Abstract
Background
The role of systemic inflammation–based markers remains uncertain in advanced or metastatic neuroendocrine tumours (nets).
Methods
Systemic inflammatory factors, such as levels of circulating white blood cells and other blood components, were combined to yield inflammation-based prognostic scores [high-sensitivity inflammation-based Glasgow prognostic score (hsgps), neutrophil:lymphocyte ratio (nlr), platelet:lymphocyte ratio (plr), high-sensitivity inflammation-based prognostic index (hspi), and prognostic nutritional index (pni)], whose individual values as prognostic markers were retrospectively determined. Univariate and multivariate analyses were used to examine the association of inflammatory markers with overall survival (os).
Results
The study included 135 patients. Univariate analysis revealed that elevated white blood cell count, elevated neutrophil count, low serum albumin, elevated high-sensitivity C-reactive protein, and elevated hspi, hsgps, and nlr scores were significantly associated with worse os. Multivariate analyses demonstrated that, apart from pathology grade and original site of the tumour, elevated hspi (p = 0.004) was an independent prognostic factor for worse os.
Conclusions
In the present study, elevated pretreatment hspi was observed to be an independent predictor of shorter os in patients with inoperable advanced or metastatic net. The hspi might thus provide additional guidance for therapeutic decision-making in such patients.
Keywords: Neuroendocrine tumours, inflammation, prognostic indices, overall survival
INTRODUCTION
Neuroendocrine tumours (nets) constitute a heterogeneous group of malignancies that originate from cells of the endocrine system, most commonly the gastrointestinal tract1. Because these tumours have been regarded as relatively rare, their biology and molecular characteristics, and the optimal treatment strategy for affected patients, are far from clear2–4. In patients with net, the clinical course varies from highly aggressive disease, with affected patients living only approximately 6 months and developing high-grade metastatic tumours, to more indolent processes, with affected patients having a median survival of approximately 20 years5. It is therefore becoming more important to identify effective prognostic factors to guide clinical treatment.
Tumour stage, tumour grade, and site of tumour origin are well established prognostic factors for patients with nets6. However, even within the same classification of those factors, response to treatment and survival vary from patient to patient7. Additionally, a growing body of evidence demonstrates that patient outcomes are also determined by the tumour microenvironment—the systemic inflammatory response in particular8.
Measurement of the systemic inflammatory response has been refined through the use of various indicators, including plasma C-reactive protein (crp) and serum albumin, and a combination of crp and albumin that is termed the Glasgow prognostic score (gps). Those indicators have been shown to be independent prognostic factors in colorectal, gastric, and renal cancer9–11. Apart from those markers, many studies have demonstrated that other hematologic components of the systemic inflammatory response and certain specific combinations—for example, neutrophil:lymphocyte ratio (nlr), platelet:lymphocyte ratio (plr), prognostic index (pi), and prognostic nutritional index (pni)—can also serve as prognostic factors and are associated with survival in cancer patients10–17.
However, the role of those inflammation-based markers remains uncertain in advanced or metastatic net. We therefore examined the value of those markers as prognostic factors and the extent to which they improve the prognostic classification of such patients.
METHODS
Patients
Our study was approved by the Medical Ethics Committee of Peking University Cancer Hospital, Beijing, P.R.C., and was performed according to principles of the Declaration of Helsinki. All study participants gave written informed consent for the storage of their information in the hospital database and its use for future research at the time of follow-up ascertainment.
Detailed clinical data for patients treated at the Gastrointestinal Oncology Department of Peking University Cancer Hospital were recorded in a regularly updated electronic database. Eligibility criteria included patients with
■ pathology-confirmed inoperable locally advanced or metastatic net.
■ a history of systemic chemotherapy, somatostatin analog, or targeted therapy use.
■ a life expectancy of 3 months or more.
Patients who received adjuvant chemotherapy within 6 months of recurrence and those showing clinical evidence of infection or other inflammatory conditions (for example, connective tissue disorders, rheumatologic diseases, and vasculitis) were excluded from the study.
Prognostic Index
Data were gathered from medical records, including general patient demographics [sex, age, and Karnofsky performance status (kps)]; tumour characteristics (location, number of metastases, degree of differentiation, and expression of somatostatin receptor); and pretreatment laboratory tests relating to the potential prognostic factors [white blood cell (wbc), neutrophil, lymphocyte, and platelet counts; serum neuron-specific enolase (nse), lactate dehydrogenase (ldh), and albumin concentrations). In addition, information about treatment methods was also extracted.
High-sensitivity crp (hs-crp) in the plasma sample collected at the patient’s first visit was tested by human antienzyme-linked immunosorbent assay kit (Beckman Coulter, Brea, CA, U.S.A.). The laboratory variables were analyzed as categorical variables based on standard thresholds. Dichotomization of the variables was based on the upper (wbc count, neutrophil count, ldh, nse, hs-crp) and lower (albumin concentration, lymphocyte count) range of normal measurements for the markers. The cut-off points for dichotomization of the nlr and plr were their median distributions, which accorded with the cut-off points used for advanced malignancies in published studies (nlr ranging from 2.5 to 5.018–21; plr ranging from 150 to 30014,19,20,22). The gps, pi, and pni were also determined using the combination of systematic inflammatory markers specified in published reports9,14–16,23. The systemic inflammation– based prognostic scores of gps, nlr, plr, hs-pi, and pni were determined as described in detail in Table I.
TABLE I.
Systemic inflammation–based prognostic scores
| Marker | Score |
|---|---|
| High-sensitivity inflammation-based Glasgow | |
| Prognostic Score | |
| High-sensitivity CRP <3 mg/L and albumin ≥35 g/L | 0 |
| High-sensitivity CRP <3 mg/L and albumin <35 g/L | 1 |
| High-sensitivity CRP ≥3 mg/L and albumin ≥35 g/L | 1 |
| High-sensitivity CRP ≥3 mg/L and albumin <35 g/L | 2 |
|
| |
| Neutrophil lymphocyte ratio | |
| Neutrophil:lymphocyte ratio <2.8 | 1 |
| Neutrophil:lymphocyte ratio ≥2.8 | 2 |
|
| |
| Platelet lymphocyte ratio | |
| Platelet:lymphocyte ratio <144 | 1 |
| Platelet:lymphocyte ratio ≥144 | 2 |
|
| |
| High-sensitivity inflammation-based prognostic index | |
| High-sensitivity CRP <3 mg/L and WBC count <11×109/L | 0 |
| High-sensitivity CRP ≥3 mg/L and WBC count <11×109/L | 1 |
| High-sensitivity CRP <3 mg/L and WBC count ≥11×109/L | 1 |
| High-sensitivity CRP ≥3 mg/L and WBC count ≥11×109/L | 2 |
|
| |
| Prognostic nutritional index | |
| Albumin (g/L) + (5 × total lymphocyte count × 109/L) ≥45 | 0 |
| Albumin (g/L) + (5 × total lymphocyte count × 109/L) <45 | 1 |
CRP = C-reactive protein; WBC = white blood cell.
