Abstract
Objective:
This study investigated the safety and efficacy of remote programming of cochlear implants.
Study Design:
Single-subject design
Setting:
Four North American clinical sites
Patients:
Forty cochlear implant recipients aged 12 years or older
Intervention:
Subjects had their cochlear implants programmed at a location that was remote from their audiologist using telecommunication with and without the support of a facilitator.
Main Outcome Measures:
Consonant-Nucleus-Consonant (CNC) word scores and the Speech, Spatial, and Qualities of Hearing Scale-C (SSQ-C) were compared using the subject's in-office MAP (program) and MAPs programmed remotely with and without the assistance of a facilitator. Additional subjective preference data were gathered from subjects and audiologists via questionnaires.
Results:
MAPs programmed via the three different models did not yield significantly different group mean CNC word scores. No device/procedure-related adverse events occurred. SSQ-C questionnaire results indicated that recipients received similar subjective benefit from familiar in-office, remote-facilitated, and remote-unassisted MAPs.
Conclusions:
Remote programming is an effective means of cochlear implant service delivery. The practice was approved by the FDA on November 17, 2017 supported by the results of this study.
Keywords: Cochlear implant, Remote programming, Telehealth, Telemedicine
INTRODUCTION
The World Health Organization estimates that 360 million people worldwide are affected by disabling hearing loss (1). Significant hearing loss can have pervasive negative effects including communication difficulties, social isolation, and reduced employment opportunities. As an intervention, cochlear implantation is considered the standard of care for people with moderate-to-profound sensorineural hearing loss when hearing aids are no longer effective. As cochlear implant (CI) technology advances and patient outcomes improve, candidacy criteria for implantation are expanding. The National Institute on Deafness and Other Communication Disorders reported that as of December 2012, approximately 324,200 devices had been implanted worldwide (2). Despite the substantial, proven benefits of cochlear implantation (3–6), a significant number of individuals who likely meet candidacy guidelines do not pursue this intervention.
One obstacle that patients report is access to ongoing hearing healthcare. Cochlear implant centers tend to be located in larger metropolitan areas. Recipients are typically seen by the audiologist five to ten times during the first year of device use and then on an annual or semiannual basis thereafter. These visits can be burdensome to recipients who travel from rural regions, but the appointments are imperative to optimizing the patient's benefit from the CI and include programming adjustments, monitoring of performance through speech perception testing, counseling regarding expectations and communication strategies, and introduction and demonstration of additional equipment such as wearing options and assistive listening devices.
Increasing patient access to healthcare providers and their services via telehealth is a growing trend in healthcare (7–9). Utilizing this model to provide remote programming of CIs may positively impact access to care for several groups of individuals, including those residing in underserved areas, those with limited transportation options, or patients who are in poor health. Additional benefits of remote care include access to specialist providers, decreased time away from work and/or school, fewer lost wages, and decreased transportation costs.
Previous studies have shown that the duration of a CI remote programming session is comparable to that of an in-office session, and that participants are generally satisfied with remote programming sessions (10,11). In their 2012 study, Hughes and colleagues (12) found no significant differences in electrode-specific measures (impedance, ECAP thresholds, and MAP levels) when tests were conducted remotely versus in-office. Samuel et al. (13) compared remote and in-office programming sessions performed on a single user on the same day and found no significant differences in audiometric thresholds or speech perception scores. The participants in this study demonstrated some variations in stimulation levels when remote and in-office programming sessions were compared, however the authors concluded that providing CI programming services remotely is safe and effective.
In the multicenter study presented here, performance with a remotely programmed MAP was evaluated in a group of adolescent and adult cochlear implant users and compared to performance with a MAP created in the traditional in-office manner. The practice of remote programming was investigated objectively for both safety and effectiveness, and also for acceptability by both the CI recipient and the audiologist.
