Abstract
Background:
Efficacy has been proven for vortioxetine in short-term and long-term treatment of major depressive disorder (MDD), with broad beneficial effects on emotional, physical and cognitive symptoms. Limited specific data on the effects of vortioxetine on depression-related physical symptoms have been published.
Methods:
A meta-analysis was carried out of five short-term multinational, double-blind, placebo-controlled studies. These studies were conducted in a total of 2105 adult MDD outpatients (18–75 years) with a major depressive episode of ⩾3 months’ duration. Only patients treated with a dose of 5 or 10 mg vortioxetine (therapeutic doses) or placebo were included in this analysis. Efficacy assessment of vortioxetine on the physical symptoms of depression included all items of the Hamilton Depression Scale (HAM-D) assessing physical symptoms, and all somatic items in the Hamilton Anxiety Scale (HAM-A). A subgroup analysis in MDD patients with coexisting anxiety symptoms (i.e. those with a HAM-A ⩾20 at baseline) was also performed.
Results:
A significant improvement (p<0.05) of vortioxetine versus placebo was observed on all HAM-D items measuring physical symptoms, except for the somatic gastrointestinal symptoms and loss of weight items. Significant effects were also observed on the HAM-A somatic items: general somatic symptoms, gastrointestinal symptoms, and autonomic symptoms. In patients with a high baseline level of anxiety, a significant effect of vortioxetine was also observed on the physical symptoms of depression.
Conclusions:
These analyses indicate that patients with MDD, including those with a high level of anxiety symptoms, have significant improvements in MDD-associated physical symptoms when treated with vortioxetine.
Keywords: Vortioxetine, major depressive disorder, physical symptoms
Introduction
Major depressive disorder (MDD) is characterized by multiple debilitating symptoms, spanning emotional, physical and cognitive domains, with serious consequences for patients’ psychosocial and occupational functioning. Core symptoms of MDD include a persistent disturbance of mood and loss of interest/pleasure in most daily activities (Otte et al., 2016). Patients may also experience physical symptoms such as fatigue/low energy, sleep and appetite disturbances, muscle tension, headaches, and general symptoms of pain, and cognitive symptoms such as impaired concentration, poor memory and difficulty in making decisions (American Psychiatric Association, 2013; Singh and Gotlib, 2014).
Full functional recovery is the ultimate treatment goal for patients with MDD, but many patients do not achieve even the more limited goal of full remission of depressive symptoms (Papadimitropoulou et al., 2017): meta-analyses of controlled clinical studies indicate that only 30–50% of patients achieve remission after 6–8 weeks of antidepressant treatment (Warden et al., 2007). Patients in partial remission may still have debilitating symptoms such as insomnia, anxiety, anhedonia, apathy and memory/concentration difficulties (Fava et al., 2006; Mattingly et al., 2016; McClintock et al., 2011). The presence of residual depressive symptoms partly accounts for the prevention of full functional recovery (Judd et al., 1998), and predicts earlier relapse, recurrence and a more chronic course of illness (Judd et al., 1998, 2000; Kennedy and Paykel, 2004).
Physical symptoms are commonly observed in patients with MDD. Depressive disorder with physical (somatic) symptoms may be the most common presentation of depression in inpatient and outpatient settings (Kapfhammer, 2006). In studies reported by Hamilton (1989) and Kirmayer et al. (1993), about 80–90% of patients experienced physical symptoms, especially somatic anxiety and fatigue (Hamilton, 1989; Kirmayer et al., 1993). Further, in a meta-analysis of 14 studies of patients with depression, 65% reported pain symptoms (Bair et al., 2003). In addition, in a separate study by Fava and colleagues (2006), physical symptoms of sleepiness/sedation were reported by over 40% of patients who responded to and were continuing with long-term antidepressant treatment (Fava et al., 2006).
The common clinical focus on the psychological symptoms of depression may obscure diagnosis in patients primarily presenting with physical symptoms, emphasizing the importance of careful clinical examination to avoid missing a diagnosis of depression (Rijavec and Grubic, 2012). The presence of somatic symptoms has a detrimental effect on the course and response to treatment (Greden, 2003). Evidence also suggests that patients with somatic symptoms have a more chronic course of MDD and greater risk of comorbid anxiety disorders (Gerrits et al., 2012; Jaracz et al., 2016).
Vortioxetine has a multimodal mechanism of action (i.e., direct modulation of receptor activity and inhibition of the serotonin transporter) and has been approved for the treatment of MDD (Sanchez et al., 2015). The efficacy and safety of vortioxetine in MDD was established as part of an extensive clinical development programme, which comprised 17 short-term placebo-controlled studies, six open-label long-term extension studies and one long-term relapse–prevention study, involving more than 9700 patients and a total exposure of over 3450 patient-years (Baldwin et al., 2016a, 2016b; Florea et al., 2015; Melander et al., 2008).
Vortioxetine significantly improves depressive symptoms as measured by the Montgomery–Åsberg Depression Rating Scale (MADRS) or by the 24-item version of the Hamilton Depression Rating Scale (HAM-D) at doses between 5 and 20 mg daily (Kelliny et al., 2015). Pooled analyses of data from short-term studies reveal significantly higher response and remission rates with vortioxetine when compared with placebo (Berhan and Barker, 2014; Kelliny et al., 2015). Further, meta-analyses of effects of vortioxetine on the single items of the MADRS scale indicate its favourable effects across a broad range of depressive symptoms (Thase et al., 2016).
Favourable effects of vortioxetine extend beyond emotional symptoms. In short-term controlled studies within the 5–20 mg dose range, vortioxetine significantly improves cognitive function (executive function, processing speed and attention/concentration) as compared to placebo, as measured by the Digit Symbol Substitution Test in patients with MDD (Mahableshwarkar et al., 2015; McIntyre et al., 2016). In addition, meta-analyses of short-term (6–8 week) studies in the same dosing range indicate improved overall functioning and functional remission, as measured by the Sheehan Disability Scale in adult MDD patients (Boulenger et al., 2014; Florea et al., 2017; Wang et al., 2015), and significant and clinically meaningful improvements in health-related quality of life (Boulenger et al., 2014; Florea et al., 2015).
So far, only limited data have been published specifically addressing the effects of vortioxetine on depression-related physical symptoms. We therefore undertook post hoc analyses of data from five short-term, placebo-controlled studies of vortioxetine in patients with MDD. We chose these studies because both the 24-item HAM-D (Hamilton, 1960; Riskind et al., 1987) and the Hamilton Anxiety Rating Scale (HAM-A) (Hamilton, 1959a) were employed; these scales cover a broad range of physical symptoms, permitting a more detailed assessment of potential effects within this domain.
Materials and methods
Clinical studies: All short-term studies where efficacy of vortioxetine on both the HAM-D and HAM-A were investigated in a comparable adult MDD population were included in this analysis. This comprised five short-term (6- or 8-week duration), randomized, double-blind, placebo-controlled, multi-centre studies evaluating the efficacy of vortioxetine versus placebo in adults with MDD (Table 1). Study NCT00735709 (Henigsberg et al., 2012) investigated fixed doses of 1, 5 and 10 mg/day vortioxetine. Study NCT00839423 (Alvarez et al., 2012) investigated fixed doses of 5 and 10 mg/day vortioxetine. Study NCT00635219 (Baldwin et al., 2012b) investigated fixed doses of 2.5, 5 and 10 mg/day vortioxetine, study NCT00672958 (Jain et al., 2013) investigated a fixed dose of 5 mg/day vortioxetine and study NCT00672620 (Mahableshwarkar et al., 2013) investigated fixed doses of 2.5 and 5 mg/day vortioxetine. From these studies, only patients treated with a dose within the therapeutic dose range (i.e. 5 or 10 mg/day) or placebo were considered for this analysis. All studies employed the MADRS, the 24-item HAM-D and the HAM-A as efficacy endpoints. The study population was defined as adults (aged 18‒75 years) with a primary diagnosis of MDD according to DSM IV-TR criteria, a current major depressive episode (MDE) of ⩾3 months’ duration (confirmed using the Mini International Neuropsychiatric Interview (Sheehan et al., 1998)) and a Montgomery–Åsberg Depression Rating Scale (MADRS) total score of ⩾ 22, 26 or 30 at screening and baseline visits (Alvarez et al., 2012; Baldwin et al., 2012b; Henigsberg et al., 2012; Jain et al., 2013; Mahableshwarkar et al., 2013).