Statistical Analysis
All analyses were performed using the IBM SPSS Statistics software application (version 21 for Windows: IBM, Armonk, NY, U.S.A.). Differences between the study groups were determined using two-sided t-tests for continuous variables and Pearson chi-square tests for categorical variables [age, sex, type of tumour, primary tumour site, kps, laboratory tests (including wbc, neutrophil, and lymphocyte counts; and nse, ldh, albumin, and hs-crp concentrations)]. Survival duration was calculated from the date of first visit to the date of death. Survival probabilities were compared for various categories of interest using the Kaplan–Meier method with log-rank test. A multivariate analysis of survival used the Cox proportional hazards model, adjusted for factors that were identified as significant (p < 0.05) on univariate analysis. All p values were two-sided, and p < 0.05 was considered significant.
RESULTS
Patient Characteristics
From 7 April 2004 to 29 April 2015, 135 patients were determined to be eligible for the study. The last follow-up visit was 3 August 2015, with 13 patients (9.6%) having been lost to follow-up. At the last follow-up visit, the median age of the 135 patients was 55 years (range: 20–85 years). Of those patients, 89 (65.9%) had tumours that originated from the gastrointestinal tract; 23 (17.0%), from the pancreas; and 23 (17.0%), from other sites such as liver (n = 4, 3.0%), gall bladder (n = 4, 3.0%), and pelvic cavity (n = 5, 3.7%). In 10 patients (7.4%), the origin was unknown. Of the 135 patients, 125 (92.6%) had metastatic disease, with 82 (60.7%) having metastases at more than 1 site.
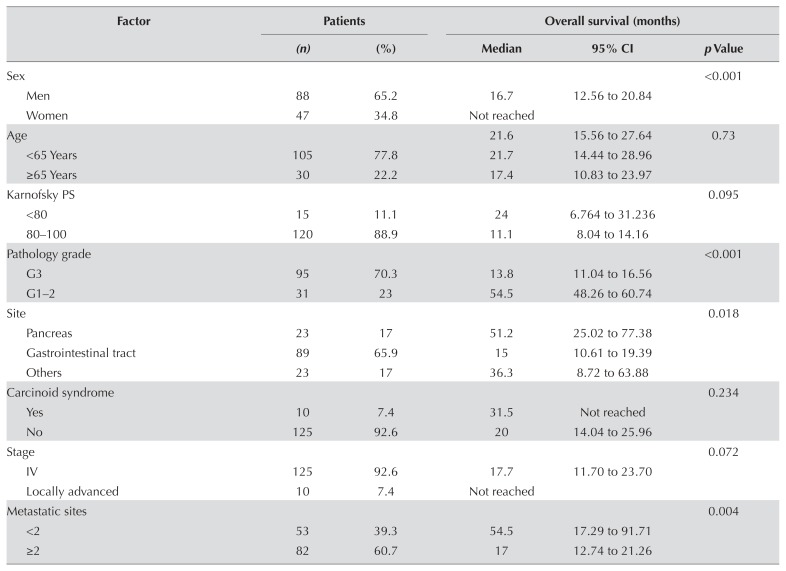
First-line treatment was chemotherapy in 101 patients (74.8%), somatostatin antagonists in 28 (20.7%), and targeted therapy in 6 (4.4%). More than half the patients died during the study period (n = 78, 57.8%), and the median survival duration was 21.6 months (95% confidence interval: 15.6 months to 27.6 months). Women, patients with carcinoid syndrome, and patients with locally advanced disease experienced longer survival (median os or the associated 95% confidence interval, or both, were not reached). Table II details the patient characteristics.
TABLE II.
Clinicopathologic and systemic inflammatory characteristics associated with overall survival
| Factor | Patients | Overall survival (months) | |||
|---|---|---|---|---|---|
|
|
|
||||
| (n) | (%) | Median | 95% CI | p Value | |
| Sex | <0.001 | ||||
| Men | 88 | 65.2 | 16.7 | 12.56 to 20.84 | |
| Women | 47 | 34.8 | Not reached | ||
|
| |||||
| Age | 21.6 | 15.56 to 27.64 | 0.73 | ||
| <65 Years | 105 | 77.8 | 21.7 | 14.44 to 28.96 | |
| ≥65 Years | 30 | 22.2 | 17.4 | 10.83 to 23.97 | |
|
| |||||
| Karnofsky PS | 0.095 | ||||
| <80 | 15 | 11.1 | 24 | 6.764 to 31.236 | |
| 80–100 | 120 | 88.9 | 11.1 | 8.04 to 14.16 | |
|
| |||||
| Pathology grade | <0.001 | ||||
| G3 | 95 | 70.3 | 13.8 | 11.04 to 16.56 | |
| G1–2 | 31 | 23 | 54.5 | 48.26 to 60.74 | |
|
| |||||
| Site | 0.018 | ||||
| Pancreas | 23 | 17 | 51.2 | 25.02 to 77.38 | |
| Gastrointestinal tract | 89 | 65.9 | 15 | 10.61 to 19.39 | |
| Others | 23 | 17 | 36.3 | 8.72 to 63.88 | |
|
| |||||
| Carcinoid syndrome | 0.234 | ||||
| Yes | 10 | 7.4 | 31.5 | Not reached | |
| No | 125 | 92.6 | 20 | 14.04 to 25.96 | |
|
| |||||
| Stage | 0.072 | ||||
| IV | 125 | 92.6 | 17.7 | 11.70 to 23.70 | |
| Locally advanced | 10 | 7.4 | Not reached | ||