MATERIALS AND METHODS
Study Design
This investigation employed a single-subject research design where each subject served as his or her own control. This design accommodates the heterogeneity that characterizes hearing-impaired populations. The study was conducted over a period of 12 months at the Medical College of Wisconsin, Rocky Mountain Ear Center, the University of Michigan, and the University of North Carolina.
Participants
Participants in this study were cochlear implant users aged 12 years or older who were deemed capable of completing the study by their primary audiologist. The subjects were recipients of Cochlear Nucleus CI24R, CI24RE, CI422, and CI500 series internal cochlear implants, and all subjects utilized either a Nucleus 5 or Nucleus 6 external sound processor. All subjects had completed an in-office programming session within the 12 months prior to Visit 1 of the study. The enrolled population consisted of 40 subjects (18 males, 22 females) including 27 adults and 13 adolescents. For the purposes of this study, adolescents are defined as those aged 12 to 21 years. The mean age at enrollment for the entire subject population was 45.2 years (range 12–88 years). Demographic information is summarized in Table 1.
TABLE 1. Demographics.
| Variable | Result |
| Gender | |
| Female | 22/40 (55.0%) |
| Male | 18/40 (45.0%) |
| Mean age at enrollment, years | 45.2 years (range 12–88 years) |
| Implanted Ear | |
| Left | 15/40 (37.5%) |
| Right | 25/40 (62.5%) |
| Internal implant model | |
| CI24R | 3/40 (7.5%) |
| CI24RE | 23/40 (57.5%) |
| CI422 | 8/40 (20.0%) |
| CI500 series | 6/40 (15.0%) |
| Sound processor model | |
| Nucleus 5 | 17/40 (42.5%) |
| Nucleus 6 | 23/40 (57.5%) |
| Hearing device contra ear | |
| Cochlear implant | 24/40 (60.0%) |
| Hearing aid | 9/40 (22.5%) |
| None | 6/40 (15.0%) |
| Other: ear plug | 1/40 (2.5%) |
Equipment
Subjects’ sound processors were programmed at a remote location by their primary audiologist using GoToMeeting video conferencing software. Each remote location was equipped with a Microsoft Surface Pro 3 Tablet, as well as a programming pod, programming cable, and backup Nucleus 6 sound processor. The tablets were loaded with Custom Sound Suite software which was password protected, allowing the audiologist exclusive access to the software and prevented any unintentional program changes by the recipient. All tablets had microphone, speaker, and camera capabilities. A wired or wireless Internet broadcasting system with a minimum connection speed of 1 megabit/s was required in both directions. The audiologist site utilized the same computer, software, and connection specifications as outlined above to enable communication between the audiologist and the subject during the remote sessions.
Methods
The study protocol was approved by each site's Institutional Review Board (IRB) prior to subject enrollment, and informed consent was obtained from each subject prior to initiation of any study-related activities.
Subjects were seen for five visits each. A visual depiction of the remote programming set-up and a visit flowchart summary are shown in Figure 1. Visit 1 took place at the primary clinic site where informed consent was obtained and the subject received orientation to the remote programming equipment and procedure with their audiologist. Speech perception with the subject's Familiar In-Office (FIO) MAP (the MAP the subject was using at study enrollment which had been programmed in-office within the past 12 months) was assessed using the CNC Monosyllabic Word Test (14) presented via recording in quiet at 60 dBA. Each subject completed two lists in the unilateral condition (cochlear implant ear only) with the contralateral ear plugged, or with the contralateral cochlear implant removed if applicable.
FIG. 1.
