Abstract
Two HIV-infected individuals on second-line atazanavir-based antiretroviral therapy presented with neuropsychiatric symptoms. Cerebrospinal fluid HIV RNA was higher than plasma HIV RNA and antiretroviral regimens’ optimization led to prompt resolution of symptoms in one. Patients on second-line atazanavir-based antiretroviral therapy with documented previous treatment failure may be at risk of symptomatic cerebrospinal fluid escape.
INTRODUCTION
Cerebrospinal fluid (CSF) HIV escape in individuals on antiretroviral therapy (ART) is defined as a detectable CSF HIV RNA with a concurrent undetectable plasma level, or CSF HIV RNA higher (any level, 0.5 or 1 log) than plasma HIV RNA. It has been reported in 0.7–27.4% asymptomatic HIV-infected individuals on ART [1].
Symptomatic CSF escape has been described in more than 100 individuals but never in limited resource countries. Patients usually present with subacute neurological symptoms, CSF HIV RNA higher than plasma levels and with resistance associated mutations (RAMs). Risk factors include low nadir CD4 cell count, incomplete plasma viral suppression and poor adherence [2, 3]. Two recent case series from India and one large cohort suggest that it may be more frequent in patients on protease inhibitors (PIs), but this may be biased by the presence of RAMs [4–6]. Treatment is usually with optimization of ART through choosing drugs according to genotype resistance test results and their central nervous system penetration/effectiveness score (CPE).
Here; we describe two Ugandan patients on PIs based second line ART for at least 7 years at the time of presentation with neuropsychiatric symptoms associated with uncontrolled central nervous system HIV compartmental replication.
CASE 1
A 62-year-old male, HIV-infected on second-line ART for 7 years with a nadir CD4 T-cell count of 158 cells/μL. He was referred to the Infectious Diseases Institute, Kampala from a mental health unit for further assessment of his neurocognitive decline. He had been well till 8 months prior to his referral when he started having; memory impairment, visual hallucinations, sleepiness, unstable gait and being scared of falling. He had no history of headache or head trauma. He was taking Donepezil 2.5 mg daily for early onset dementia for 8 months by the time of his referral. He had no history of substance abuse and no family history of mental illness. His cognitive decline had impaired his daily activities. He stopped working due to memory impairment and was unable to move unsupported. He needed help for his daily activities including; washing himself, dressing up and ambulation. He could not recognize anyone in his family including his wife and children.
He initiated ART 18 years prior to his referral. The antiretroviral regimen he had taken for the first 4 years was unknown after which he was switched to tenofovir, lamivudine and efavirenz which he took with inconsistent adherence for 7 years before being switched to second line therapy due to viral failure (plasma HIV RNA; 4014 copies/mL). His second line regimen consisted of zidovudine, lamivudine and ritonavir-boosted atazanavir. He achieved plasma virological suppression (HIV RNA < 75 copies/mL) in the first 5 years of this second line regimen. He had no prior history of an AIDS-defining opportunistic illness.
Clinically, he was slightly overweight (BMI 26 kg/m2), afebrile, the blood pressure was 158/96 mmHg and a pulse of 96 beats per minute; his speech was characterized by confabulating, challenges in maintaining attention, had intentional tremors and a shuffling gait.
A lumbar puncture was performed as part of his investigations for his neurocognitive decline. CSF had normal opening pressure at 11cmH2O, with a normal white cell count (<5 cells/μL), normal total protein (12 mg/dL), an elevated glucose level of 11.7 mmol/L as compared to plasma 7.6 mmol/L and the veneral disease research laboratory (VDRL) test was negative. The CSF HIV RNA was 4 895 copies/mL (3.69 log units) and plasma HIV RNA was 277 copies/mL. Other laboratory findings included; CD4 T-cell count of 503 cells/μL normal renal and liver function tests, hyponatremia (127 mmol/L), HbA1C 6.6%, and elevated fasting triglycerides (2.2 mmol/L).