|
| |||||
| Metastatic sites | 0.004 | ||||
| <2 | 53 | 39.3 | 54.5 | 17.29 to 91.71 | |
| ≥2 | 82 | 60.7 | 17 | 12.74 to 21.26 | |
|
| |||||
| SSR scintigraphy | 0.289 | ||||
| Negative | 36 | 26.7 | 15.5 | 10.48 to 20.52 | |
| Positive | 76 | 56.3 | 25.6 | 20.62 to 30.58 | |
|
| |||||
| Body mass index | 0.049 | ||||
| <18.5 | 11 | 8.1 | 10.6 | 3.97 to 17.23 | |
| ≥18.5 | 124 | 91.9 | 22.5 | 15.67 to 29.33 | |
|
| |||||
| Albumin (g/L) | 0.025 | ||||
| <35 | 9 | 6.7 | 10.3 | 0.07 to 20.53 | |
| ≥35 | 125 | 92.6 | 21.7 | 15.39 to 28.02 | |
|
| |||||
| Lactate dehydrogenase (U/L) | <0.001 | ||||
| <240 | 96 | 71.1 | 26.4 | 21.76 to 31.04 | |
| ≥240 | 37 | 27.4 | 9.6 | 3.11 to 16.09 | |
|
| |||||
| Neuron-specific enolase (ng/mL) | <0.001 | ||||
| <15.2 | 37 | 27.4 | 51.2 | Not reached | |
| ≥15.2 | 75 | 55.6 | 13.8 | 9.71 to 15.89 | |
|
| |||||
| White blood cell count | <0.001 | ||||
| <10×109 | 117 | 86.7 | 24.2 | 18.90 to 29.50 | |
| ≥10×109 | 15 | 11.1 | 5.8 | 1.76 to 9.84 | |
|
| |||||
| Neutrophil count | <0.001 | ||||
| <8×109 | 121 | 89.6 | 24.2 | 17.86 to 30.55 | |
| ≥8×109 | 11 | 8.1 | 11 | 3.23 to 18.77 | |
|
| |||||
| Lymphocyte count | 0.502 | ||||
| < 1×109 | 15 | 11.1 | 16.7 | 6.20 to 27.20 | |
| ≥1×109 | 117 | 86.7 | 21.7 | 14.88 to 28.52 | |
|
| |||||
| High-sensitivity CRP (mg/L) | <0.001 | ||||
| < 3 | 42 | 31.1 | 51.2 | 30.72 to 71.68 | |
| ≥3 | 54 | 40 | 12.8 | 9.71 to 15.89 | |
|
| |||||
| Prognostic nutritional index | 0.116 | ||||
| <45 | 116 | 85.9 | 21.7 | 15.52 to 27.88 | |
| ≥45 | 16 | 11.9 | 13.8 | 1.07 to 26.53 | |
|
| |||||
| High-sensitivity PI | <0.001 | ||||
| 0 | 41 | 30.4 | 51.2 | 30.70 to 71.70 | |
| 1 | 46 | 34.1 | 16.7 | 8.23 to 25.17 | |
| 2 | 9 | 6.7 | 4.7 | 2.36 to 7.04 | |
|
| |||||
| High-sensitivity GPS | <0.001 | ||||
| 0 | 41 | 30.4 | 51.2 | 30.69 to 71.71 | |
| 1 | 51 | 37.8 | 12.9 | 9.03 to 16.77 | |
| 2 | 4 | 3 | 1.4 | 0 to 2.90 | |
|
| |||||
| Neutrophil:lymphocyte ratio | 0.003 | ||||
| <2.8 | 66 | 48.9 | 26.6 | 10.77 to 42.43 | |
| ≥2.8 | 66 | 48.9 | 15.4 | 11.28 to 19.52 | |
|
| |||||
| Platelet:lymphocyte ratio | 0.184 | ||||
| <144 | 66 | 48.9 | 26.5 | 20.15 to 32.85 | |
| ≥144 | 66 | 48.9 | 15.4 | 11.18 to 19.62 | |
CI = confidence interval; PS = performance status; G3 = poorly differentiated; G1–2 = well or intermediately differentiated; SSR = somatostatin receptor; CRP = C-reactive protein; PI = inflammation-based prognostic index; GPS = inflammation-based Glasgow Prognostic Score.
Inflammation-Based Variables
In 54 patients (40%), hs-crp was elevated (≥3 mg/L). The median nlr and plr were 2.8 and 144 respectively. The hs-pi score was 0 in 41 patients (30.4%), 1 in 46 patients (34.1%), and 2 in 9 patients (6.7%). Similarly, the gps score was 0 in 41 patients (30.4%), 1 in 51 patients (37.8%), and 2 in 4 patients (3.0%). Accordingly, 16 patients (11.9%) had an elevated pni (≥45). Table II presents the details.
Univariate Survival Analysis
The univariate analyses demonstrated that sex, tumour grade, original tumour site, presence of metastasis, number of metastases, body mass index, serum albumin, ldh, nse, hs-crp, wbc count, neutrophil count, lymphocyte count, hs-pi, hs-gps, and nlr were significantly associated with os (all p < 0.05). No significant correlation of os with age, kps, carcinoid syndrome, metastasis or not, somatostatin receptor scintigraphy status, lymphocyte count, pni, or plr was observed (all p > 0.05, Table II).
