Abstract
A 64-year-old gentleman initially presented with nephrotic syndrome and membranous nephropathy with positive staining for C1q, which was suspicious for lupus membranous nephritis. Investigation led to the simultaneous diagnosis of colorectal cancer (CRC). The CRC was surgically excised and the patient’s nephrotic syndrome resolved. The patient subsequently presented with classic systemic lupus erythematosus (SLE) including positive serological markers, mouth-ulcers and a photosensitive maculopapular rash. Two months later the patient represented with an SLE flare encompassing the full-hand of renal-pulmonary syndrome and vasculitic-neuropathy, importantly at this presentation occult recurrence of CRC was proven with tissue biopsy. Major histocompatibility class II haplotyping demonstrated HLA-DRB1*03, a known predisposition for SLE. This case depicts the scenario of tumour transformation triggering SLE development in a predisposed individual after an initial paraneoplastic manifestation in the form of membranous nephropathy (plus C1q). This supports the potential role of tumourgenesis in the development of SLE in a primed individual.
INTRODUCTION
The expression of neoantigens and the anti-tumour immune response which accompanies the malignant transformation of cells may elicit an immune response against self-antigens expressed by cancer cells leading to the development of rheumatological syndromes in predisposed individuals. We report a case of systemic lupus erythematosus (SLE) which presented as a progressive autoimmune disorder associated with metastatic colorectal cancer (CRC).
CASE REPORT
A 64-year-old gentleman with a history of ulcerative colitis (lost to follow-up) initially presented with nephrotic syndrome, characterized by gross generalized oedema, 8.74 g of proteinuria and glomerular red cells. A kidney biopsy diagnosed membranous glomerulonephritis and stained positive for IgG, C3 and C1q but negative for anti-phospholipase-A2-receptor antibody. The C1q stain was strongly suspicious for SLE-related membranous nephropathy [1, 2], however, serum was negative for anti-dsDNA and systemic symptoms of SLE were absent (Fig. 1). At this time, colonoscopy led to the diagnosis of CRC. A total colectomy was performed and revealed three left-sided colonic tumours and one lymph-node positive for metastatic tumour. As whole-body CT was negative for radiological metastatic disease, the final stage was T3N1aM0. The patient received two courses of post-adjuvant chemotherapy with XELOX (oxaliplatin, capecitabine), but withdrew from further treatment due to malaise. The nephrotic syndrome was managed medically with an angiotensin-converting-enzyme inhibitor (ACEi) and resolved following surgical resection of the CRC.
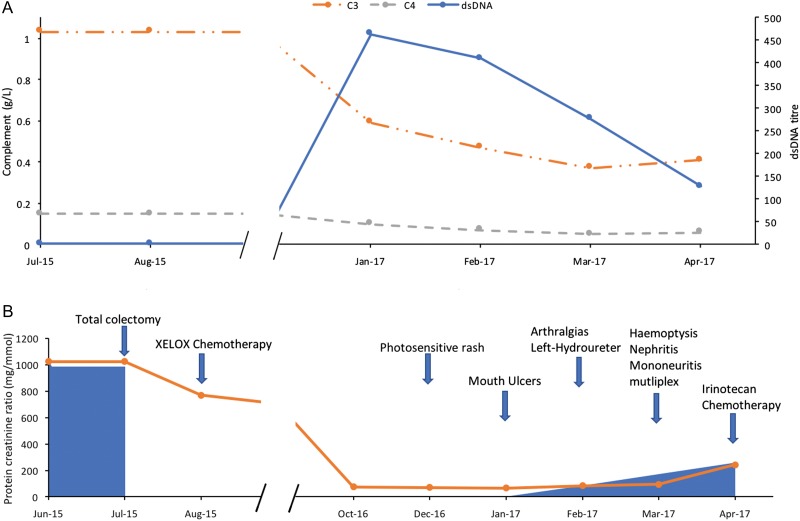
Figure 1:
Laboratory and clinical features of SLE according to time. (A) Solid blue line = anti-dsDNA antibody titre (IU/mL, normal <100 IU/mL), dashed grey line = C4 complement levels (g/L, normal: 0.16–0.47 g/L), dotted orange line = C3 complement levels (g/L, normal: 0.9–1.8 g/L). (B) Solid blue shade = apparent tumour volume, solid orange line = protein creatinine ratio (mg/mmol), blue arrows = development of relevant clinical symptoms and signs.
Seven months later, the patient presented with polyarthritis, mouth-ulcers, a photosensitive maculopapular rash on his face and trunk, palpable purpura in the lower extremities, strongly positive tests for ANA (1:2560) and anti-dsDNA (463IU/mL) and lymphopaenia. A diagnosis of SLE was made. Imaging at this time identified left-sided hydroureter and CRC recurrence was suspected, however, there was no demonstrable mass on CT or ultrasound. The SLE was treated with prednisolone and hydroxychloroquine.