Table 1.
Summary characteristics of the five short-term, placebo-controlled studies of vortioxetine in patients with MDD included in the meta-analyses.
| NCT identifier | Treatment period (weeks) | Dose(s) of vortioxetine tested (mg/day)a | Key inclusion criteria for MDD | Literature citation |
|---|---|---|---|---|
| NCT00839423 | 6 | 5 or 10 | Between 18 and 65 years MADRS total score ⩾30 |
Alvarez et al. 2012 |
| NCT00635219 | 8 | 2.5, 5 or 10 | Between 18 and 75 years MADRS total score ⩾26 |
Baldwin et al. 2012b |
| NCT00735709 | 8 | 1,5 or 10 | Between 18 and 75 years MADRS total score ⩾26 |
Henigsberg et al. 2012 |
| NCT00672958 | 6 | 5 | Between 18 and 75 years MADRS total score ⩾30 |
Jain et al. 2013 |
| NCT00672620 | 8 | 2.5 or 5 | Between 18 and 75 years MADRS total score ⩾22 |
Mahableshwarkar et al. 2013 |
For the post hoc meta-analyses, only the 5 and 10 mg doses, approved as per current prescribing information, were included.
Detailed descriptions of the five clinical trial designs, methods and primary efficacy analyses have been published (Alvarez et al., 2012; Baldwin et al., 2012b; Henigsberg et al., 2012; Jain et al., 2013; Mahableshwarkar et al., 2013). All trials were conducted according to the principles of Good Clinical Practice (International Conference on Harmonization, 1996) the Declaration of Helsinki (World Medical Association, 1964 and 2008), and adhered to the requirements of all applicable local or regional regulations.
The meta-analysis did not include the long-term open-label studies, nor the dedicated study in elderly patients with MDD, or the study conducted for regulatory submission in Japan. The open-label studies (Alam et al., 2014; Baldwin et al., 2012a; Florea et al., 2012; Jacobsen et al., 2015a) were excluded as these by definition do not have a comparator and thus prevent establising efficacy. The dedicated elderly study (Katona et al., 2012) and the Japanese study (Inoue et al., 2018) were excluded as they did not include comparable study populations to the five global studies conducted in an adult MDD population; thus preventing a pooled analysis.
Clinical assessments: Complete details of all study assessments in the five studies are provided in Alvarez et al. (2012), Baldwin et al. (2012b), Henigsberg et al. (2012), Jain et al. (2013) and Mahableshwarkar et al. (2013). This analysis is based on HAM-D items assessing the physical symptoms of depression, namely insomnia (early (item 4), middle (item 5) and late (item 6)), anxiety somatic (item 11), somatic symptoms gastrointestinal (item 12), somatic symptoms general (include both muscular pain, headache and lack of energy) (item 13), genital symptoms (include both loss of libido and menstrual disturbances) (item 14) and loss of weight (item 16) (Fava, 2003; Hamilton, 1960; Hung et al., 2006), as well as the physical symptoms measured by the HAM-A, namely items of general somatic symptoms (muscular pain) (item 7), general somatic symptoms (sensory) (item 8), cardiovascular symptoms (item 9), respiratory symptoms (item 10), gastrointestinal symptoms (item 11), genitourinary symptoms (item 12) and autonomic symptoms (item 13) (Hamilton, 1959b).
Statistical analysis: To investigate the efficacy of vortioxetine on the physical symptoms of depression, a meta-analysis was performed, including data from all five studies. The statistical analyses were based on the full analysis set (FAS), as defined in each study separately. All statistical tests were two-sided. Nominal p-values less than 5% were considered statistically significant. Changes from baseline in HAM-D and HAM-A single items were, for each study and item separately, analyzed using a mixed model for repeated measurements (MMRM) approach, including treatment and site as factors and baseline value as covariate, with treatment-by-week and baseline-by-week interactions, and using an unstructured variance–covariance matrix. The MMRM analyses included all dose groups included in each study, but results were re-analyzed to align the model across studies before applying the meta-analysis. Standard random effects meta-analyses were carried out using the HAM-D and HAM-A results from the studies, and standardized mean differences to placebo were derived. The standardized estimates (SES) were obtained by applying a Cohen’s D approach in the MMRM setting, with the relevant denominator being derived directly from the MMRM standard error to obtain the same p-values for the SES as for the original estimates.
The same meta-analysis was repeated but only including the data from the three studies – NCT00839423, NCT00635219 and NCT00735709 – that separated from placebo on the primary endpoint using the same analysis applied in our research, namely MMRM (Alvarez et al., 2012; Baldwin et al., 2012b; Henigsberg et al., 2012). As the 10 mg dose was only investigated in these three studies, the results for the 10 mg group are identical to those in the meta-analysis considering all five studies. In addition, patients with a significant level of anxiety symptoms (i.e. those with a HAM-A ⩾20 at baseline) were analyzed as a subgroup. MDD patients with coexisting anxiety symptoms are not only common but typically also more difficult to treat than MDD patients without prominent anxiety, hence form a clinically relevant subgroup for this analysis (Hirschfeld, 2001).
The trial was registered at ClinicalTrials.gov identifier: NCT00839423, NCT00635219, NCT00735709, NCT00672958, NCT00672620.
Results
Baseline characteristics: Across studies, a total of 2105 patients were randomized to double-blind treatment with placebo (n = 850), vortioxetine 5 mg (n = 861) or vortioxetine 10 mg (n = 394). Of these, 2089 received study medication and 1729 completed the 6/8-week treatment period. Premature discontinuation rates were 17.7%, 16.6% and 17.7% in the placebo, vortioxetine 5 mg and vortioxetine 10 mg groups, respectively, across studies.
Demographic and baseline clinical characteristics of the study population in all five studies are summarized in Table 2. Baseline demographic and clinical characteristics were similar across treatment groups. In the five studies patients had a mean age of approximately 44 years, all groups comprised a greater proportion of women than men and patients were predominantly Caucasian. The mean baseline MADRS total score and HAM-A total score were approximately 32 and 21, respectively, across all treatment groups in all five studies indicating a patient population with moderate to severe MDD and a significant level of anxiety.
Table 2.
Demographic and baseline characteristics (all randomized patients).