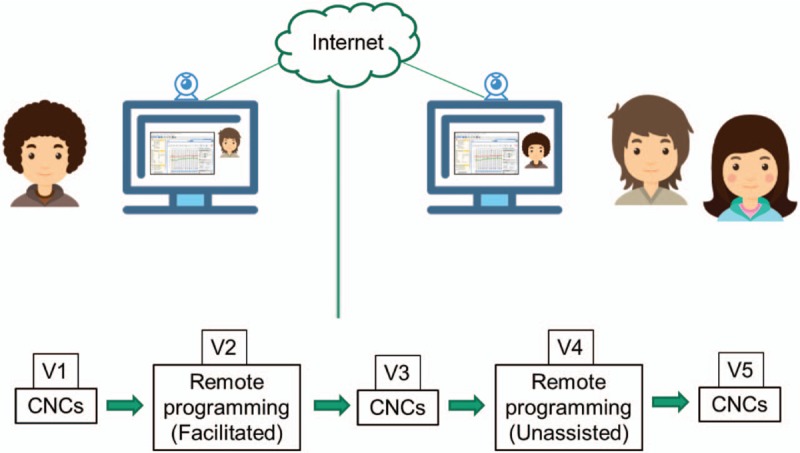
Remote programming set up and visit flow summary.
Visit 2 was completed within one month of Visit 1 and was performed at a location that was remote from the audiologist. A trained facilitator assisted the subject in connecting the sound processor to the tablet and launching the teleconferencing software. Through this software, the audiologist gained remote access to the tablet and completed the tasks of a typical device programming session including measuring impedance telemetry and assessing electrical thresholds and comfort levels. During this session, the audiologist and the subject communicated via video, audio, and typing chat functions. The new program, labeled “Facilitated Remote MAP” (FR MAP), was saved to the sound processor and subjects used this MAP until Visit 3. Following programming, subjects completed the Remote Programming Satisfaction Survey, which is a rating scale developed by Cochlear Americas to gather subjects’ opinions regarding the ease, quality, and comfort of the remote programming sessions.
Visit 3 occurred at the primary clinic two to four weeks after Visit 2, and included speech perception testing using the subject's FR MAP completed in identical fashion to Visit 1. Subjects also completed the Speech, Spatial, and Qualities of Hearing Scale-C (SSQ-C) (15) comparing their FIO MAP and FR MAP. The SSQ-C is the “comparative” version of the SSQ and can be used for comparing two different hearing technologies across a variety of domains such as naturalness of sounds, listening in crowds, or hearing from a distance.
Visit 4 was completed two to four weeks after Visit 3. The subject was again at a remote location from the audiologist, but at this visit they completed the remote programming setup process independently. The audiologist again gained remote access to the tablet and completed the programming session. The new “Unassisted Remote MAP” (UR MAP) was saved to the sound processor and participants used this MAP until Visit 5. Following device programming, subjects completed the Remote Programming Satisfaction Survey for this unassisted visit.
Visit 5 occurred at the primary clinic two to four weeks after Visit 4, and consisted of speech perception testing using the subject's UR MAP in identical fashion to Visits 1 and 3. Subjects completed the SSQ-C comparing their FIO and UR MAPs, as well as a separate questionnaire developed by Cochlear Americas to gather their opinions of the overall telemedicine experience.
Endpoints and Data Analysis
Sample size was determined based on the variability observed in earlier studies using PASS (NCSS Statistical Software). A minimum of 26 subjects was necessary to achieve 90% power for hypothesis testing of the two co-primary endpoints at the 0.025 alpha level. Thus, the sample size of 39 subjects provided adequate power for hypothesis testing. Pooling data from study sites is justified because all sites had the same protocol, the sites were monitored to assure protocol compliance, and the data gathering mechanism (case report forms and data acquisition) were the same across all study sites.
The primary safety endpoint was to characterize the safety profile of device and/or procedure-related adverse events associated with each of the three programming conditions: FR MAP programming, UR MAP programming, and FIO MAP programming.
The two primary efficacy endpoints aimed to demonstrate noninferiority of FR MAP and UR MAP performance compared to FIO MAP performance for word recognition in quiet as evaluated with the CNC Monosyllabic Word test. Success for both endpoints was based on rejection of the null hypothesis of inferiority, with a noninferiority margin of 10%. The treatment effect was evaluated using a one sample t-test, with statistical significance being met if the upper limit of the two-sided 95% confidence bound is <10. A secondary efficacy endpoint compared the FR MAP to the UR MAP.