Multivariate Survival Analysis
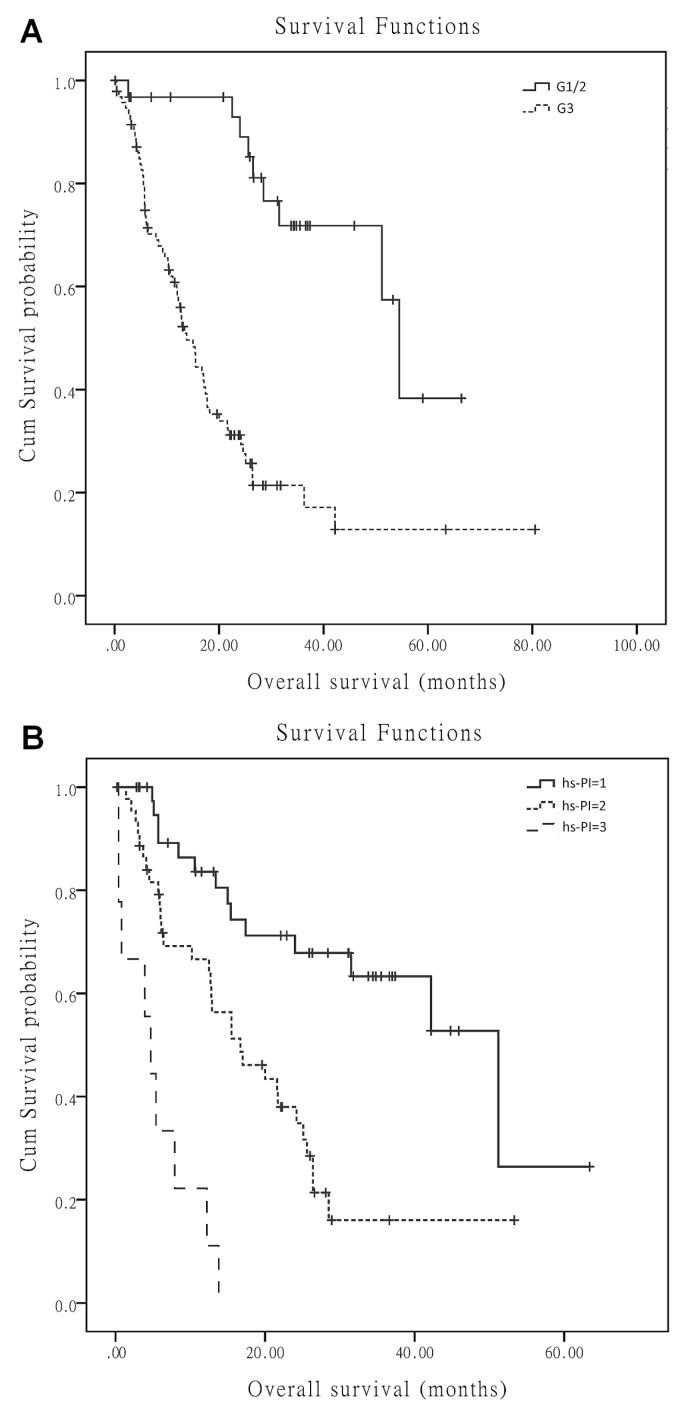
The variables sex, tumour grade, original tumour site, kps, presence of metastasis, number of metastases, body mass index, serum albumin, ldh, nse, hs-crp, wbc count, neutrophil count, lymphocyte count, hs-pi, gps, and nlr were included in the multivariate analyses. The results demonstrated that pathology grade (p < 0.001), original tumour site (p = 0.01), and hs-pi (p = 0.004) were independent prognostic factors for survival (Table III). Figure 1 shows the survival curves for patients by original tumour site, pathology grade, and hs-pi.
TABLE III.
Multivariate analyses of overall survival in 96 patients
| Factor | HR | 95% CI | p Value |
|---|---|---|---|
| Pathology grade | |||
| G3 | 9.6 | 3.03–30.41 | <0.001 |
| G1–2 | Reference | ||
|
| |||
| Site | |||
| Pancreas | Reference | 0.01 | |
| Gastrointestinal tract | 3.259 | 1.129 to 9.402 | |
| Others | 0.785 | 0.184 to 3.346 | |
|
| |||
| High-sensitivity PI | 0.004 | ||
| 0 | Reference | ||
| 1 | 1.504 | 0.747 to 3.027 | |
| 2 | 4.75 | 1.83 to 12.33 | |
HR = hazard ratio; CI = confidence interval; G3 = poorly differentiated; G1–2 = well or intermediately differentiated; PI = inflammation-based prognostic index.
FIGURE 1.
Overall survival by (A) pathologic grade (G1/2 = well or intermediately differentiated neuroendocrine tumours; G3 = poorly differentiated neuroendocrine tumours) and (B) high-sensitivity inflammation-based prognostic index (hs-PI).
Correlation Between hs-PI and Clinicopathologic Characteristics
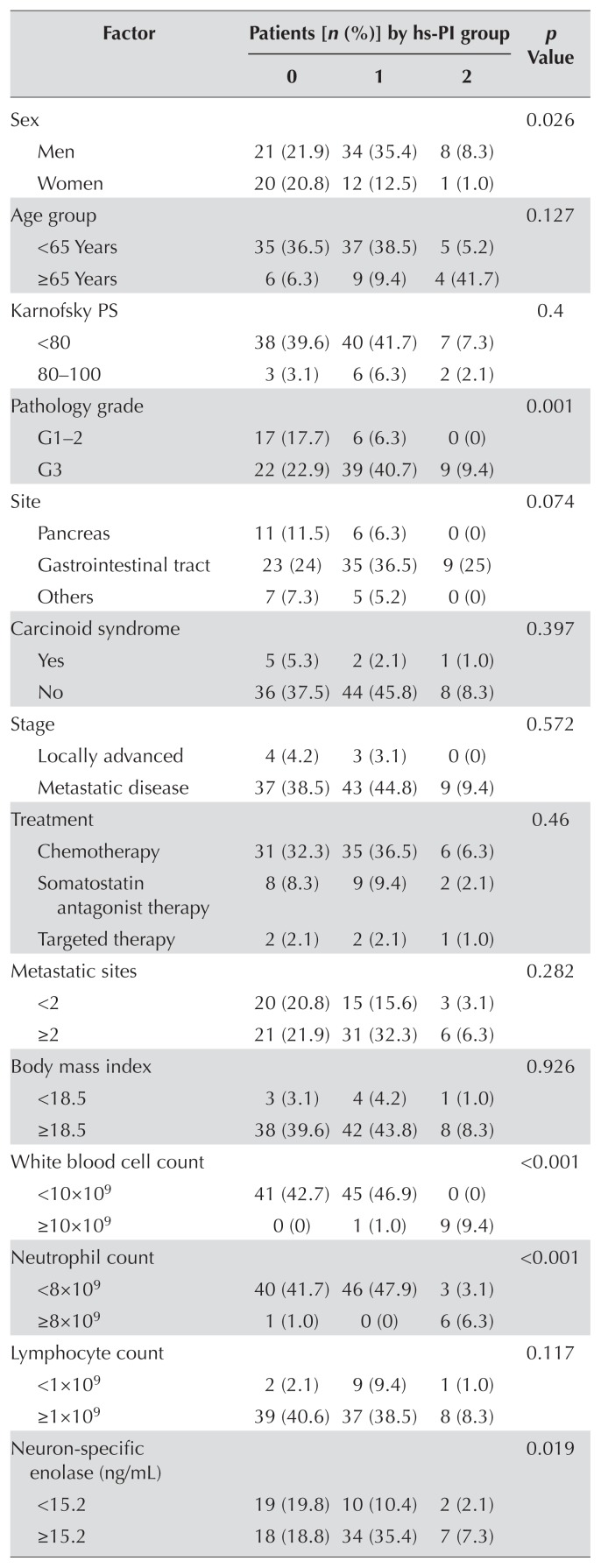
No statistically significant correlation was observed between the hs-pi and patient age, kps, original tumour site, carcinoid syndrome, presence of metastasis, body mass index, lymphocyte count, serum albumin, somatostatin receptor scintigraphy, pni, or plr. However, sex (p = 0.026), tumour grade (p = 0.001), wbc count (p < 0.001), neutrophil count (p < 0.001), nse (p = 0.019), ldh (p = 0.005), hs-crp (p < 0.001), gps (p < 0.001), and nlr (p = 0.006) were significantly different between patients with different hs-pi scores. Patients with a higher hs-pi score tended to have more severe disease and worse overall condition, which could be associated with worse outcomes (Table IV).