| Variable |
All studies
(NCT00839423, NCT00635219, NCT00735709, NCT00672958, NCT00672620) |
|||
|---|---|---|---|---|
| Placebo (n=850) |
Vortioxetine 5 mg (n=861) |
Vortioxetine 10 mg (n=394) |
Total (n=2105) |
|
| Age, years | ||||
| Mean (SD) | 43.3 (12.6) | 43.9 (13.0) | 44.9 (12.9) | 43.8 (12.8) |
| Range | 18-75 | 18-75 | 18-75 | 18-75 |
| Gender, n (%) | ||||
| Male | 333 (39.2) | 306 (35.5) | 141 (35.8) | 780 (37.1) |
| Female | 517 (60.8) | 555 (64.5) | 253 (64.2) | 1325 (62.9) |
| Race (grouped) n (%) | ||||
| Caucasian | 673 (79.2) | 662 (76.9) | 321 (81.5) | 1656 (78.7) |
| Black | 112 (13.2) | 125 (14.5) | 6 (1.5) | 243 (11.5) |
| Asian | 61 (7.2) | 69 (8.0) | 64 (16.2) | 194 (9.2) |
| American | 3 (0.4) | 1 (0.1) | 0 | 4 (0.2) |
| 1 (0.1) | 2 (0.2) | 3 (0.8) | 6 (0.3) | |
| BMI, kg/m 2 | ||||
| Mean (SD) | 28.3 (6.9) | 28.4 (7.5) | 25.5 (4.8) | 27.8 (6.9) |
| No. previous MDEs | ||||
| Mean (SD) | 2.3 (2.6) | 2.4 (3.1) | 1.7 (2.1) | 2.2 (2.7) |
| Range | 0–23 | 0–45 | 0–20 | 0–45 |
| Duration of current MDE, weeks | ||||
| Mean (SD) | 35.5 (57.4) | 40.8 (92.0) | 30.6 (64.3) | 36.8 (74.7) |
| MADRS total score | ||||
| Mean (SD) | 32.3 (4.0) | 32.6 (4.1) | 32.3 (3.7) | 32.4 (4.0) |
| CGI-S score | ||||
| Mean (SD) | 4.8 (0.7) | 4.8 (0.7) | 4.9 (0.7) | 4.8 (0.7) |
| HAM-D, mean (SD) | ||||
| Item 4: Insomnia Early1 | 1.5 (0.7) | 1.5 (0.8) | 1.5 (0.7) | 1.5 (0.8) |
| Item 5: Insomnia Middle2 | 1.5 (0.7) | 1.5 (0.7) | 1.5 (0.7) | 1.5 (0.7) |
| Item 6: Insomnia Late3 | 1.4 (0.8) | 1.3 (0.8) | 1.3 (0.7) | 1.3 (0.8) |
| Item 11: Anxiety Somatic4 | 1.6 (0.8) | 1.6 (0.8) | 1.7 (0.7) | 1.6 (0.8) |
| Item 12: Somatic Symptoms: Gastrointestinal5 |
0.8 (0.6) | 0.8 (0.6) | 0.8 (0.6) | 0.8 (0.6) |
| Item 13: Somatic Symptoms: General6 | 1.6 (0.6) | 1.6 (0.6) | 1.5 (0.6) | 1.6 (0.6) |
| Item 14: Genital Symptoms7 | 1.3 (0.8) | 1.3 (0.8) | 1.2 (0.8) | 1.3 (0.8) |
| Item 16: Loss of Weight8 | 0.3 (0.7) | 0.3 (0.7) | 0.5 (0.7) | 0.4 (0.7) |
| Total score (24 items) | 31.0 (5.5) | 31.5 (5.6) | 31.1 (5.5) | 31.2 (5.5) |
| HAM-A, Mean (SD) | ||||
| Item 7: Somatic Muscular9 | 1.2 (0.9) | 1.2 (0.9) | 1.3 (0.9) | 1.3 (0.9) |
| Item 8: Somatic Sensory10 | 0.8 (0.9) | 0.8 (0.9) | 1.1 (0.9) | 0.9 (0.9) |
| Item 9: Cardiovascular11 | 0.8 (0.9) | 0.7 (0.9) | 1.1 (0.9) | 0.8 (0.9) |
| Item 10: Respiratory12 | 0.7 (0.8) | 0.7 (0.8) | 0.9 (0.9) | 0.7 (0.8) |
| Item 11: Gastrointestinal13 | 1.0 (0.9) | 1.0 (0.9) | 1.3 (0.9) | 1.1 (0.9) |
| Item 12: Genitourinary14 | 1.5 (1.1) | 1.5 (1.0) | 1.5 (1.0) | 1.5 (1.0) |
| Item 13: Autonomic15 | 1.2 (0.9) | 1.1 (0.9) | 1.3 (0.9) | 1.2 (0.9) |
| Total score | 20.4 (6.3) | 20.3 (6.1) | 22.3 (6.6) | 20.7 (6.3) |
Item 4: Insomnia Early = complains of occasional difficulty falling asleep, i.e. more than ½ hour, or complains of nightly difficulty falling asleep;
Item 5: Insomnia Middle = patient complains of being restless and disturbed during the night or waking during the night;
Item 6: Insomnia Late = waking in early hours of the morning but goes back to sleep or unable to fall asleep again if getting out of bed;
Item 11: Anxiety Somatic = physiological concomitants of anxiety, such as gastrointestinal – dry mouth, wind, indigestion, diarrhoea, cramps, belching, cardiovascular (palpitations, headache), respiratory (hyperventilation, sighing, urinary frequency, sweating);
Item 12: Somatic Symptoms – Gastrointestinal = loss of appetite but eating without staff encouragement, heavy feelings in abdomen, difficulty eating without staff urging, requests or requires laxatives or medication for bowels or medication for GI symptoms;
Item 13: Somatic Symptoms – General = heaviness in limbs, back or head, backaches, headache, muscle aches, loss of energy and fatigability;
Item 14: Genital Symptoms = symptoms such as loss of libido or menstrual disturbances;
Item 16: Loss of Weight = probable weight loss associated with present illness or definite (according to patient) weight loss.
HAM-A: 9Item 7: Somatic Muscular = includes weakness, stiffness, soreness merging into real pain, which is more or less diffusely localized in the muscles;
Item 8: Somatic Sensory = includes increased fatigability and weakness merging into real functional disturbances of the senses;
Item 9: Cardiovascular = includes tachycardia, palpitations, oppression, chest pain, throbbing in the blood vessels and feelings of fainting;
Item 10: Respiratory = includes feelings of constriction or contraction in throat or chest, dyspnoea merging into choking sensations and sighing respiration;
Item 11: Gastrointestinal = includes difficulties in swallowing, “sinking” sensation of the stomach, dyspepsia (heartburn or burning sensations in the stomach, abdominal pains related to meals, fullness, nausea and vomiting), abdominal rumbling and diarrhoea;
Item 12: Genitourinary = includes non-organic or psychic symptoms such as frequent or more pressing passing of urine, menstrual irregularities, anorgasmia, dyspareunia, premature ejaculation, loss of erection;
Item 13: Autonomic = includes dryness of mouth, blushing or pallor, sweating and dizziness.
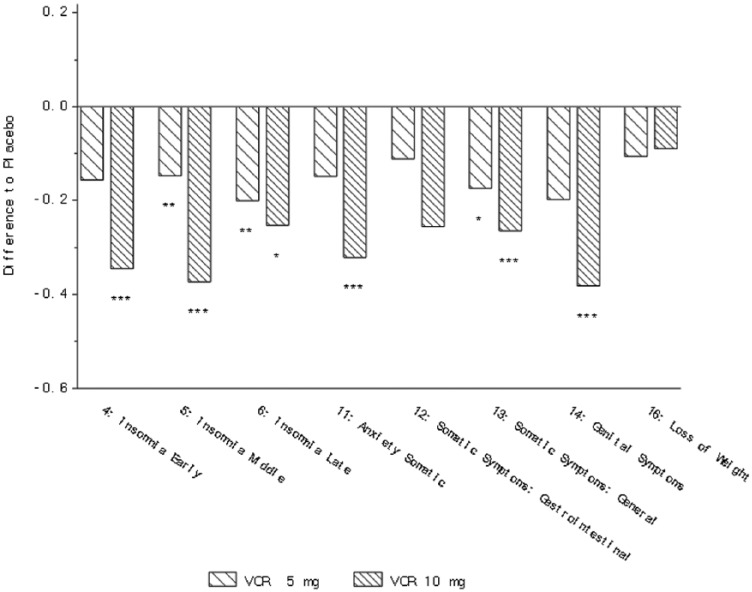
Clinical outcomes: In the analysis of the five studies (NCT00839423, NCT00635219, NCT00735709, NCT00672958 and NCT00672620), a significant effect of vortioxetine versus placebo was observed in change from baseline on the HAM-D items of early insomnia (10 mg), middle and late insomnia (5 and 10 mg), anxiety somatic (10 mg), somatic symptoms general (5 and 10 mg) and genital symptoms (10 mg) (Table 3, Figure 1). For physical symptoms as measured by the HAM-A scale, a significant effect of vortioxetine versus placebo was observed on the somatic muscular item (5 mg), genitourinary item (5 and 10 mg) and autonomic item (10 mg) (Table 4). In the subgroup of MDD patients with a high baseline level of anxiety, a significant effect of vortioxetine versus placebo was observed on the HAM-D scale for insomnia early and middle (5 and 10 mg), insomnia late (5 mg), anxiety somatic (5 and 10 mg), somatic symptoms gastrointestinal (5 mg), somatic symptoms general (5 and 10 mg) and genital symptoms (5 and 10 mg) (Table 5). For the HAM-A scale a significant effect was observed on the somatic muscular (5 mg) and genitourinary items (5 and 10 mg) in patients with coexisting anxiety (Table 5).
Table 3.
