7:205–210. doi: 10.1002/alr.21861. [DOI] [PubMed] [Google Scholar]
- 21.Musy PY, Reibel JF, Levine PA. Sinonasal undifferentiated carcinoma: The search for a better outcome. Laryngoscope. 2002;112:1450–1455. doi: 10.1097/00005537-200208000-00023. [DOI] [PubMed] [Google Scholar]
- 22.Kim BS, Vongtama R, Juillard G. Sinonasal undifferentiated carcinoma: Case series and literature review. Am J Otolaryngol. 2004;25:162–166. doi: 10.1016/j.amjoto.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Wolf GT, Fisher SG, Hong WK, et al. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324:1685–1690. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 24.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 25.Bossi P, Saba NF, Vermorken JB, et al. The role of systemic therapy in the management of sinonasal cancer: A critical review. Cancer Treat Rev. 2015;41:836–843. doi: 10.1016/j.ctrv.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Amit M, Tam S, Abdelmeguid AS, et al. Role of adjuvant treatment in sinonasal mucosal melanoma. J Neurol Surg B Skull Base. 2017;78:512–518. doi: 10.1055/s-0037-1604350. [DOI] [PMC free article] [PubMed] [Google Scholar]