RESULTS
Forty subjects were enrolled into the study. One subject withdrew in order to upgrade to the next generation sound processor mid-study, leaving 39 subjects that completed the study protocol.
Primary Safety Endpoint
Neither device-related nor procedure-related adverse events were reported during this study.
Primary Efficacy Endpoints—CNC Scores
The group mean CNC score was 70.5% (± 18.6) for the FIO MAP and 72.4% (± 18.7) for the FR MAP. This difference of 1.8 percentage points (± 8.7) meets the defined noninferiority margin at a p-value of <0.001, demonstrating noninferiority of FR MAPs compared to FIO MAPs.
The group mean CNC score obtained with the FIO MAP was 1.9 (± 8.9) percentage points poorer than the UR MAP group mean score of 72.5% (± 21.3). This meets the defined noninferiority margin at a p-value of <0.001 and demonstrates noninferiority of UR MAPs compared to FIO MAPs.
Secondary Efficacy Endpoint
The group mean CNC score of 72.4% (±18.7) from the FR session was compared to the group mean CNC score of 72.5% (± 21.3) from the UR session resulting in a difference of 0.1 (± 9.2). This comparison also met the definition of noninferiority with a p-value of <0.001.
Within-Subject Differences in CNC Test Scores
The critical differences (0.05 level of confidence) adapted from Thornton and Raffin were used for comparison of CNC word recognition scores (16). The number of subjects with significantly lower, significantly higher, and similar CNC word scores based on the binomial model can be found in Tables 2–4.
TABLE 2.
Within-subject differences in CNC test scores for facilitated remote (FR) vs familiar in-office (FIO) MAPs (N = 39)
| Difference in Scores | N | Percent |
| Significantly higher | 3 | 7.7 |
| Significantly lower | 2 | 5.1 |
| Similar | 34 | 87.2 |
CNC indicates Consonant-Nucleus-Consonant.
TABLE 4.
Within-subject differences in CNC test scores for unassisted remote (UR) vs facilitated remote (FR) MAPs (N = 39)
| Difference in Scores | N | Percent |
| Significantly higher | 2 | 5.1 |
| Significantly lower | 1 | 2.6 |
| Similar | 36 | 92.3 |
CNC indicates Consonant-Nucleus-Consonant.
Subjective Questionnaires
Remote Programming Satisfaction Survey
At the completion of Visits 2 and 4, the subjects completed the Remote Programming Satisfaction Survey. When asked if they were able to communicate easily with the audiologist, 92.5% of participants indicated that they agreed or strongly agreed for the FR session (N = 40) and 92.3% agreed or strongly agreed for the UR session (N = 39). All subjects agreed or strongly agreed that they were comfortable with the care provided and were satisfied with the programming session for both the FR and UR sessions.
Speech, Spatial, and Qualities of Hearing Scale-C (SSQ-C)
At Visits 3 and 5, the SSQ-C was completed to compare the FR and UR MAPs to the FIO MAP. The survey's scale ranges from −5 (poorer) to +5 (better). A more negative number indicates a preference for the FIO programmed MAP, a more positive number indicates a preference for the remotely programmed MAP, and a number close to zero indicates that the subject found the MAPs to be somewhat equivalent. Group mean results for the subscales of the SSQ-C can be found in Tables 5 and 6, and indicate that on average, subjects reported that the FR and UR MAPs were similar to the FIO MAP.
TABLE 5.
Group mean responses on SSQ-C comparing FR MAP to FIO MAP (N = 39)
| Variable | ResultMean ± SD |
| Speech and Hearing Scale | 0.6 ± 1.3 |
| Spatial Rating Scale | 0.6 ± 1.0 |
| Sound Quality Rating Scale | 0.9 ± 1.3 |
| Average | 0.7 ± 1.1 |
FIO indicates Familiar In-Office; FR, Facilitated Remote; SSQ-C Speech, Spatial, and Qualities of Hearing Scale-C.