Meta-analysis of change from baseline in HAM-D single items at week 6/8 (FAS, MMRM, SES).
| Item | Treatment a |
All studies analysis
(NCT00839423, NCT00635219, NCT00735709, NCT00672958, NCT00672620) |
Three studies analysis
(NCT00839423, NCT00635219, NCT00735709) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | ∆ Placebo | SE | p-value |
Heterogeneity
p-value |
N | ∆ Placebo | SE | p-value |
Heterogeneity
p-value |
||
| 4: Insomnia Early b | Placebo | 691 | · | · | · | 338 | · | · | · | ||
| VOR 5 mg | 714 | −0.16 | 0.09 | 0.086 | 0.025 | 350 | −0.28 | 0.08 | <0.001 | 0.358 | |
| VOR 10 mg | 324 | −0.35 | 0.08 | <0.001 | 0.386 | 324 | −0.35 | 0.08 | <0.001 | 0.386 | |
| 5: Insomnia Middle b | Placebo | 691 | · | · | · | 338 | · | · | · | ||
| VOR 5 mg | 714 | −0.15 | 0.05 | 0.006 | 0.576 | 350 | −0.23 | 0.08 | 0.003 | 0.985 | |
| VOR 10 mg | 324 | −0.37 | 0.08 | <0.001 | 0.815 | 324 | −0.37 | 0.08 | <0.001 | 0.815 | |
| 6: Insomnia Late b | Placebo | 691 | 338 | ||||||||
| VOR 5 mg | 714 | −0.20 | 0.07 | 0.002 | 0.214 | 350 | −0.29 | 0.09 | <0.001 | 0.269 | |
| VOR 10 mg | 324 | −0.25 | 0.12 | 0.038 | 0.088 | 324 | −0.25 | 0.12 | 0.038 | 0.088 | |
| 11: Anxiety Somatic b | Placebo | 691 | · | · | · | 338 | · | · | · | ||
| VOR 5 mg | 714 | −0.15 | 0.08 | 0.059 | 0.078 | 350 | −0.28 | 0.08 | <0.001 | 0.678 | |
| VOR 10 mg | 324 | −0.32 | 0.08 | <0.001 | 0.356 | 324 | −0.32 | 0.08 | <0.001 | 0.356 | |
| 12: Somatic Symptoms: Gastrointestinal b | Placebo | 691 | · | · | · | 338 | · | · | · | ||
| VOR 5 mg | 714 | −0.11 | 0.07 | 0.091 | 0.205 | 350 | −0.23 | 0.08 | 0.003 | 0.908 | |
| VOR 10 mg | 324 | −0.26 | 0.18 | 0.153 | 0.005 | 324 | −0.26 | 0.18 | 0.153 | 0.005 | |
| 13: Somatic Symptoms: General b | Placebo | 691 | · | · | · | 338 | · | · | · | ||
| VOR 5 mg | 714 | −0.17 | 0.07 | 0.013 | 0.154 | 350 | −0.28 | 0.08 | <0.001 | 0.411 | |
| VOR 10 mg | 324 | −0.27 | 0.08 | <0.001 | 0.408 | 324 | −0.27 | 0.08 | <0.001 | 0.408 | |
| 14: Genital Symptoms b | Placebo | 691 | · | · | · | 338 | · | · | · | ||
| VOR 5 mg | 714 | −0.20 | 0.10 | 0.052 | 0.007 | 350 | −0.37 | 0.08 | <0.001 | 0.853 | |
| VOR 10 mg | 324 | −0.38 | 0.08 | <0.001 | 0.803 | 324 | −0.38 | 0.08 | <0.001 | 0.803 | |
| 16: Loss of Weight b | Placebo | 691 | · | · | · | 338 | · | · | · | ||
| VOR 5 mg | 714 | −0.11 | 0.08 | 0.208 | 0.050 | 350 | −0.04 | 0.08 | 0.620 | 0.941 | |
| VOR 10 mg | 324 | −0.09 | 0.08 | 0.249 | 0.830 | 324 | −0.09 | 0.08 | 0.249 | 0.830 | |
| HAM-D24 total score | Placebo | 691 | 338 | ||||||||
| VOR 5 mg | 714 | −0.32 | 0.11 | 0.003 | 0.004 | 350 | −0.47 | 0.09 | <0.001 | 0.230 | |
| VOR 10 mg | 324 | −0.58 | 0.15 | <0.001 | 0.026 | 324 | −0.58 | 0.15 | <0.001 | 0.026 | |
The 10 mg vortioxetine (VOR) dose was tested only in the three positive studies; thus, the data in the 10 mg dose rows for the ‘All studies’ and the ‘Three studies’ analyses are identical.
Definition of item provided in Table 2.
Figure 1.
Change from baseline in HAM-D single items at week 6/8 (FAS, MMRM, SES) – all five studies (Alvarez et al., 2012; Baldwin et al., 2012b; Henigsberg et al., 2012; Jain et al., 2013; Mahableshwarkar et al., 2013). *p<0.05; **p<0.01; ***p<0.001. Definition of each item provided in Table 2.
Table 4.
Meta-analysis of change from baseline in HAM-A single items at week 6/8 (FAS, MMRM, SES).
| Item | Treatment a |
All studies analysis
(NCT00839423, NCT00635219, NCT00735709, NCT00672958, NCT00672620) |
Three studies analysis
(NCT00839423, NCT00635219, NCT00735709) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | ∆ Placebo | SE | p-value |
Heterogeneity
p-value |
N | ∆ Placebo | SE | p-value |
Heterogeneity
p-value |
||
| 7: Somatic Muscular b | Placebo | 695 | · | · | · | 335 | · | ||||
| VOR 5 mg | 711 | −0.15 | 0.06 | 0.021 | 0.239 | 344 | −0.21 | 0.08 | 0.005 | 0.740 | |
| VOR 10 mg | 319 | −0.15 | 0.08 | 0.055 | 0.837 | 319 | −0.15 | 0.08 | 0.055 | 0.837 | |
| 8: Somatic Sensory b | Placebo | 695 | · | · | · | 335 | · | ||||
| VOR 5 mg | 711 | −0.10 | 0.05 | 0.052 | 0.866 | 344 | −0.15 | 0.08 | 0.050 | 0.765 | |
| VOR 10 mg | 319 | −0.18 | 0.11 | 0.126 | 0.121 | 319 | −0.18 | 0.11 | 0.126 | 0.121 | |
| 9: Cardiovascular b | Placebo | 695 | · | · | · | 335 | · | ||||
| VOR 5 mg | 711 | −0.04 | 0.12 | 0.722 | 0.001 | 344 | −0.18 | 0.11 | 0.097 | 0.140 | |
| VOR 10 mg | 319 | −0.17 | 0.13 | 0.182 | 0.073 | 319 | −0.17 | 0.13 | 0.182 | 0.073 | |
| 10: Respiratory b | Placebo | 694 | · | · | · | 335 | · | ||||
| VOR 5 mg | 711 | −0.16 | 0.09 | 0.074 | 0.035 | 344 | −0.24 | 0.11 | 0.030 | 0.133 | |
| VOR 10 mg | 319 | −0.19 | 0.11 | 0.084 | 0.131 | 319 | −0.19 | 0.11 | 0.084 | 0.131 | |
| 11: Gastrointestinal b | Placebo | 695 | · | · | · | 335 | · | ||||
| VOR 5 mg | 711 | −0.10 | 0.05 | 0.060 | 0.540 | 344 | −0.20 | 0.08 | 0.011 | 0.990 | |
| VOR 10 mg | 319 | −0.22 | 0.12 | 0.069 | 0.089 | 319 | −0.22 | 0.12 | 0.069 | 0.089 | |
| 12: Genitourinary b | Placebo | 695 | · | · | · | 335 | · | ||||
| VOR 5 mg | 711 | −0.15 | 0.06 | 0.020 | 0.251 | 344 | −0.24 | ·0.08 | 0.002 | 0.457 | |
| VOR 10 mg | 319 | −0.28 | 0.08 | <.001 | 0.553 | 319 | −0.28 | 0.08 | <0.001 | 0.553 | |
| 13: Autonomic b | Placebo | 695 | · | · | · | 335 | · | ||||
| VOR 5 mg | 711 | −0.01 | 0.07 | 0.897 | 0.206 | 344 | −0.12 | 0.08 | 0.123 | 0.536 | |
| VOR 10 mg | 319 | −0.27 | 0.12 | 0.025 | 0.103 | 319 | −0.27 | 0.12 | 0.025 | 0.103 | |
| HAM-A total score | Placebo | 695 | 335 | ||||||||
| VOR 5 mg | 711 | −0.25 | 0.10 | 0.012 | 0.012 | 344 | −0.39 | 0.08 | <0.001 | 0.370 | |
| VOR 10 mg | 319 | −0.47 | 0.12 | <.001 | 0.119 | 319 | −0.47 | 0.12 | <0.001 | 0.119 | |
The 10 mg vortioxetine (VOR) dose was tested only in the three positive studies; thus, the data in the 10 mg dose rows for the ‘All studies’ and the ‘Three studies’ analyses are identical.