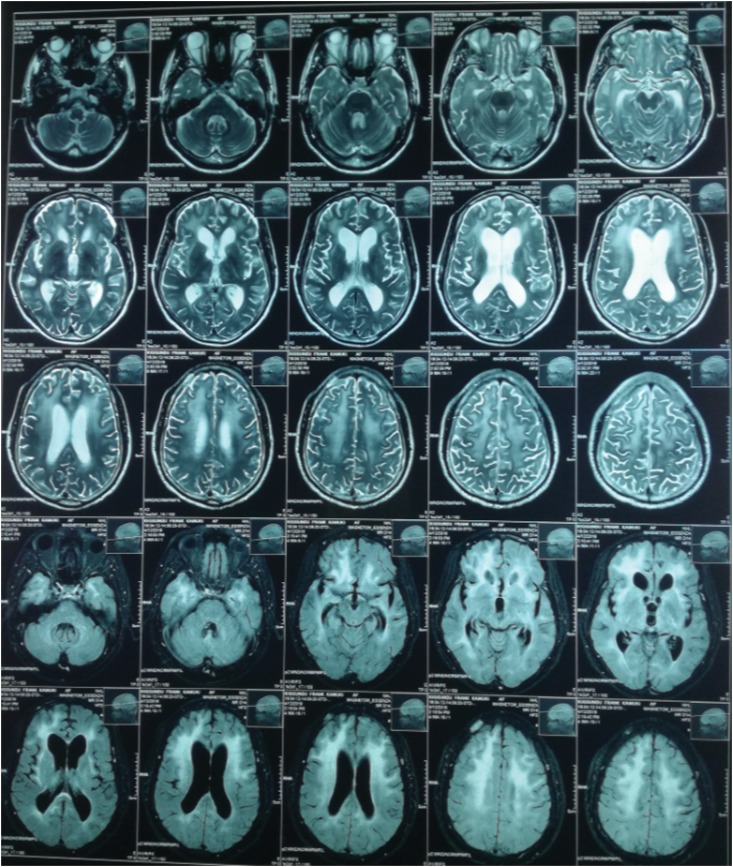
He had a normal brain CT scan. However, magnetic resonance imaging (MRI) of his brain showed neuroparenchymal atrophy and hyperintensities involving bilateral periventricular white matter; features suggestive of HIV encephalitis (Fig. 1).
Figure 1:
Magnetic resonance image of the brain: symmetric T2/FLAIR hyperintensities involving bilateral periventricular white matter, fronto-temporo-parietal sub-cortical white matter and centrum semiovale. Neuroparenchymal atrophy. Dilated and prominent cerebral ventricles, sulci and cisterns. Features usually representative of HIV associated encephalitis.
Plasma drug sensitivity tests done showed several RAMs: I50L and others (L10V, I13V, K14R, I15V, E35D, M36I, R41K, R57K, I64V, H69K, T74A, L89M) in the protease gene, several mutations in the reverse transcriptase gene both for NRTIs (T215F, K219E, D67H, K70R, L74I, M184V) and NNRTIs (K103N, V179T, P225H). CSF HIV drug sensitivity testing was not available.
Basing on the resistance profile results, his ART regimen was changed to dolutegravir, lamivudine and ritonavir boosted lopinavir. This increased his central nervous penetration effectiveness score from 5 to 8. He is registering on-going improvement in his subsequent follow-up visits. Gait normalizing, memory impairment resolving within 2 months of follow up. His sodium improved to 137 mmol/L. His plasma HIV RNA was suppressed within a month of ART change. He was now able to move without support and recognize family members. He returned to work with no one escorting him in his third month of ART change. Donepezil was tapered off.