TABLE IV.
Correlation between the high-sensitivity inflammationbased prognostic index (hs-PI) and clinicopathologic parameters in 96 patients
| Factor | Patients [n (%)] by hs-PI group | p Value | ||
|---|---|---|---|---|
|
| ||||
| 0 | 1 | 2 | ||
| Sex | 0.026 | |||
| Men | 21 (21.9) | 34 (35.4) | 8 (8.3) | |
| Women | 20 (20.8) | 12 (12.5) | 1 (1.0) | |
|
| ||||
| Age group | 0.127 | |||
| <65 Years | 35 (36.5) | 37 (38.5) | 5 (5.2) | |
| ≥65 Years | 6 (6.3) | 9 (9.4) | 4 (41.7) | |
|
| ||||
| Karnofsky PS | 0.4 | |||
| <80 | 38 (39.6) | 40 (41.7) | 7 (7.3) | |
| 80–100 | 3 (3.1) | 6 (6.3) | 2 (2.1) | |
|
| ||||
| Pathology grade | 0.001 | |||
| G1–2 | 17 (17.7) | 6 (6.3) | 0 (0) | |
| G3 | 22 (22.9) | 39 (40.7) | 9 (9.4) | |
|
| ||||
| Site | 0.074 | |||
| Pancreas | 11 (11.5) | 6 (6.3) | 0 (0) | |
| Gastrointestinal tract | 23 (24) | 35 (36.5) | 9 (25) | |
| Others | 7 (7.3) | 5 (5.2) | 0 (0) | |
|
| ||||
| Carcinoid syndrome | 0.397 | |||
| Yes | 5 (5.3) | 2 (2.1) | 1 (1.0) | |
| No | 36 (37.5) | 44 (45.8) | 8 (8.3) | |
|
| ||||
| Stage | 0.572 | |||
| Locally advanced | 4 (4.2) | 3 (3.1) | 0 (0) | |
| Metastatic disease | 37 (38.5) | 43 (44.8) | 9 (9.4) | |
|
| ||||
| Treatment | 0.46 | |||
| Chemotherapy | 31 (32.3) | 35 (36.5) | 6 (6.3) | |
| Somatostatin antagonist therapy | 8 (8.3) | 9 (9.4) | 2 (2.1) | |
| Targeted therapy | 2 (2.1) | 2 (2.1) | 1 (1.0) | |
|
| ||||
| Metastatic sites | 0.282 | |||
| <2 | 20 (20.8) | 15 (15.6) | 3 (3.1) | |
| ≥2 | 21 (21.9) | 31 (32.3) | 6 (6.3) | |
|
| ||||
| Body mass index | 0.926 | |||
| <18.5 | 3 (3.1) | 4 (4.2) | 1 (1.0) | |
| ≥18.5 | 38 (39.6) | 42 (43.8) | 8 (8.3) | |
|
| ||||
| White blood cell count | <0.001 | |||
| <10×109 | 41 (42.7) | 45 (46.9) | 0 (0) | |
| ≥10×109 | 0 (0) | 1 (1.0) | 9 (9.4) | |
|
| ||||
| Neutrophil count | <0.001 | |||
| <8×109 | 40 (41.7) | 46 (47.9) | 3 (3.1) | |
| ≥8×109 | 1 (1.0) | 0 (0) | 6 (6.3) | |
|
| ||||
| Lymphocyte count | 0.117 | |||
| <1×109 | 2 (2.1) | 9 (9.4) | 1 (1.0) | |
| ≥1×109 | 39 (40.6) | 37 (38.5) | 8 (8.3) | |
|
| ||||
| Neuron-specific enolase (ng/mL) | 0.019 | |||
| <15.2 | 19 (19.8) | 10 (10.4) | 2 (2.1) | |
| ≥15.2 | 18 (18.8) | 34 (35.4) | 7 (7.3) | |
|
| ||||
| Albumin (g/L) | 0.05 | |||
| < 35 | 1 (1.0) | 2 (2.1) | 2 (2.1) | |
| ≥35 | 40 (41.7) | 44 (45.8) | 7 (7.3) | |
|
| ||||
| Lactate dehydrogenase (U/L) | 0.005 | |||
| <240 | 35 (36.5) | 31 (32.3) | 3 (3.1) | |
| ≥240 | 6 (6.3) | 15 (15.6) | 6 (6.3) | |
|
| ||||
| High-sensitivity CRP (mg/L) | <0.001 | |||
| <3 | 41 (42.7) | 1 (1.0) | 0 (0) | |
| ≥3 | 0 (0) | 45 (46.9) | 9 (9.4) | |
|
| ||||
| SSR scintigraphy | 0.082 | |||
| Negative | 7 (7.3) | 17 (17.7) | 3 (3.1) | |
| Positive | 30 (31.2) | 24 (25) | 4 (41.7) | |
|
| ||||
| Prognostic nutritional index | 0.017 | |||
| <45 | 1 (1.0) | 6 (6.3) | 3 (3.1) | |
| ≥45 | 40 (41.7) | 40 (41.7) | 6 (6.3) | |
|
| ||||
| High-sensitivity GPS | <0.001 | |||
| 0 | 40 (41.7) | 1 (1.0) | 0 () | |
| 1 | 1 (1.0) | 43 (44.8) | 7 (7.3) | |
| 2 | 0 (0) | 2 (2.1) | 2 (2.1) | |
|
| ||||
| Neutrophil:lymphocyte ratio | 0.006 | |||
| <2.8 | 26 (27.1) | 18 (18.8) | 1 (1.0) | |
| ≥2.8 | 15 (15.6) | 28 (29.2) | 8 (8.3) | |
|
| ||||
| Platelet:lymphocyte ratio | 0.121 | |||
| <144 | 25 (26.0) | 18 (18.8) | 4 (41.7) | |
| ≥144 | 16 (16.7) | 28 (29.2) | 5 (5.2) | |
PS = performance status; G1–2 = well or intermediately differentiated; G3 = poorly differentiated; CRP = C-reactive protein; SSR = somatostatin receptor; PI = inflammation-based prognostic index; GPS = inflammation-based Glasgow Prognostic Score.