Definition of item provided in Table 2.
Table 5.
Meta-analysis of change from baseline in HAM-D and HAM-A single items at week 6/8 in MDD patients with a high level of baseline anxiety (HAM-A total score ⩾20) (FAS, MMRM, SES).
| Item | Treatment a |
All studies analysis
(NCT00839423, NCT00635219, NCT00735709, NCT00672958, NCT00672620) |
Three studies analysis
(NCT00839423, NCT00635219, NCT00735709) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | ∆ Placebo | SE | p-value |
Heterogeneity
p-value |
N | ∆ Placebo | SE | p-value |
Heterogeneity
p-value |
||
| HAM-D | |||||||||||
| 4: Insomnia Early b | Placebo | 325 | · | · | · | 196 | · | · | · | ||
| VOR 5 mg | 315 | −0.20 | 0.09 | 0.022 | 0.303 | 178 | −0.29 | 0.13 | 0.029 | 0.189 | |
| VOR 10 mg | 183 | −0.23 | 0.10 | 0.025 | 0.643 | 183 | −0.23 | 0.10 | 0.025 | 0.643 | |
| 5: Insomnia Middle b | Placebo | 325 | · | · | · | 196 | · | · | · | ||
| VOR 5 mg | 315 | −0.19 | 0.08 | 0.018 | 0.772 | 178 | −0.24 | 0.10 | 0.022 | 0.614 | |
| VOR 10 mg | 183 | −0.39 | 0.10 | <0.001 | 0.652 | 183 | −0.39 | 0.10 | <0.001 | 0.652 | |
| 6: Insomnia Late b | Placebo | 325 | · | · | 196 | · | · | ||||
| VOR 5 mg | 315 | ·–0.26 | 0.10 | 0.009 | 0.195 | 178 | −0.29 | 0.18 | 0.098 | 0.058 | |
| VOR 10 mg | 183 | −0.19 | 0.19 | 0.316 | 0.032 | 183 | −0.19 | 0.19 | 0.316 | 0.032 | |
| 11: Anxiety Somatic b | Placebo | 325 | · | · | · | 196 | · | · | · | ||
| VOR 5 mg | 315 | −0.21 | 0.10 | 0.039 | 0.165 | 178 | −0.32 | 0.13 | 0.012 | 0.218 | |
| VOR 10 mg | 183 | −0.26 | 0.10 | 0.012 | 0.869 | 183 | −0.26 | 0.10 | 0.012 | 0.869 | |
| 12: Somatic Symptoms: Gastrointestinal b | Placebo | 325 | · | · | · | 196 | · | · | · | ||
| VOR 5 mg | 315 | −0.17 | 0.08 | 0.032 | 0.555 | 178 | −0.17 | 0.13 | 0.168 | 0.230 | |
| VOR 10 mg | 183 | −0.21 | 0.20 | 0.315 | 0.021 | 183 | −0.21 | 0.20 | 0.315 | 0.021 | |
| 13: Somatic Symptoms: General b | Placebo | 325 | · | · | · | 196 | · | · | · | ||
| VOR 5 mg | 315 | −0.27 | 0.08 | 0.002 | 0.341 | 178 | −0.33 | 0.10 | 0.001 | 0.591 | |
| VOR 10 mg | 183 | −0.28 | 0.10 | 0.006 | 0.617 | 183 | −0.28 | 0.10 | 0.006 | 0.617 | |
| 14: Genital Symptoms b | Placebo | 325 | · | · | · | 196 | · | · | · | ||
| VOR 5 mg | 315 | −0.21 | 0.08 | 0.007 | 0.692 | 178 | −0.30 | 0.10 | 0.004 | 0.776 | |
| VOR 10 mg | 183 | −0.38 | 0.10 | <0.001 | 0.929 | 183 | −0.38 | 0.10 | <0.001 | 0.929 | |
| 16: Loss of Weight b | Placebo | 325 | · | · | · | 196 | · | · | · | ||
| VOR 5 mg | 315 | −0.11 | 0.08 | 0.148 | 0.434 | 178 | −0.07 | 0.11 | 0.523 | 0.316 | |
| VOR 10 mg | 183 | −0.13 | 0.10 | 0.190 | 0.878 | 183 | −0.13 | 0.10 | 0.190 | 0.878 | |
| HAM-D24 total score | Placebo | 325 | 196 | ||||||||
| VOR 5 mg | 315 | −0.37 | 0.11 | 0.001 | 0.088 | 178 | −0.49 | 0.18 | 0.005 | 0.060 | |
| VOR 10 mg | 183 | −0.52 | 0.18 | 0.004 | 0.044 | 183 | −0.52 | 0.18 | 0.004 | 0.044 | |
| Item | Treatment | N | ∆ Placebo | SE | p-value |
Heterogeneity
p-value |
N | ∆ Placebo | SE | p-value |
Heterogeneity
p-value |
| HAM-A | |||||||||||
| 7: Somatic Muscular b | Placebo | 327 | · | · | · | 196 | · | · | · | ||
| VOR 5 mg | 316 | −0.20 | 0.08 | 0.011 | 0.784 | 178 | −0.19 | 0.10 | 0.066 | 0.641 | |
| VOR 10 mg | 183 | −0.06 | 0.10 | 0.541 | 0.897 | 183 | −0.06 | 0.10 | 0.541 | 0.897 | |
| 8: Somatic Sensory b | Placebo | 327 | · | · | · | 196 | · | · | · | ||
| VOR 5 mg | 316 | −0.11 | 0.08 | 0.162 | 0.655 | 178 | −0.15 | 0.10 | 0.142 | 0.472 | |
| VOR 10 mg | 183 | −0.11 | 0.15 | 0.457 | 0.125 | 183 | −0.11 | 0.15 | 0.457 | 0.125 | |
| 9: Cardiovascular b | Placebo | 327 | · | · | · | 196 | |||||
| VOR 5 mg | 316 | −0.10 | 0.14 | 0.476 | 0.017 | 178 | −0.23 | 0.14 | 0.088 | 0.182 | |
| VOR 10 mg | 183 | −0.17 | 0.14 | 0.237 | 0.154 | 183 | −0.17 | 0.14 | 0.237 | 0.154 | |
| 10: Respiratory b | Placebo | 326 | · | · | · | 196 | · | · | · | ||
| VOR 5 mg | 316 | −0.14 | 0.11 | 0.187 | 0.133 | 178 | −0.23 | 0.15 | 0.126 | 0.135 | |
| VOR 10 mg | 183 | −0.24 | 0.14 | 0.084 | 0.173 | 183 | −0.24 | 0.14 | 0.084 | 0.173 | |
| 11: Gastrointestinal b | Placebo | 327 | · | · | · | 196 | · | · | · | ||
| VOR 5 mg | 316 | −0.12 | 0.08 | 0.120 | 0.441 | 178 | −0.17 | 0.10 | 0.096 | 0.633 | |
| VOR 10 mg | 183 | −0.27 | 0.15 | 0.072 | 0.129 | 183 | −0.27 | 0.15 | 0.072 | 0.129 | |
| 12: Genitourinary b | Placebo | 327 | · | · | · | 196 | · | · | · | ||
| VOR 5 mg | 316 | −0.17 | 0.08 | 0.029 | 0.853 | 178 | −0.18 | 0.10 | 0.090 | 0.543 | |
| VOR 10 mg | 183 | −0.25 | 0.12 | 0.035 | 0.273 | 183 | −0.25 | 0.12 | 0.035 | 0.273 | |
| 13: Autonomic b | Placebo | 327 | · | · | · | 196 | · | · | · | ||
| VOR 5 mg | 316 | −0.06 | 0.09 | 0.536 | 0.246 | 178 | −0.16 | 0.14 | 0.244 | 0.179 | |
| VOR 10 mg | 183 | −0.28 | 0.16 | 0.077 | 0.098 | 183 | −0.28 | 0.16 | 0.077 | 0.098 | |
| HAM-A total score | Placebo | 327 | 0. | 196 | |||||||
| VOR 5 mg | 316 | −0.28 | 12 | 0.021 | 0.063 | 178 | −0.42 | 0.17 | 0.013 | 0.076 | |
| VOR 10 mg | 183 | −0.44 | 0.18 | 0.017 | 0.045 | 183 | −0.44 | 0.18 | 0.017 | 0.045 | |
The 10 mg vortioxetine (VOR) dose was tested only in the three positive studies; thus, the data in the 10 mg dose rows for the ‘All studies’ and the ‘Three studies’ analyses are identical.
