Abstract
Nivolumab, an anti–PD-1 antibody, is now considered an important therapeutic agent in several advanced malignancies. However, immune-related adverse events such as endocrinopathies have been reported with its use. Thyroid disorder and isolated adrenocorticotropic hormone deficiency have frequently been reported as nivolumab-induced immune-related adverse events. Another endocrinopathy is nivolumab-induced type 1 diabetes mellitus (t1dm), described as diabetes mellitus with rapid onset and complete insulin insufficiency, at times leading to fulminant t1dm.
We report the case of a 68-year-old woman who developed pancreatic islet–related autoantibody-negative t1dm, possibly induced by nivolumab, under continuous glucocorticoid administration. She was treated with nivolumab for advanced malignant melanoma, concomitant with 10 mg prednisolone daily for thrombophlebitis tapered to 5 mg after 13 courses of nivolumab therapy.
At approximately the 27th course of nivolumab therapy, she showed elevated plasma glucose levels despite preserved insulin secretion. A month later, she developed diabetic ketoacidosis. Her insulin secretion decreased and finally was exhausted. She was diagnosed with acute-onset rather than fulminant t1dm because of a rapidly progressive course to diabetic ketoacidosis during just more than 1 week. She is currently receiving insulin replacement. There has been no recurrence of the melanoma.
Thus, nivolumab might induce autoimmune diabetes mellitus, with patients having t1dm-sensitive human leucocyte antigen being more susceptible even when receiving glucocorticoids. Physicians should be aware that nivolumab could potentially induce t1dm as a critical immune-related adverse event.
Keywords: Melanoma, nivolumab, autoimmunity, adverse drug events, diabetes mellitus, type 1 diabetes
INTRODUCTION
Anti–PD-1 antibodies activate an antitumour immunologic response by abrogating PD-1–related T cell inhibition. They reportedly improve the prognosis of patients with several advanced malignancies1. Although nivolumab, an anti–PD-1 antibody, has improved prognosis and become a popular agent in several advanced malignancies, various immune-related adverse events (iraes)2, including endocrinopathies3, have been reported.
Several cases of nivolumab-induced type 1 diabetes mellitus (t1dm) have been reported as endocrinologic iraes. The patients in most of those cases had a genetically susceptible background for t1dm4 and experienced rapidly progressive fulminant t1dm5–7. However, the clinical course of their disrupted insulin secretion was not studied.
We describe a case of acute-onset t1dm, probably induced by nivolumab, in which the patient’s insulin secretion was monitored throughout the clinical course. A progressive decline of insulin secretion that exhausted within a month was observed in this patient, indicating that t1dm with a slower clinical course rather than fulminant t1dm can develop as an irae. Thus, in the case of a hyperglycemic event, physicians should consider t1dm, a critical irae, even when insulin secretion is initially reported to be in the normal range.
The patient’s written informed consent was obtained for the publication of this case report. The Institutional Review Board of Kyushu University Hospital waived the need for ethics approval.
CASE DESCRIPTION
A 68-year-old woman presented to our endocrine department complaining of general fatigue. She had been diagnosed 3 years earlier with vaginal malignant melanoma and had undergone total abdominal hysterectomy, bilateral salpingo-oophorectomy, and sentinel lymph node resection. Although interferon therapy was given after the surgical procedure, the melanoma progressed 1 year later, with relapse in intra-abdominal lymph nodes.
The patient was then started on nivolumab 3 mg/kg every 3 weeks. She had a 10-year history of Graves disease treated with potassium iodide 100 mg daily. She did not have any other past or family history of diabetes mellitus, endocrine, or autoimmune disease. After administration of the 13th course of nivolumab, prednisolone 10 mg daily was prescribed to treat thrombophlebitis in her left lower thigh and was tapered to 5 mg daily after 1 week.