DISCUSSION
Study findings suggest that systemic inflammation–based scores—and, in particular, an elevated pretreatment hs-pi score—are independent predictors of shorter os in patients with inoperable advanced or metastatic net. The hazard ratio for death was elevated by a factor of 1.5 for the hs-pi 1 group and by a factor of 4.75 for the hs-pi 2 group compared with the hs-pi 0 group.
Despite advances in the accuracy of clinical staging, established prognostic factors are of limited prognostic value in this disease because of the rarity of this tumour type and the tremendous patient heterogeneity24. The 2010 World Health Organization classification of tumours of the gastrointestinal tract, liver, and pancreas, a grading scheme for nets of the digestive tract that is based on expert consensus and endorsed by the European Neuroendocrine Tumor Society25, has resulted in improvements in tumour prognostication, treatment planning, and comparison of data from different institutions26. However, many studies support the concept that the current World Health Organization G3 category is heterogeneous27,28. Tumours at the lower end of the G3 range are, in fact, well-differentiated nets with an elevated proliferative rate (that is, high-grade, well-differentiated nets), and prognosis appears to be significantly better for those patients than for patients with poorly differentiated high-grade tumours29. The current World Health Organization high-grade neuroendocrine carcinoma category might therefore have to be refined and a new prognostic index developed4.
Additionally, chromogranin A is the most frequently used noninvasive serum marker, especially in the management of patients with well-differentiated nets. However, it has some limitations, given that various assays are available, and international standardization is lacking. In addition, elevated chromogranin A can be caused by renal or liver failure and the use of proton pump inhibitors30,31. In patients with net, nse is elevated and correlates with tumour size32,33. It has been considered to be a useful marker for follow-up in several studies of net34–36 and a generic marker for both neurons and neuroendocrine carcinoma. Although it has high sensitivity, its specificity is low. Similarly, elevated ldh is considered to be an adverse prognostic factor in various solid tumours, including nets37–39. In our analysis, abnormally increased levels of nse and ldh also predicted worse outcome in univariate analyses (p < 0.001), but lost statistical significance in Cox proportional hazards regression modelling, appearing to be less robust as prognostic markers in advanced or metastatic nets. More efficient factors therefore have to be uncovered.
It is now becoming clear that the tumour microenvironment, which is largely orchestrated by inflammatory cells, is an indispensable participant in the neoplastic process. Many blood components, including acute-phase crp40–43, lymphocytes44–46, wbcs47,48, and neutrophils49–51, have been identified as markers that reflect the systemic inflammatory response. Moreover, to further refine prognostic accuracy, a variety of indices based on a combination of various inflammatory markers or a combination of inflammatory factors and albumin or platelet count have been proposed. Evidence has shown that systematic inflammatory factors such as gps, pi, pni, nlr, and plr provide superior prognostic value for cancers of the lung, breast, colorectum, and stomach9,13,15,19. In the report by Salman et al.20, it was also demonstrated that nlr and plr can serve as factors to reliably predict survival in gastroenteropancreatic nets. However, that study focused mainly on the roles of nlr and plr in gastroenteropancreatic nets. Because the relationship between crp-based systemic inflammation–related prognostic scores and advanced or metastatic nets has not been examined, we further considered the individual markers of crp, lymphocyte count, wbc count, neutrophil count, and the combined markers of gps, hs-pi, pni, nlr, and plr, analyzing their correlation with os and clinicopathologic parameters. To our knowledge, the present study is the first to investigate the roles of hs-pi, pni, and gps in nets. In addition to the traditional clinicopathologic parameters such as grade and tumour location, hs-pi was also found to be an independent predictor of reduced survival in patients with advanced or metastatic net, and it was a predictor superior to gps, nlr, plr, and pni.
Being a combination of plasma crp and wbc count, a hs-pi score that is elevated at the time of net diagnosis is independently associated with shorter os in advanced or metastatic net. A high hs-pi score indicates elevated crp and an increased number of wbcs. Those increases reflect imbalance in the immune response, which impairs normal antitumour functions. White blood cells are described as main sources of the inflammatory response and important participants in the production of circulating angiogenic growth factors that promote tumour progression. The acute-phase protein crp is produced by hepatocytes, predominantly under the control of circulating interleukin 6. Some cancer cell lines produce interleukin 6, which is strongly associated with serum crp. Increased serum crp has been recognized to be a prognostic factor for poor outcome in several malignant tumours, including nets. In a prospective cohort study, Siemes et al.52 hypothesized that increased serum crp and crp gene variations are associated with an altered risk of colorectal, lung, breast, and prostate cancers. In addition, crp modulates both innate and adaptive immunity. Because the peripheral wbc count and hs-crp test are convenient, hs-pi can be considered to be a useful marker for predicting immune status in patients with advanced or metastatic net.
As part of our study, we considered the relationships of hs-pi with other clinicopathologic parameters, finding that hs-pi is not only associated with hs-crp, wbc count, and other crp-based inflammation-related scores (such as gps), but also with other systemic factors—for example, neutrophil count, pni, and nlr. Furthermore, a higher hs-pi score was relevant to the biologic characteristics of the tumour, such as poorer differentiation, and was found more often in male patients than in female patients. Moreover, an elevated hs-pi was associated with higher levels of nse and ldh, both of which are associated with worse outcomes. The statistically significant correlation between hs-pi and those related factors further suggests that hs-pi could be considered to be a reliable prognostic marker in advanced or metastatic net.
CONCLUSIONS
In summary, based on widely available, cost-effective, and easy-to-perform measurements, we developed a novel hs-pi. Using serum crp and the wbc count, the hs-pi identifies 3 subgroups of patients with advanced or metastatic net who have distinct prognoses. This newly proposed hs-pi might improve the accuracy of survival predictions in patients with advanced or metastatic net. It might also provide information that can complement other prognostic models, such as those based on gene profiling, potentially aiding in treatment decision-making and influencing a revised staging system.