Definition of item provided in Table 1.
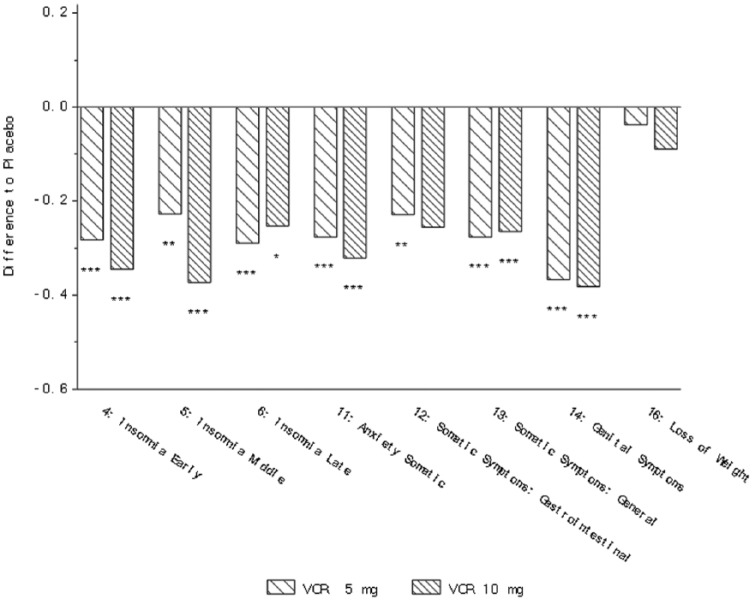
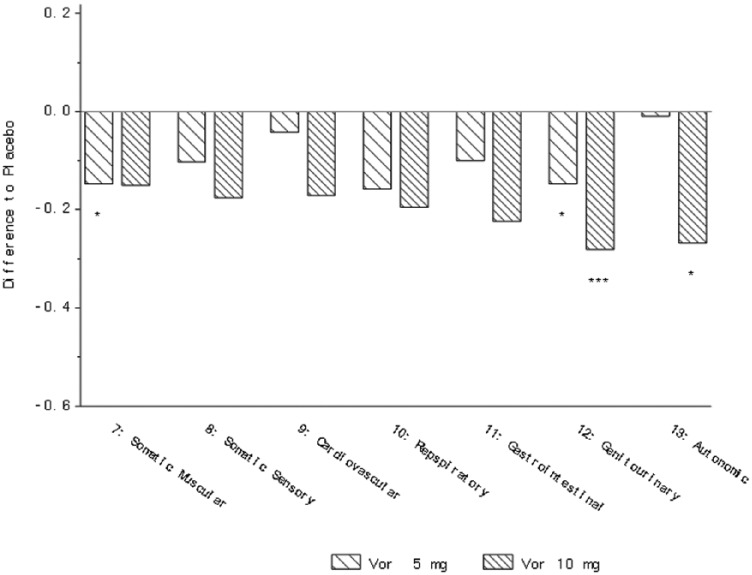
In the analysis of the three studies NCT00839423, NCT00635219 and NCT00735709, a statistically significant improvement with vortioxetine (5 and 10 mg) versus placebo was observed in change from baseline on all three insomnia items of the HAM-D (insomnia early, middle and late), two somatic items (gastrointestinal (5 mg only) and general), anxiety somatic and genital symptoms (Table 3, Figure 2). For physical symptoms measured by the HAM-A scale, a significant improvement with vortioxetine versus placebo was observed on the somatic muscular, respiratory, and gastrointestinal items for 5 mg vortioxetine, genitourinary item (5 and 10 mg vortioxetine) and autonomic item (10 mg vortioxetine) (Table 4, Figure 3). A borderline significant effect was also observed on the somatic sensory item (p=0.05). In the subgroup of MDD patients with a high baseline level of anxiety, significant favourable effects versus placebo for both 5 and 10 mg vortioxetine were observed on the HAM-D items of early and middle insomnia, general somatic and somatic anxiety symptoms, and genital symptoms (Table 5).
Figure 2.
Change from baseline in HAM-D single items at week 6/8 (FAS, MMRM, SES) – three studies (Alvarez et al., 2012; Baldwin et al., 2012b; Henigsberg et al., 2012). *p<0.05; **p<0.01; ***p<0.001. Definition of each item provided in Table 2.
Figure 3.
Change from baseline in HAM-A single items at week 6/8 (FAS, MMRM, SES) – five studies (Alvarez et al., 2012; Baldwin et al., 2012b; Henigsberg et al., 2012; Jain et al., 2013; Mahableshwarkar et al., 2013). *p<0.05; **p<0.01; ***p<0.001. Definition of each item provided in Table 2.
Discussion
In this meta-analysis of five short-term randomized clinical trials in patients with MDD, vortioxetine significantly improved most of the physical symptoms of depression, as measured by the HAM-D and HAM-A. Further, in the subset of MDD patients with high baseline anxiety levels, improvements in physical symptoms were also observed.
The presence of physical symptoms in MDD patients is a significant predictor of a more chronic course of disease, with a lower probability of treatment response and remission of depressive symptoms (Gerrits et al., 2012; Jaracz et al., 2016). Residual physical symptoms may also increase the risk of recurrence (Greden, 2003).
Since comorbid anxiety disorders are observed in a substantial proportion of MDD patients (with ranges of ~30–50% reported depending upon population sampled; Kessler et al., 2015), the efficacy of vortioxetine on the physical symptoms of depression in MDD patients with marked coexisting anxiety symptoms is encouraging. A recent meta-analysis of 10 short-term randomized, placebo-controlled trials of vortioxetine in MDD patients with high levels of anxiety indicated efficacy in reducing depressive and anxiety symptoms in this group of patients (Baldwin et al., 2016b). Together with the results reported here on the physical symptoms of depression, vortioxetine also seems to be a rational treatment option in patients with MDD and high anxiety, who often do not respond satisfactorily to alternative antidepressant therapy.
Although much remains uncertain about the pathophysiology of depression, abnormalities in serotonin (5-HT) and norepinephrine (NE) neurotransmission are probably involved in psychological and physical depressive symptoms (Fava, 2003). Pain control, for instance, appears to be influenced by both 5-HT and NE; this is consistent with reports that their analgesic effects seem to be mediated via common descending pain pathways (Fava, 2003; Jones, 1991; Richardson, 1990; Willis and Westlund, 1997). The 5-HT7 receptor has been shown in preclinical studies to play a key role in regulation of circadian rhythmicity and sleep – physiological functions that often are disturbed in patients with MDD (Hedlund, 2009; Monti and Jantos, 2014). Non-clinical studies with vortioxetine have shown that the compound modulates several neurotransmitter systems, including GABAergic, glutamatergic, serotonergic, norepinephrinergic, dopaminergic, histaminergic and cholinergic systems through complex mechanisms involving SERT inhibition and modulation of several 5-HT receptor subtypes, including the 5-HT7 receptor (Sanchez et al., 2015). Further, in rodent preclinical models of analgesic activity, vortioxetine showed potential for mitigating centrally mediated pain, though no activity was observed against inflammatory pain (Committee for Medicinal Products for Human Use (CHMP), 2013). Modulation of neurotransmitters involved in neural pain pathways may mediate an analgesic response and consequently relief of painful physical symptoms associated with depression (Kelliny et al., 2015; Kurian et al., 2009; Mork et al., 2012).
Sexual dysfunction is a common physical symptom of depression as well as common side effect of many antidepressants. In the clinical development programme of vortioxetine, treatment-emergent sexual dysfunction (TESD) was prospectively captured by the Arizona Sexual Dysfunction Scale and compared with placebo. Vortioxetine 5–20 mg was associated with an approximately 5% increase in incidence of TESD, a relatively low level compared with other antidepressants (Jacobsen et al., 2016; Kennedy et al., 2016). In a recent randomized, double-blind trial in which well-treated MDD patients experiencing selective serotonin reuptake inhibitor (SSRI)-related sexual dysfunction were switched to either vortioxetine or escitalopram, significant clinical improvements in sexual functioning were observed for vortioxetine versus escitalopram, thus confirming its clinical value for this specific yet important physical symptom of depression (Jacobsen et al., 2015b).