Two months later, the patient presented with anaemia, lymphopaenia, haemoptysis, left-sided foot-drop, right-upper limb dysaesthesia, acute kidney injury, hypocomplementaemia and progressive left-sided hydroureter. The patient had an episode of diffuse alveolar haemorrhage requiring ICU admission. Bronchoalveolar lavage revealed blood throughout the bronchial tree, pneumocystis jiroveci PCR and cultures were negative. Nerve conduction studies were consistent with mononeuritis multiplex. A kidney biopsy performed for worsening kidney function demonstrated membranous lupus nephritis class V with extensive mesangial and subepithelial immune-deposits. Although CT failed to reveal a cause for the hydronephrosis, a PET-scan demonstrated a left peri-ureteric metastasis and fluorodeoxyglucose avid mesorectal and abdominal wall lymph-nodes. Lymph-node biopsy confirmed CRC recurrence. Major histocompatibility class II haplotyping was positive for HLA-DRB1*03, which confers a strong susceptibility to SLE [3]. Serum testing for ANCA was negative at initial diagnosis and subsequent representation.
DISCUSSION
We hypothesize that this case represents SLE triggered by tumour antigen, which is supported by the compelling relationship between SLE progression, CRC dissemination and the genetic predisposition of the individual. Isolated case reports have described SLE in association with leukaemia, cholangiocarcinoma, breast, ovarian and thyroid cancer [4–6].
Several theories for the pathological mechanism of paraneoplastic rheumatological diseases have been proposed [7, 8]. First, rheumatological diseases may result from the direct effect of cytotoxins produced by malignant cells that trigger inflammation at target sites such as the synovium. Second, both the rheumatological disease and malignancy may be independent consequences of a common underlying trigger such as viral infection or chemical exposure. Third, paraneoplastic rheumatological diseases may develop secondary to the anti-tumour immune response which elicits the production of autoantibodies against autoantigens, including self-antigens expressed by apoptotic tumour cells. The presence of autoantibodies to nuclear proteins, double-stranded DNA and other tissue associated antigens lends support to this third mechanism. The haplotype of the individual detailed in this report may have predisposed him to such a sensitizing immunological event following the development of his CRC leading to his initial membranous nephropathy, with positive C1q and negative anti-phospholipase-A2-receptor antibody suggesting early seronegative SLE [1], a known clinical phenomena [9].
In most instances, paraneoplastic rheumatological syndromes tend to improve or resolve completely with treatment of the underlying malignancy. This is straightforward, when the cancer may be removed surgically, however, in metastatic disease the appropriate therapeutic strategy requires judicial use of immunosuppression which may inadvertently accelerate cancer development or recurrence. In our case the improvement of the patients’ membranous glomerulonephritis following CRC resection supports this finding, although his treatment with adjuvant-chemotherapy and an ACEi are confounders. His subsequent recurrent metastatic CRC with secondary exposure to tumour antigen most likely led to the propagation of his autoimmune condition, eventually culminating in fulminant SLE with the full hand of renal-pulmonary syndrome, vasculitic-neuropathy and positive serology. Monitoring this patient as he proceeds with CRC therapy will provide further insight, particularly as immunosuppression for the symptoms of SLE may influence CRC biology.
ACKNOWLEDGEMENTS
Nil.
CONFLICT OF INTEREST STATEMENT
No conflicts of interest.
FUNDING
The authors have received no financial support for the research, authorship and/or publication of this article.
ETHICAL APPROVAL
No specific ethical approval was required.
CONSENT
Written informed consent was obtained from the patient for the submission of this manuscript.
GUARANTOR
Matthew James Rees is the guarantor of this article.
REFERENCES
- 1. Jennette JC, Hipp CG. Immunohistopathologic evaluation of C1q in 800 renal biopsy specimens. Am J Clin Pathol 1985;83:415–20. [DOI] [PubMed] [Google Scholar]
- 2. Jennette JC, Iskandar SS, Dalldorf FG. Pathologic differentiation between lupus and nonlupus membranous glomerulopathy. Kidney Int 1983;24:377–85. [DOI] [PubMed] [Google Scholar]
- 3. Niu Z, Zhang P, Tong Y. Value of HLA-DR genotype in systemic lupus erythematosus and lupus nephritis: a meta-analysis. Int J Rheum Dis 2015;18:17–28. [DOI] [PubMed] [Google Scholar]
- 4. Racanelli V, Prete M, Minoia C, Favoino E, Perosa F. Rheumatic disorders as paraneoplastic syndromes. Autoimmunity Rev 2008;7:352–8. [DOI] [PubMed] [Google Scholar]
- 5. Sola D, Sainaghi PP, Pirisi M. Paraneoplastic systemic lupus erythematosus associated with papillary thyroid carcinoma. Br J Hosp Med (Lond) 2013;74:530–1. [DOI] [PubMed] [Google Scholar]
- 6. Gonzalez Amores Y, Hernando Rebollar S, Casado Bernabeu A. Lupus as a paraneoplastic manifestation of cholangiocarcinoma. Rev Esp Enferm Dig 2016;108:292. [DOI] [PubMed] [Google Scholar]
- 7. Szekanecz E, Andras C, Sandor Z, Antal-Szalmas P, Szanto J, Tamasi L, et al. Malignancies and soluble tumor antigens in rheumatic diseases. Autoimmunity Rev 2006;6:42–7. [DOI] [PubMed] [Google Scholar]
- 8. Bernatsky S, Ramsey-Goldman R, Clarke A. Malignancy and autoimmunity. Curr Opin Rheumatol 2006;18:129–34. [DOI] [PubMed] [Google Scholar]
- 9. Beck LH Jr., Salant DJ. Treatment of membranous lupus nephritis: where are we now? Clin J Am Soc Nephrol 2009;20:690–1. [DOI] [PubMed] [Google Scholar]