ACKNOWLEDGMENTS
The authors thank their colleagues and all the subjects who participated in the study for their contributions.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Ramage JK, Davies AH, Ardill J, et al. on behalf of the UK-NETwork for Neuroendocrine Tumours. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours. Gut. 2005;54(suppl 4):iv1–16. doi: 10.1136/gut.2004.053314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basuroy R, Srirajaskanthan R, Prachalias A, Quaglia A, Ramage JK. Review article: the investigation and management of gastric neuroendocrine tumours. Aliment Pharmacol Ther. 2014;39:1071–84. doi: 10.1111/apt.12698. [DOI] [PubMed] [Google Scholar]
- 3.Basuroy R, Srirajaskanthan R, Ramage JK. Neuroendocrine tumors. Gastroenterol Clin North Am. 2016;45:487–507. doi: 10.1016/j.gtc.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Oberg K, Modlin IM, De Herder W, et al. Consensus on biomarkers for neuroendocrine tumour disease. Lancet Oncol. 2015;16:e435–46. doi: 10.1016/S1470-2045(15)00186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pape UF, Berndt U, Muller-Nordhorn J, et al. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer. 2008;15:1083–97. doi: 10.1677/ERC-08-0017. [DOI] [PubMed] [Google Scholar]
- 6.Zeng YJ, Liu L, Wu H, et al. Clinicopathological features and prognosis of gastroenteropancreatic neuroendocrine tumors: analysis from a single-institution. Asian Pac J Cancer Prev. 2013;14:5775–81. doi: 10.7314/APJCP.2013.14.10.5775. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Lu M, Lu Z, et al. Irinotecan plus cisplatin followed by octreotide long-acting release maintenance treatment in advanced gastroenteropancreatic neuroendocrine carcinoma: ipo–nec study. Oncotarget. 2016;8:25669–78. doi: 10.18632/oncotarget.12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–81. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 9.Li QQ, Lu ZH, Yang L, et al. Neutrophil count and the inflammation-based Glasgow prognostic score predict survival in patients with advanced gastric cancer receiving first-line chemotherapy. Asian Pac J Cancer Prev. 2014;15:945–50. doi: 10.7314/APJCP.2014.15.2.945. [DOI] [PubMed] [Google Scholar]
- 10.Unal D, Eroglu C, Kurtul N, Oguz A, Tasdemir A. Are neutrophil/ lymphocyte and platelet/lymphocyte rates in patients with non-small cell lung cancer associated with treatment response and prognosis? Asian Pac J Cancer Prev. 2013;14:5237– 42. doi: 10.7314/APJCP.2013.14.9.5237. [DOI] [PubMed] [Google Scholar]
- 11.Grimes N, Tyson M, Hannan C, Mulholland C. A systematic review of the prognostic role of hematologic scoring systems in patients with renal cell carcinoma undergoing nephrectomy with curative intent. Clin Genitourin Cancer. 2016;14:271–6. doi: 10.1016/j.clgc.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Shi P, Meng X, Ni M, Sun X, Xing L, Yu J. Association between serum tumor markers and metabolic tumor volume or total lesion glycolysis in patients with recurrent small cell lung cancer. Oncol Lett. 2015;10:3123–8. doi: 10.3892/ol.2015.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asano Y, Kashiwagi S, Onoda N, et al. Platelet–lymphocyte ratio as a useful predictor of the therapeutic effect of neoadjuvant chemotherapy in breast cancer. PloS One. 2016;11:e0153459. doi: 10.1371/journal.pone.0153459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin HL, Ohara K, Kiberu A, Van Hagen T, Davidson A, Khattak MA. Prognostic value of systemic inflammation– based markers in advanced pancreatic cancer. Intern Med J. 2014;44:676–82. doi: 10.1111/imj.12453. [DOI] [PubMed] [Google Scholar]
- 15.Kasymjanova G, MacDonald N, Agulnik JS, et al. The predictive value of pre-treatment inflammatory markers in advanced non-small-cell lung cancer. Curr Oncol. 2010;17:52–8. doi: 10.3747/co.v17i4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40:440–3. doi: 10.1007/s00595-009-4065-y. [DOI] [PubMed] [Google Scholar]
- 17.Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98:268–74. doi: 10.1002/bjs.7305. [DOI] [PubMed] [Google Scholar]
- 18.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation–based neutrophil– lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–30. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Kwon HC, Kim SH, Oh SY, et al. Clinical significance of preoperative neutrophil–lymphocyte versus platelet–lymphocyte ratio in patients with operable colorectal cancer. Biomarkers. 2012;17:216–22. doi: 10.3109/1354750X.2012.656705. [DOI] [PubMed] [Google Scholar]
- 20.Salman T, Kazaz SN, Varol U, et al. Prognostic value of the pretreatment neutrophil-to-lymphocyte ratio and plateletto-lymphocyte ratio for patients with neuroendocrine tumors: an Izmir Oncology Group Study. Chemotherapy. 2016;61:281–6. doi: 10.1159/000445045. [DOI] [PubMed] [Google Scholar]
- 21.Szkandera J, Stotz M, Eisner F, et al. External validation of the derived neutrophil to lymphocyte ratio as a prognostic marker on a large cohort of pancreatic cancer patients. PloS One. 2013;8:e78225. doi: 10.1371/journal.pone.0078225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S, Oh SY, Kim SH, et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with folfox chemotherapy. BMC Cancer. 2013;13:350. doi: 10.1186/1471-2407-13-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeno S, Hashimoto T, Shibata R, et al. Improvement of high-sensitivity inflammation-based Glasgow prognostic score by gastrectomy is a favorable prognostic factor in patients with gastric cancer. Anticancer Res. 2014;34:5695–702. [PubMed] [Google Scholar]
- 24.Ilett EE, Langer SW, Olsen IH, Federspiel B, Kjaer A, Knigge U. Neuroendocrine carcinomas of the gastroenteropancreatic system: a comprehensive review. Diagnostics (Basel) 2015;5:119–76. doi: 10.3390/diagnostics5020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scoazec JY, Couvelard A. The new who classification of digestive neuroendocrine tumors [French] Ann Pathol. 2011;31:88–92. doi: 10.1016/j.annpat.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Kulke MH, Shah MH, Benson AB, 3rd, et al. on behalf of the National Comprehensive Cancer Network. Neuroendocrine tumors, version 1.2015. J Natl Compr Canc Netw. 2015;13:78–108. doi: 10.6004/jnccn.2015.0011. [DOI] [PubMed] [Google Scholar]