Antidepressants with proven efficacy across multiple symptom domains may provide clinicians with important options to fill the existing unmet needs in the treatment of MDD. Vortioxetine has proven effective across a broad range of depressive symptoms as measured by MADRS or the HAM-D (Kelliny et al., 2015). In addition, vortioxetine significantly improves cognitive symptoms known to be impacted in MDD such as executive function, attention/speed of processing and memory (Harrison et al., 2016; McIntyre et al., 2016) as well as functional capacity (Christensen et al., 2018; Mahableshwarkar et al., 2015). These improvements, along with beneficial effects on physical symptoms, may confer the MDD patient the best chance for a full functional recovery.
There are some limitations to these analyses that affect the interpretation of data. All analyses were conducted post hoc using data from five short-term studies originally designed to assess a different primary outcome. In these studies, the assessment of somatic symptoms was not a specific endpoint, nor was a specific scale used for the evaluation of somatic symptoms such as measures of pain or insomnia. Nevertheless, among the commonly used scales for measuring broad antidepressant effect in clinical registration trials such as the MADRS or HAM-D, the HAM-D captures the most physical symptoms of depression in a broad MDD population. Additionally, vortioxetine is an approved antidepressant in the dose range of 5, 10, 15 and 20 mg. The HAM-D scale was only used as a measure of antidepressant effect in the studies investigating the efficacy of vortioxetine 5 and 10 mg, and therefore this study could not investigate the efficacy on physical symptoms of depression at the doses 15 and 20 mg. Nevertheless, the clinical development programme for vortioxetine demonstrated a dose–response relationship for overall efficacy, and single-item analysis of the MADRS scale confirmed this dose–response relationship across the dose range (Thase et al., 2016). Finally, our analysis is based on studies of short duration and study participants are not necessarily representative of patients with MDD in usual clinical practice.
In conclusion, the findings of these analyses indicate that patients with MDD (and patients with MDD and a high level of anxiety symptoms) can have significant improvements in MDD-associated physical symptoms during vortioxetine treatment. These findings are important in the treatment of MDD patients for the therapeutic goals of providing broad symptom relief and achieving full functional recovery.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: IF, AL and MCC are employees of H. Lundbeck A/S. DSB did not receive any financial support from H. Lundbeck A/S or other sources in regard to this manuscript. DSB has received personal honoraria from H. Lundbeck A/S for participation in an advisory board relating to vortioxetine, and the University of Southampton has received grants from H. Lundbeck A/S to undertake research relating to vortioxetine and escitalopram (both manufactured by H. Lundbeck A/S).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Data for this manuscript were derived from clinical studies sponsored by H. Lundbeck A/S, Valby, Denmark and Takeda Pharmaceuticals Inc., Deerfield, Illinois.
ORCID iD: Michael Cronquist Christensen  https://orcid.org/0000-0002-3605-7223
https://orcid.org/0000-0002-3605-7223
References
- Alam MY, Jacobsen PL, Chen Y, et al. (2014) Safety, tolerability, and efficacy of vortioxetine (Lu AA21004) in major depressive disorder: Results of an open-label, flexible-dose, 52-week extension study. Int Clin Psychopharmacol 29: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez E, Perez V, Dragheim M, et al. (2012) A double-blind, randomized, placebo-controlled, active reference study of Lu AA21004 in patients with major depressive disorder. Int J Neuropsychopharmacol 15: 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders DSM-5. Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Bair MJ, Robinson RL, Katon W, et al. (2003) Depression and pain comorbidity: A literature review. Arch Intern Med 163: 2433–2445. [DOI] [PubMed] [Google Scholar]
- Baldwin DS, Chrones L, Florea I, et al. (2016. a) The safety and tolerability of vortioxetine: Analysis of data from randomized placebo-controlled trials and open-label extension studies. J Psychopharmacol 30: 242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin DS, Florea I, Jacobsen PL, et al. (2016. b) A meta-analysis of the efficacy of vortioxetine in patients with major depressive disorder (MDD) and high levels of anxiety symptoms. J Affect Disord 206: 140–150. [DOI] [PubMed] [Google Scholar]
- Baldwin DS, Hansen T, Florea I. (2012. a) Vortioxetine (Lu AA21004) in the long-term open-label treatment of major depressive disorder. Curr Med Res Opin 28: 1717–1724. [DOI] [PubMed] [Google Scholar]
- Baldwin DS, Loft H, Dragheim M. (2012. b) A randomised, double-blind, placebo controlled, duloxetine-referenced, fixed-dose study of three dosages of Lu AA21004 in acute treatment of major depressive disorder (MDD). Eur Neuropsychopharmacol 22: 482–491. [DOI] [PubMed] [Google Scholar]
- Berhan A, Barker A. (2014) Vortioxetine in the treatment of adult patients with major depressive disorder: A meta-analysis of randomized double-blind controlled trials. BMC Psychiatry 14: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulenger JP, Loft H, Olsen CK. (2014) Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day: A randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. Int Clin Psychopharmacol 29: 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen MC, Loft H, McIntyre RS. (2018) Vortioxetine improves symptomatic and functional outcomes in major depressive disorder: A novel dual outcome measure in depressive disorders. J Affect Disord 227: 787–794. [DOI] [PubMed] [Google Scholar]
- Committee for Medicinal Products for Human Use (2013) Brintellix: Assessment report for an initial marketing authorisation application. London: European Medicines Agency; vailable from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002717/WC500159447.pdf (accessed 4 July 2018). [Google Scholar]
- Fava M. (2003) The role of the serotonergic and noradrenergic neurotransmitter systems in the treatment of psychological and physical symptoms of depression. J Clin Psychiatry 64(Suppl 13): 26–29. [PubMed] [Google Scholar]
- Fava M, Graves LM, Benazzi F, et al. (2006) A cross-sectional study of the prevalence of cognitive and physical symptoms during long-term antidepressant treatment. J Clin Psychiatry 67: 1754–1759. [DOI] [PubMed] [Google Scholar]
- Florea I, Danchenko N, Brignone M, et al. (2015) The effect of vortioxetine on health-related quality of life in patients with major depressive disorder. Clin Ther 37: 2309–2323. e2306. [DOI] [PubMed] [Google Scholar]
- Florea I, Dragheim M, Loft H. (2012) The multimodal antidepressant Lu AA21004: Open-label long-term safety and tolerability study in major depressive disorder. Eur Neuropsychopharmacol 22(Suppl 2): S255–S256. [Google Scholar]
- Florea I, Loft H, Danchenko N, et al. (2017) The effect of vortioxetine on overall patient functioning in patients with major depressive disorder. Brain Behav 7: e00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits MM, Vogelzangs N, van Oppen P, et al. (2012) Impact of pain on the course of depressive and anxiety disorders. Pain 153: 429–436. [DOI] [PubMed] [Google Scholar]
- Greden JF. (2003) Physical symptoms of depression: Unmet needs. J Clin Psychiatry 64(Suppl 7): 5–11. [PubMed] [Google Scholar]
- Hamilton M. (1959. a) The assessment of anxiety states by rating. Br J Med Psychol 32: 50–55. [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. (1989) Frequency of symptoms in melancholia (depressive illness). Br J Psychiatry 154: 201–206. [DOI] [PubMed] [Google Scholar]
- Hamilton V. (1959. b) Eysenck’s theories of anxiety and hysteria: A methodological critique. Br J Psychol 50: 48–63. [DOI] [PubMed] [Google Scholar]
- Harrison JE, Lophaven S, Olsen CK. (2016) Which cognitive domains are improved by treatment with vortioxetine? Int J Neuropsychopharmacol. Epub ahead of print 8 June 2016. DOI: 10.1093/ijnp/pyw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB. (2009) The 5-HT7 receptor and disorders of the nervous system: An overview. Psychopharmacology (Berl) 206: 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henigsberg N, Mahableshwarkar AR, Jacobsen P, et al. (2012) A randomized, double-blind, placebo-controlled 8-week trial of the efficacy and tolerability of multiple doses of Lu AA21004 in adults with major depressive disorder. J Clin Psychiatry 73: 953–959. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RM. (2001) The comorbidity of major depression and anxiety disorders: Recognition and management in primary care. Prim Care Companion J Clin Psychiatry 3: 244–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CI, Weng LJ, Su YJ, et al. (2006) Depression and somatic symptoms scale: A new scale with both depression and somatic symptoms emphasized. Psychiatry Clin Neurosci 60: 700–708. [DOI] [PubMed] [Google Scholar]