At approximately the 27th course of nivolumab, the patient’s plasma glucose increased to 11.3 mmol/L and 18.2 mmol/L (before dinner), 27 and 13 days respectively before a ketoacidosis episode. At the time, endogenous insulin secretion and HbA1c were within the normal range (Figure 1). Fasting plasma glucose, C-peptide, and anti-glutamic acid decarboxylase antibody 26 days before the ketoacidosis episode were 5.1 mmol/L, 0.5 nmol/L, and less than 0.5 U/mL respectively (Table I, Figure 1).
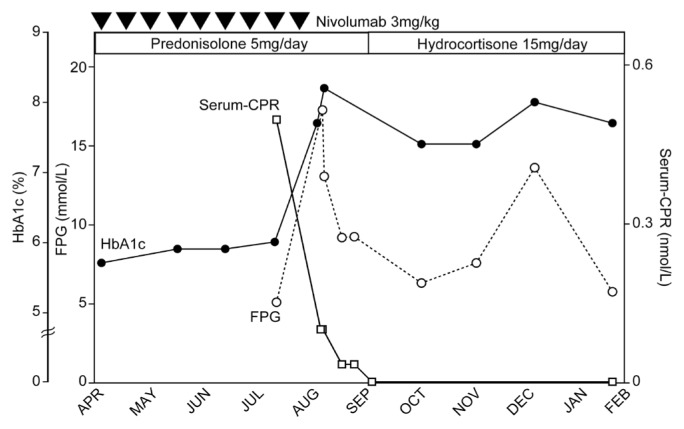
FIGURE 1.
Patient’s clinical course in the present case. On day 26, before diabetic ketoacidosis, insulin secretion is preserved, based on serum C-peptide (CPR) 0.5 nmol/L and fasting plasma glucose (FPG) 5.1 mmol/L. At the time of admission, FPG was highly elevated, and insulin secretion had declined, but not become exhausted (day 1 CPR 0.1 nmol/L and FPG 13.0 mmol/L). Continuous insulin replacement was required to manage plasma glucose. The patient’s insulin secretion declined steadily, leading to exhaustion 9 days after the ketoacidosis event. Her insulin secretion had not recovered by 5 months later.
TABLE I.
Laboratory data at patient admission
| Variable | Value | Reference range |
|---|---|---|
| Diabetes-related | ||
| Plasma glucose (mmol/L) | 17.4 | 4.1–6.1 |
| HbA1c (%) | 8.2 | 4.9–6.0 |
| Glycated albumin (%) | 30.3 | 11–16 |
| Immunoreactive insulin (mU/L) | 1.5 | 1.0–18 |
| Serum C-peptide (nmol/L) | 0.2 | 0.4–1.1 |
| Urinary C-peptide (nmol/day) | 0.3 | 9.7–55.3 |
| Urinary ketones | 3+ | — |
| Total ketone bodies (μmol/L) | 7728 | <130 |
| Acetoacetic acid (μmol/L) | 1048 | <55 |
| 3-Hydroxybutyric acid (μmol/L) | 6680 | <85 |
| Amylase (IU/L) | 24 | 44–142 |
| Lipase (IU/L) | 25 | 16–51 |
|
| ||
| Blood gases | ||
| pH | 7.22 | 7.35–7.45 |
| pCO2 (kPa) | 3.50 | 4.26–5.59 |
| pO2 (kPa) | 13.19 | 10.64–13.30 |
| HCO3− (mmol/L) | 10.5 | 20.0–24.0 |
|
| ||
| Type 1 diabetes–related antibodies | ||
| Anti-GAD (IU/mL) | <5.0 | <5.0 |
| Anti-IA2 (IU/mL) | <0.4 | <0.4 |
| Anti-ZnT8 (IU/mL) | <10 | <15.0 |
| Anti-ICA (IU/mL) | <1.25 | <1.25 |
| Anti-insulin (%) | <0.4 | <0.4 |
|
| ||
| Endocrine function | ||
| TSH (mIU/L) | 0.01 | 0.27–4.2 |
| Free triiodothyronine (pmol/L) | 6.64 | 3.38–6.76 |
| Free thyroxine (pmol/L) | 29.4 | 12.9–23.2 |
| TSH receptor antibody (IU/mL) | 52.4 | <2.0 |
| Thyroid-stimulating antibody (%) | 331 | <120 |
| Anti-TPO antibody (IU/mL) | 8.3 | <30.0 |
| Anti-Tg antibody (IU/mL) | 59.4 | <30.0 |
| ACTH (pmol/L) | 0.24 | 1.58–13.86 |
| Cortisol (nmol/L) | 44.1 | 110.4–504.9 |
GAD = glutamic acid decarboxylase; IA2 = insulinoma-associated antigen 2; ZnT8 = zinc transporter 8; ICA = islet cell antibodies; TSH = thyroid-stimulating hormone; TPO = thyroid peroxidase; Tg = thyroglobulin; ACTH = adrenocorticotropic hormone.