CASE 2
A 58-year-old female diagnosed with HIV and cryptococcal meningitis 12 years prior to her admission to Mulago National Referral Hospital. Her initial ART regimen included zidovudine, lamivudine and nevirapine and had a nadir CD4 T-cell count of 263 cell/μL (current). Nine years following ART initiation, she was diagnosed with sputum positive pulmonary tuberculosis for which she received anti-tuberculous therapy for 6 months and was declared cured. Following her pulmonary tuberculosis treatment, she was found to have virologic failure (plasma HIV RNA of 170 694 copies/mL) and was switched to tenofovir, lamivudine and ritonavir boosted atazanavir. Plasma HIV RNA was <75 copies/mL a year after her switch, and low viremia 2 years after the switch (HIV RNA; 251 copies/mL) and an elevated fasting blood sugar (8.9 mmol/L) was found for which metformin 500 mg daily was added to her treatment.
She was admitted to Mulago National Referral Hospital with urinary incontinence and irritability but no constitutional symptoms. Of note, she had progressively had lower limb weakness over a year resulting in inability to move, and had been bedridden for 3 months. This was associated with short term memory loss and abnormal picking on herself articles that were never seen by other individuals and unusual posturing of her neck (mannerisms). She was always in and out of confusion for over 2 months.
Clinically, she had an axillary temperature of 36.2°C, fast regular normal volume pulse of 111 bpm, normal blood pressure 104/88 mmHg, saturating at 98% on room air, respiratory rate of 20 bpm. She had mannerisms and poor eye contact. She was paraplegic with a staggering gait on moving with support.
A lumbar puncture was performed; CSF was under normal opening pressure at 14 cmH2O with normal CSF protein 48 mg/dL, elevated white cell count 220 cells/μL 100% lymphocytes. Negative gram stain, cryptococcal antigen, MTB/Rif GeneXpert and ultra Xpert and no fungal or bacterial or mycobacterial growth on culture. Her CSF HIV RNA was 31 896 copies/mL (4.50 log units) with a plasma HIV RNA of 1804 copies/mL (3.26 log units). She was diagnosed with symptomatic CSF HIV escape. We await her plasma HIV drug sensitivity test results to optimize her ART.
DISCUSSION
We here report the first two cases of symptomatic CSF HIV escape from Uganda; it should be noted that CSF HIV RNA is not usually measured in the routine work up for unexplained neurological symptoms in HIV-infected individuals. In many resource limited countries, the application of World Health Organization guidelines may result into the ‘perfect’ scenario for the compartmental replication of HIV. The use of low genetic barrier first-line therapy, delayed antiretroviral switch due to infrequent viral load monitoring and at a higher cut off (1000 copies/mL) as well as the recycling of some nucleotide reverse transcriptase inhibitors (NRTIs) for second line treatment might favour selection of RAMs.
Of the PIs routinely available in Uganda, atazanavir has the least CPE score of 2 compared to lopinavir and darunavir each with a CPE score of 3 [7]. Atazanavir has been associated with more reports of CSF HIV escape than lopinavir [8]. In Uganda, however, atazanavir is preferred to lopinavir for second line antiretroviral regimens due to its affordability, gastrointestinal tolerability and reduced pill burden [9]. The presence of several RAMs may therefore expose patients to atazanavir monotherapy. Several studies reported the possibility of symptomatic CSF escape in patients with low nadir CD4 T-cell count on PI monotherapy. Low CD4 nadir seems the strongest risk factor for symptomatic CSF escape: in one the two Indian studies its incidence was 9.5 per 10 000 person-months in patients with nadir CD4 cell count below 200/μL versus 0.49 per 10 000 person-months in those ones with higher levels [4]. However, CSF escape is rare in individuals on robust highly active ART with atazanavir as one of the drugs unless they also have RAMs.
The two patients described had their HIV diagnoses 18 and 12 years ago with current CD4 counts of 504 and 263, respectively. They were both on ritonavir boosted atazanavir based ART. Their characteristics are similar to other patients previously described with CSF HIV escape [5, 10]. Cases 1 and 2 had a CSF/plasma HIV RNA log difference of 1.25 log units and 1.24 log units respectively characteristic of CSF HIV escape.