- Inoue T, Nishimura A, Sasai K, et al. (2018) Randomized, 8-week, double-blind, placebo-controlled trial of vortioxetine in Japanese adults with major depressive disorder, followed by a 52-week open-label extension trial. Psychiatry Clin Neurosci 72: 103–115. [DOI] [PubMed] [Google Scholar]
- International Conference on Harmonization (1996) https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf (accessed 4 July 2018). [Google Scholar]
- Jacobsen PL, Harper L, Chrones L, et al. (2015. a) Safety and tolerability of vortioxetine (15 and 20 mg) in patients with major depressive disorder: Results of an open-label, flexible-dose, 52-week extension study. Int Clin Psychopharmacol 30: 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen PL, Mahableshwarkar AR, Chen Y, et al. (2015. b) Effect of vortioxetine versus escitalopram on sexual functioning in adults with well-treated major depressive disorder experiencing SSRI-induced sexual dysfunction. J Sex Med 12: 2036–2048. [DOI] [PubMed] [Google Scholar]
- Jacobsen PL, Mahableshwarkar AR, Palo WA, et al. (2016) Treatment-emergent sexual dysfunction in randomized trials of vortioxetine for major depressive disorder or generalized anxiety disorder: A pooled analysis. CNS Spectr 21: 367–378. [DOI] [PubMed] [Google Scholar]
- Jain R, Mahableshwarkar AR, Jacobsen PL, et al. (2013) A randomized, double-blind, placebo-controlled 6-wk trial of the efficacy and tolerability of 5 mg vortioxetine in adults with major depressive disorder. Int J Neuropsychopharmacol 16: 313–321. [DOI] [PubMed] [Google Scholar]
- Jaracz J, Gattner K, Jaracz K, et al. (2016) Unexplained painful physical symptoms in patients with major depressive disorder: Prevalence, pathophysiology and management. CNS Drugs 30: 293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SL. (1991) Descending noradrenergic influences on pain. Prog Brain Res 88: 381–394. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Maser JD, et al. (1998) Major depressive disorder: A prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord 50: 97–108. [DOI] [PubMed] [Google Scholar]
- Judd LL, Paulus MJ, Schettler PJ, et al. (2000) Does incomplete recovery from first lifetime major depressive episode herald a chronic course of illness? Am J Psychiatry 157: 1501–1504. [DOI] [PubMed] [Google Scholar]
- Kapfhammer HP. (2006) Somatic symptoms in depression. Dialogues Clin Neurosci 8: 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona C, Hansen T, Olsen CK. (2012) A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol 27: 215–223. [DOI] [PubMed] [Google Scholar]
- Kelliny M, Croarkin PE, Moore KM, et al. (2015) Profile of vortioxetine in the treatment of major depressive disorder: An overview of the primary and secondary literature. Ther Clin Risk Manag 11: 1193–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy N, Paykel ES. (2004) Residual symptoms at remission from depression: Impact on long-term outcome. J Affect Disord 80: 135–144. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Lam RW, McIntyre RS, et al. (2016) Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Section 3. Pharmacological treatments. Can J Psychiatry 61: 540–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sampson NA, Berglund P, et al. (2015) Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci 24: 210–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirmayer LJ, Robbins JM, Dworkind M, et al. (1993) Somatization and the recognition of depression and anxiety in primary care. Am J Psychiatry 150: 734–741. [DOI] [PubMed] [Google Scholar]
- Kurian BT, Greer TL, Trivedi MH. (2009) Strategies to enhance the therapeutic efficacy of antidepressants: Targeting residual symptoms. Expert Rev Neurother 9: 975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahableshwarkar AR, Jacobsen PL, Chen Y. (2013) A randomized, double-blind trial of 2.5 mg and 5 mg vortioxetine (Lu AA21004) versus placebo for 8 weeks in adults with major depressive disorder. Curr Med Res Opin 29: 217–226. [DOI] [PubMed] [Google Scholar]
- Mahableshwarkar AR, Zajecka J, Jacobson W, et al. (2015) A randomized, placebo-controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder. Neuropsychopharmacology 40: 2025–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly G, Anderson RH, Mattingly SG, et al. (2016) The impact of cognitive challenges in major depression: The role of the primary care physician. Postgrad Med 128: 665–671. [DOI] [PubMed] [Google Scholar]
- McClintock SM, Husain MM, Wisniewski SR, et al. (2011) Residual symptoms in depressed outpatients who respond by 50% but do not remit to antidepressant medication. J Clin Psychopharmacol 31: 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Harrison J, Loft H, et al. (2016) The effects of vortioxetine on cognitive function in patients with major depressive disorder: A meta-analysis of three randomized controlled trials. Int J Neuropsychopharmacol. Epub ahead of print 24 August 2016. DOI: 10.1093/ijnp/pyw055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melander H, Salmonson T, Abadie E, et al. (2008) A regulatory Apologia: a review of placebo-controlled studies in regulatory submissions of new-generation antidepressants. Eur Neuropsychopharmacol 18: 623–627. [DOI] [PubMed] [Google Scholar]
- Monti JM, Jantos H. (2014) The role of serotonin 5-HT7 receptor in regulating sleep and wakefulness. Rev Neurosci 25: 429–437. [DOI] [PubMed] [Google Scholar]
- Mork A, Pehrson A, Brennum LT, et al. (2012) Pharmacological effects of Lu AA21004: A novel multimodal compound for the treatment of major depressive disorder. J Pharmacol Exp Ther 340: 666–675. [DOI] [PubMed] [Google Scholar]
- Otte C, Gold SM, Penninx BW, et al. (2016) Major depressive disorder. Nat Rev Dis Primers 2: 16065. [DOI] [PubMed] [Google Scholar]
- Papadimitropoulou K, Vossen C, Karabis A, et al. (2017) Comparative efficacy and tolerability of pharmacological and somatic interventions in adult patients with treatment-resistant depression: A systematic review and network meta-analysis. Curr Med Res Opin 33: 701–711. [DOI] [PubMed] [Google Scholar]
- Richardson BP. (1990) Serotonin and nociception. Ann N Y Acad Sci 600: 511–519; discussion 519–520. [DOI] [PubMed] [Google Scholar]
- Rijavec N, Grubic VN. (2012) Depression and pain: Often together but still a clinical challenge: A review. Psychiatr Danub 24: 346–352. [PubMed] [Google Scholar]
- Riskind JH, Beck AT, Brown G, et al. (1987) Taking the measure of anxiety and depression. Validity of the reconstructed Hamilton scales. J Nerv Ment Dis 175: 474–479. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Asin KE, Artigas F. (2015) Vortioxetine, a novel antidepressant with multimodal activity: Review of preclinical and clinical data. Pharmacol Ther 145: 43–57. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl 20): 22–33; quiz 34–57. [PubMed] [Google Scholar]
- Singh MK, Gotlib IH. (2014) The neuroscience of depression: Implications for assessment and intervention. Behav Res Ther 62: 60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thase ME, Mahableshwarkar AR, Dragheim M, et al. (2016) A meta-analysis of randomized, placebo-controlled trials of vortioxetine for the treatment of major depressive disorder in adults. Eur Neuropsychopharmacol 26: 979–993. [DOI] [PubMed] [Google Scholar]
- Wang G, Gislum M, Filippov G, et al. (2015) Comparison of vortioxetine versus venlafaxine XR in adults in Asia with major depressive disorder: A randomized, double-blind study. Curr Med Res Opin 31: 785–794. [DOI] [PubMed] [Google Scholar]
- Warden D, Rush AJ, Trivedi MH, et al. (2007) The STAR*D Project results: A comprehensive review of findings. Curr Psychiatry Rep 9: 449–459. [DOI] [PubMed] [Google Scholar]
- Willis WD, Westlund KN. (1997) Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol 14: 2–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association. Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects (1964, 2008). Ferney-Voltaire: The World Medical Association, Inc; https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed 4 July 2018). [Google Scholar]