TABLE 6.
Group mean responses on SSQ-C comparing UR MAP to FIO MAP (N = 39)
| Variable | ResultMean ± SD |
| Speech and Hearing Scale | 0.7 ± 1.5 |
| Spatial Rating Scale | 0.7 ± 1.4 |
| Sound Quality Rating Scale | 0.9 ± 1.5 |
| Average | 0.7 ± 1.4 |
FIO indicates Familiar In-Office; SSQ-C Speech, Spatial, and Qualities of Hearing Scale-C; UR, Unassisted Remote.
Telemedicine Experience Questionnaire
At Visit 5, the Telemedicine Experience Questionnaire was completed. When asked if they would choose telehealth over in-office programming, 56% of the subjects responded Likely, 39% responded Neutral, and 5% responded Not Likely. Analysis of these responses by age showed that 55% of subjects aged 18 and younger (N = 11), 69% of subjects between ages 19 and 64 (N = 16), and 50% of subjects aged 65 and older (N = 12) reported they were Likely to choose telehealth. Analysis of these responses by roundtrip mileage showed that 46% of subjects who drove <50 miles (N = 28), 83% of subjects who drove 50 to 100 miles (N = 6), and 80% of subjects who drove 100+ miles (N = 5) reported they were Likely to choose telehealth. Figure 2 shows a breakdown of responses.
FIG. 2.
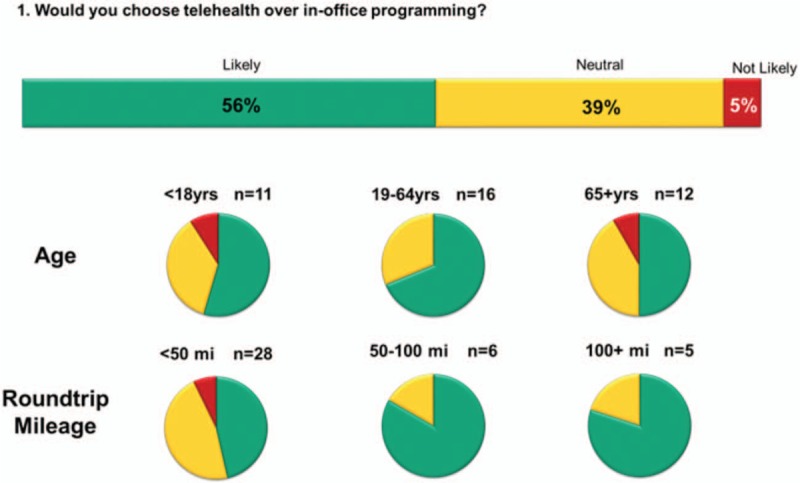
Subject responses to Telemedicine Experience Questionnaire.
When asked if they would use telehealth if they lived > 2 hours away, in the event of inclement weather, if it were difficult to arrange transportation, or if sessions were offered during evening/weekend hours, 80% of subjects responded that they were Likely to choose telehealth, 17% said they were Neutral, and 3% said they were Not Likely (N = 39).
When asked if they would recommend telehealth to another recipient, 90% of participants reported they were Likely to recommend it, and 10% reported they were Neutral (N = 39).
Investigator Responses
After each remote programming session, the audiologists reported on the ease and effectiveness of the session.
Visit 2—Facilitated Remote Programming Session Investigator Experience
Data were available for 40 sessions. Audiologists reported that they agreed or strongly agreed that they were able to communicate easily with the subject in 35/40 of the sessions. Additionally, audiologists reported that they agreed or strongly agreed that they were satisfied with the programming session in 38/40 of the sessions. Aggregate data are shown in Table 7.
TABLE 7.