Symptomatic neurocognitive impairment has been reported in 35.1% of individuals with CSF HIV escape [11]. The more commonly described symptoms in individuals with CSF HIV escape include; memory impairment, gait disturbances, sleep disorders and inability to concentrate. These symptoms were noted in both patients we have described. Case 2 had worse symptoms compared to case 1. This could be as a result of overwhelming inflammation from the higher CSF HIV RNA level noted compared to that found in case 1.
In a setting where adults with HIV infection, even with ART, are more likely to have cognitive dysfunction than those without HIV infection [12], it is only prudent that CSF HIV RNA evaluation is added to their routine diagnostic evaluation. This will facilitate optimization of their ART to better central nervous system penetrating/effective regimens.
ACKNOWLEDGEMENTS
We acknowledge Drs Noela Orwawo, Ddungu Ahmed and Lumu Ivan for clinical advice. Opio Patrick and Babirye Dorothy for providing prompt laboratory services. We thank the patients’ families.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
No funding was got for this work.
CONSENT
Surrogate informed consent was sought from the next of kin of each of the patients described above.
REFERENCES
- 1. Joseph J, Cinque P, Colosi D, Dravid A, Ene L, Fox H, et al. . Highlights of the Global HIV-1 CSF Escape Consortium Meeting, 9 June 2016, Bethesda, MD, USA. J virus Erad 2016;2:243–50. [PMC free article] [PubMed] [Google Scholar]
- 2. Peluso MJ, Ferretti F, Peterson J, Lee E, Fuchs D, Boschini A, et al. . Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS 2012;26:1765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Canestri A, Jaureguiberry S, Moulignier A, Amiel C, Peytavin G, Tubiana R, et al. . Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis 2010;50:773–8. [DOI] [PubMed] [Google Scholar]
- 4. Patel AK, Patel KK, Gohel S, Kumar A, Letendre S. Incidence of symptomatic CSF viral escape in HIV infected patients receiving atazanavir/ritonavir (ATV/r)-containing ART: a tertiary care cohort in western India. J Neurovirol 2018;4:498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mukerji SS, Misra V, Lorenz D, Cervantes-Arslanian AM, Lyons J, Chalkias S, et al. . Temporal patterns and drug resistance in CSF viral escape among ART-experienced HIV-1 infected adults. J Acquir Immune Defic Syndr 2017;75:246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dravid AN, Natrajan K, Kulkarni MM, Saraf CK, Mahajan US, Kore SD, et al. . Discordant CSF/plasma HIV-1 RNA in individuals on virologically suppressive antiretroviral therapy in Western India. Medicine 2018;97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Letendre S. Letendre 2011 HAND and HAART. Top Antivir Med [Internet] 2011;19:137–42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22156215. [PMC free article] [PubMed] [Google Scholar]
- 8. Smurzynski M, Wu K, Letendre S, Robertson K, Bosch RJ. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS 2011;25:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Uganda Ministry of Health Consolidated Guidelines for Prevention and Treatment of HIV in Uganda 2018; April. https://www.prepwatch.org/wp-content/uploads/2017/08/consolidated_guidelines_hiv_prevention_uganda.pdf
- 10. Bingham R, Ahmed N, Rangi P, Johnson M, Tyrer M, Green J. HIV encephalitis despite suppressed viraemia: a case of compartmentalized viral escape. Int J STD AIDS 2011;22:608–9. [DOI] [PubMed] [Google Scholar]
- 11. Mukerji SS, Misra V, Lorenz DR, Uno H, Morgello S, Franklin D, et al. . Impact of antiretroviral regimens on CSF viral escape in a prospective multicohort study of ART-experienced HIV-1 infected adults in the United States. Clin Infect Dis [Internet] 2018;2018:1–14. Available from: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciy267/4959767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakasujja N, Allebeck P, Agren H, Musisi S, Katabira E. Cognitive dysfunction among HIV positive and HIV negative patients with psychosis in Uganda. PLoS One 2012;7:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]