- 27.Basturk O, Yang Z, Tang LH, et al. The high-grade (who G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogeneous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol. 2015;39:683–90. doi: 10.1097/PAS.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reid MD, Balci S, Saka B, Adsay NV. Neuroendocrine tumors of the pancreas: current concepts and controversies. Endocr Pathol. 2014;25:65–79. doi: 10.1007/s12022-013-9295-2. [DOI] [PubMed] [Google Scholar]
- 29.Sorbye H, Strosberg J, Baudin E, Klimstra DS, Yao JC. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer. 2014;120:2814–23. doi: 10.1002/cncr.28721. [DOI] [PubMed] [Google Scholar]
- 30.Giusti M, Sidoti M, Augeri C, Rabitti C, Minuto F. Effect of short-term treatment with low dosages of the proton-pump inhibitor omeprazole on serum chromogranin A levels in man. Eur J Endocrinol. 2004;150:299–303. doi: 10.1530/eje.0.1500299. [DOI] [PubMed] [Google Scholar]
- 31.Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M. Chromogranin A—biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol. 2010;17:2427–43. doi: 10.1245/s10434-010-1006-3. [DOI] [PubMed] [Google Scholar]
- 32.Baudin E, Gigliotti A, Ducreux M, et al. Neuron-specific enolase and chromogranin A as markers of neuroendocrine tumours. Br J Cancer. 1998;78:1102–7. doi: 10.1038/bjc.1998.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oberg K, Knigge U, Kwekkeboom D, Perren A on behalf of the esmo Guidelines Working Group. Neuroendocrine gastroentero-pancreatic tumors: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl 7):vii124–30. doi: 10.1093/annonc/mds295. [DOI] [PubMed] [Google Scholar]
- 34.van Adrichem RC, Kamp K, Vandamme T, Peeters M, Feelders RA, de Herder WW. Serum neuron-specific enolase level is an independent predictor of overall survival in patients with gastroenteropancreatic neuroendocrine tumors. Ann Oncol. 2016;27:746–7. doi: 10.1093/annonc/mdv626. [DOI] [PubMed] [Google Scholar]
- 35.Kasprzak A, Zabel M, Biczysko W. Selected markers (chromogranin A, neuron-specif ic enolase, synaptophysin, protein gene product 9.5) in diagnosis and prognosis of neuroendocrine pulmonar y tumours. Pol J Pathol. 2007;58:23–33. [PubMed] [Google Scholar]
- 36.Korse CM, Taal BG, Vincent A, et al. Choice of tumour markers in patients with neuroendocrine tumours is dependent on the histological grade. A marker study of chromogranin A, neuron specific enolase, progastrin-releasing peptide and cytokeratin fragments. Eur J Cancer. 2012;48:662–71. doi: 10.1016/j.ejca.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Wang X, Huang J. Irinotecan plus fluorouracil-based regimen as second or third-line chemotherapy for recurrent or metastatic esophageal squamous cell carcinoma. Thorac Cancer. 2016;7:246–50. doi: 10.1111/1759-7714.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrelli F, Cabiddu M, Coinu A, et al. Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta Oncol. 2015;54:961–70. doi: 10.3109/0284186X.2015.1043026. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Yao YH, Li BG, Yang Q, Zhang PY, Wang HT. Prognostic value of pretreatment serum lactate dehydrogenase level in patients with solid tumors: a systematic review and meta-analysis. Sci Rep. 2015;5:9800. doi: 10.1038/srep09800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci. 2011;48:155–70. doi: 10.3109/10408363.2011.599831. [DOI] [PubMed] [Google Scholar]
- 41.Asegaonkar SB, Asegaonkar BN, Takalkar UV, Advani S, Thorat AP. C-Reactive protein and breast cancer: new insights from old molecule. Int J Breast Cancer. 2015;2015 doi: 10.1155/2015/145647. 145647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shrotriya S, Walsh D, Bennani-Baiti N, Thomas S, Lorton C. C-Reactive protein is an important biomarker for prognosis tumor recurrence and treatment response in adult solid tumors: a systematic review. PloS One. 2015;10:e0143080. doi: 10.1371/journal.pone.0143080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szkandera J, Stotz M, Absenger G, et al. Validation of C-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. Br J Cancer. 2014;110:183–8. doi: 10.1038/bjc.2013.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kou F, Lu Z, Li J, et al. Pretreatment lymphopenia is an easily detectable predictive and prognostic marker in patients with metastatic esophagus squamous cell carcinoma receiving first-line chemotherapy. Cancer Med. 2016;5:778–86. doi: 10.1002/cam4.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porrata LF, Markovic SN. Is absolute lymphocyte count just another prognostic factor in cancer? SRX Med. 2010;2010:1–8. doi: 10.3814/2010/812304. [DOI] [Google Scholar]
- 46.Milne K, Alexander C, Webb JR, et al. Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytes. J Transl Med. 2012;10:33. doi: 10.1186/1479-5876-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kruse AL, Luebbers HT, Gratz KW. Evaluation of white blood cell count as a possible prognostic marker for oral cancer. Head Neck Oncol. 2011;3:13. doi: 10.1186/1758-3284-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mandrekar SJ, Schild SE, Hillman SL, et al. A prognostic model for advanced stage nonsmall cell lung cancer. Pooled analysis of North Central Cancer Treatment Group trials. Cancer. 2006;107:781–92. doi: 10.1002/cncr.22049. [DOI] [PubMed] [Google Scholar]
- 49.Mantas D, Kostakis ID, Machairas N, Markopoulos C. White blood cell and platelet indices as prognostic markers in patients with invasive ductal breast carcinoma. Oncol Lett. 2016;12:1610–14. doi: 10.3892/ol.2016.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donskov F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin Cancer Biol. 2013;23:200–7. doi: 10.1016/j.semcancer.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 51.Bahig H, Taussky D, Delouya G, et al. Neutrophil count is associated with survival in localized prostate cancer. BMC Cancer. 2015;15:594. doi: 10.1186/s12885-015-1599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siemes C, Visser LE, Coebergh JW, et al. C-Reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol. 2006;24:5216–22. doi: 10.1200/JCO.2006.07.1381. [DOI] [PubMed] [Google Scholar]