Investigator experience with facilitated remote (FR) programming sessions (N = 40)
| Statement | Responses |
| I was able to communicate easily with the subject | Strongly agree = 19/40 |
| Agree = 16/40 | |
| Neither agree nor disagree = 3/40 | |
| Disagree = 2/40 | |
| I was satisfied with the programming session | Strongly agree = 21/40 |
| Agree = 17/40 | |
| Neither agree nor disagree = 1/40 | |
| Strongly disagree = 1/40 |
In FR programming sessions, audiologists noted issues with Internet speed, signal delay and/or the applications on the tablet. Many of these issues were remedied by restarting the Internet connection or the tablet. A summary of reported issues is shown in Table 8.
TABLE 8.
Investigator report of issues in facilitated remote (FR) programming sessions
| Description of Issue | Occurrences/Session |
| Internet related | 18/40 |
| Device/Application related | 10/40 |
| Other | 3/40 |
| No issue | 13/40 |
Visit 4—Unassisted Remote Programming Session Investigator Experience
Data were available for 39 sessions. Audiologists reported that they agreed or strongly agreed that they were able to communicate easily with the subject in 36/39 of the UR programming sessions. Additionally, audiologists reported that they agreed or strongly agreed that they were satisfied with the programming session in 38/39 of the UR programming sessions. Aggregate data are shown in Table 9.
TABLE 9.
Investigator experience with unassisted remote (UR) programming sessions (N = 39)
| Statement | Responses |
| I was able to communicate easily with the subject | Strongly agree = 21/39 |
| Agree = 16/39 | |
| Neither agree nor disagree = 1/39 | |
| Disagree = 1/39 | |
| I was satisfied with the programming session | Strongly agree = 20/39 |
| Agree = 18/39 | |
| Disagree = 1/39 |
In UR programming sessions, audiologists noted issues with Internet speed, signal delay and/or the applications on the tablet. A summary of reported issues is shown in Table 10.
TABLE 10.
Investigator report of issues in unassisted remote (UR) programming sessions
| Description of Issue | Occurrences/Session |
| Internet related | 6/39 |
| Device/Application related | 12/39 |
| Other | 1/39 |
| No issue | 21/39 |
DISCUSSION
Healthcare is evolving including changes in technology, service delivery, and service providers. Most changes are geared toward providing better care at lower costs. Telehealth has been a growing factor in our healthcare systems with many states, large hospital networks, and even the Centers for Medicare and Medicaid Services working toward better access to care through remote providers. The results from this multicenter study demonstrate that remote programming of cochlear implants via telecommunication is safe, yields similar speech perception outcomes for recipients as traditional in-office programming, and is generally procedurally acceptable to both patients and audiologists. There were no device- nor procedure-related adverse events during the course of this study, similar to reports by investigators of other related studies (11,17–19).
The participants in this study were preselected for enrollment by their audiologists and may not represent the patient population seen on an average day in a CI clinic. Recipients who need more direct attention, in-depth counseling, or extensive device troubleshooting may not be managed as easily as those who are experienced CI users requiring routine maintenance or check-ups. Thus, remote programming will not be appropriate for all cochlear implant recipients. For example, Wesarg and colleagues caution against using teleprogramming with individuals who have a propensity towards facial nerve stimulation, visual impairments, or cognitive delay as these populations may be better served by the additional support that in-person care provides (19).
In-office and remote programming sessions yielded similar speech perception outcomes in this study, which aligns with previous findings by other investigators (11,17,19–21). In one study, poorer speech perception was noted when individuals were tested at the remote site; however, this difference was attributed to the fact that the remote site did not have a sound booth (12). Not surprisingly, speech perception testing at remote locations may not always be as well controlled as traditional in-booth testing. The findings in this controlled study, where speech perception was carried out in the same calibrated test booth, indicate no difference in outcomes when comparing the use of remote and in-office maps. While this study did not focus on psychophysical measurements, others have found no significant difference which would logically result in comparable speech perception scores (12,19,22).
Previous investigations have found that satisfaction with the experience of using telemedicine techniques for CI care is overwhelmingly positive for patients and audiologists (10,11,18,19,20,22). In this study, issues with Internet speed, signal delay, and/or the applications on the tablet occurred in approximately half of the remote sessions. However, audiologists indicated with high consensus that they were satisfied with the programming sessions.
Clinical validation has found that remote programming of cochlear implants is both feasible and safe. Despite positive responses from patients and clinicians, barriers to implementation persist. On November 17, 2017, remote programming of Nucleus cochlear implants was approved by the FDA for follow-up appointments of recipients 12 years of age and older. However, reimbursement for the procedure is not yet provided by most commercial insurance carriers, and most states do not have reciprocity for licensure which makes providing distance care to patients who live in other states unrealistic without dual licensure. Remote speech perception testing may be less controlled and therefore more variable than in-office assessment, indicating that some in-office visits will be necessary for validation of programming and proper device function.
An increasing patient population and rising costs associated with healthcare necessitate the development of innovative solutions for providing cochlear implant care. Current barriers to implementation of remote programming services can be overcome with additional research and proactive administrative and legislative endorsement for procedures and policies that will enable the easing of financial, time, and travel burdens for patients. Expanding these solutions is important for patients who are medically fragile, geographically isolated from their CI clinics, or significantly taxed by transportation costs and scheduling. Changes in healthcare accessibility and increasing demand for cochlear implant services require a transformation in the care delivery model.
Limitations of this study include the prescription of programming visit schedules per the protocol to control variables, whereas in practice, patients are reprogrammed per the clinic protocol or at the subject's request. This study did not provide reprogramming as a way to troubleshoot specific complaints from the subjects, but was focused on demonstrating safety of the process and equivalency of programming methods. Additionally, the study did not directly require any equipment checks prior to speech perception testing, and aided audiometric thresholds were not evaluated. Future studies could include a longer term protocol to follow subjects through their standard process of care, and include a variety of remote care settings.
TABLE 3.
Within-subject differences in CNC test scores for unassisted remote (UR) vs familiar in-office (FIO) MAPs (N = 39)
| Difference in Scores | N | Percent |
| Significantly lower | 1 | 2.6 |
| Similar | 38 | 97.4 |
CNC indicates Consonant-Nucleus-Consonant.
Acknowledgments
The authors gratefully acknowledge the Cochlear Implant teams at the University of Michigan, Medical College of Wisconsin, University of North Carolina and Rocky Mountain Ear Center for data collection.
Footnotes
Source of funding: This clinical study was sponsored by Cochlear Americas, the manufacturer of the Nucleus cochlear implants and sound processors referenced in this document.
Conflicts of Interest:
H.S. is employed by the University of Michigan and has served as a consultant for Cochlear Americas for projects unrelated to this study.
J.J. is employed by the Medical College of Wisconsin.
K.K. is employed by the Medical College of Wisconsin.
H.T. is employed by the University of North Carolina and has served as a consultant for Cochlear Americas for projects unrelated to this study.
L.P. is employed by the University of North Carolina.
A.B. is employed by Rocky Mountain Ear Center and has served as a consultant for Cochlear Americas for projects unrelated to this study.
M.M. is employed by Cochlear Americas.
REFERENCES
- 1.World Health Organization Deafness and Hearing Loss fact sheet, updated February 2017. Available at: http://www.who.int/mediacentre/factsheets/fs300/en/ Accessed December 11, 2017. [Google Scholar]
- 2.NIDCD, Quick Statistics About Hearing, December 2016. Available at: https://www.nidcd.nih.gov/health/statistics/quick-statistics-hearing Accessed December 11, 2017. [Google Scholar]
- 3.Tyler RS, Parkinson AJ, Woodworth GG. Performance over time of adult patients using the Ineraid or Nucleus cochlear implant. J Am Acoust Soc Am 1997; 102:508–522. [DOI] [PubMed] [Google Scholar]
- 4.Cohen NH, Waltzman SB, Roland JT. Cochlear implants: do they benefit children? Pediatrics 1999; 104:729. [Google Scholar]
- 5.Svirksy MA, Robbins AM, Kirk KI, et al. Language development in profoundly deaf children with cochlear implants. Psychol Sci Mar 2000; 11:153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vermeire K, Brokx JP, Wuyts FL, et al. Quality-of-life benefit from cochlear implantation in the elderly. Otol Neurotol 2005; 26:188–195. [DOI] [PubMed] [Google Scholar]
- 7.Uscher-Pines L, Mehrotra A. Analysis of Teladoc use seems to indicate expanded access to care for patients without prior connection to a provider. Health Affairs 2014; 33:258–264. [DOI] [PubMed] [Google Scholar]
- 8.Darkins A, Foster L, Anderson C, et al. The design, implementation, and operational management of a comprehensive quality management program to support national telehealth networks. Telemed J E Health 2013; 19:557–564. [DOI] [PubMed] [Google Scholar]
- 9.Mashima PA, Doarn CR. Overview of telehealth activities in speech-language pathology. Telemed J E Health 2008; 14:1101–1117. [DOI] [PubMed] [Google Scholar]
- 10.Kuzovkov V, Yanov Y, Levin S, et al. Remote programming of MED-EL cochlear implants: users’ and professionals’ evolution of the remote programming experience. Acta Otolaryngol 2014; 134:709–716. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez C, Ramos A, Falcon JC, et al. Use of telemedicine in the remote programming of cochlear implants. Cochlear Implants Int 2010; 11:461–464. [DOI] [PubMed] [Google Scholar]
- 12.Hughes ML, Goehring JL, Baudhuin JL, et al. Use of telehealth for research and clinical measures in cochlear implant recipients: a validation study. J Speech Lang Hear Res 2012; 55:1112–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samuel PA, Goffi-Gomez MVS, Bittencourt AG, et al. Remote programming of cochlear implants. CoDAS 2014; 26:481–486. [DOI] [PubMed] [Google Scholar]
- 14.Peterson GE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Disord 1962; 27:62–70. [DOI] [PubMed] [Google Scholar]
- 15.Gatehouse S, Noble W. The speech, spatial and qualities of hearing scale (SSQ). Int J Audiol 2004; 43:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thornton AR, Raffin MJM. Speech-discrimination scores modeled as a binomial variable. J Speech Hearing Res 1978; 21:507–513. [DOI] [PubMed] [Google Scholar]
- 17.McElveen JT, Blackburn EL, Green JD, et al. Remote programming of cochlear implants: a telecommunications model. Otol Neurotol 2010; 31:1035–1040. [DOI] [PubMed] [Google Scholar]
- 18.Wasowski A, Skarzynski PH, Lorens A, et al. Remote fitting of cochlear implant system. Cochlear Implants Int 2010; 11:489–492. [DOI] [PubMed] [Google Scholar]
- 19.Wesarg T, Wasowski A, Skarzynski H, et al. Remote fitting in nucleus cochlear implant recipients. Acta Otolaryngol 2010; 130:1379–1388. [DOI] [PubMed] [Google Scholar]
- 20.Eikelboom RH, Jayakody D, Swanepoel DW, et al. Validation of remote mapping of cochlear implants. J Telemed Telecare 2014; 20:171–177. [DOI] [PubMed] [Google Scholar]
- 21.Ramos A, Rodriguez C, Martinez-Beneyto P, et al. Use of telemedicine in the remote programming of cochlear implants. Acta Otolaryngol 2009; 129:533–540. [DOI] [PubMed] [Google Scholar]
- 22.Goehring JL, Hughes ML. Measuring sound processor threshold levels for pediatric cochlear implant recipients using conditioned play audiometry via telepractice. J Speech Lang Hearing Res 2017; 60:732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]