Seven days before the ketoacidosis episode and after administration of the 28th course of nivolumab, the patient complained of nausea and general fatigue. On the day of admission, she complained of progressive fatigue. She was alert, with no abnormality on physical examination. Table I presents her laboratory results. She was diagnosed with diabetic ketoacidosis, thyrotoxicosis, and adrenal insufficiency secondary to prednisolone treatment for thrombophlebitis. Whole-body computed tomography imaging revealed mild diffuse shrinkage of the pancreas, with no progression compared with prior imaging. Human leucocyte antigen (hla) typing revealed that she had the acute-onset t1dm-sensitive genotype DRB1*09:01. Serum C-peptide values show the time course of the decline in her insulin secretion (Figure 1).
The patient initially received isotonic saline and continuous insulin infusion intravenously, which was changed to daily subcutaneous insulin injections because of the persistence of endogenous insulin secretion exhaustion8 (Table II, Figure 1). Thyrotoxicosis attributed to Graves disease was improved by increasing the dose of potassium iodide to 100 mg daily. At 20 days after admission, because the patient had recovered from thrombophlebitis, her prednisolone 5 mg daily was changed to hydrocortisone 15 mg daily, which was continued as replacement therapy (Figure 1).
TABLE II.
Evaluation of insulin secretion by glucagon loading testa
| Variable | Day 8 | Day 15 | ||||
|---|---|---|---|---|---|---|
| Time (minutes) | 0 | 6 | 10 | 0 | 6 | 10 |
| Plasma glucose (mmol/L) | 5.4 | 5.4 | 6.2 | 16.1 | 15.9 | 16.0 |
| Serum C-peptide (nmol/L) | <0.03 | <0.03 | <0.03 | 0.03 | 0.03 | 0.03 |
The residual ability of pancreatic beta cells to secrete insulin was tested by intravenous administration of 1 mg glucagon, a highly sensitive and efficient method for diagnosing insulin insufficiency even in the presence of high blood glucose levels8.
The patient has had no recurrence of melanoma since the foregoing event.
DISCUSSION
We describe a case of acute-onset t1dm probably induced by nivolumab. Nivolumab progressively disturbed the patient’s insulin secretion, leading to exhaustion within a month. This case is the first in which a time-dependent decline of insulin secretion, leading to exhaustion, has been documented during the development of acute-onset t1dm. Despite daily glucocorticoid administration, nivolumab possibly induced t1dm in this patient who had a t1dm-sensitive genetic background.
Previous reports of t1dm induced by immune check-point inhibitors, and the present case, are summarized in supplemental Table 1. Diabetic ketoacidosis episodes6,7,9–15 similar to the episode in the present case were observed in 24 of 35 patients (69%), and 7 patients were diagnosed with fulminant t1dm6,7,14. Nivolumab was the main t1dm-inducing immune checkpoint inhibitor, but the period of treatment with immune checkpoint inhibitors before the development of t1dm varied between 1 week10 and 12 months6 (supplemental Table 1).
Our patient developed t1dm after 60 weeks and 28 courses of nivolumab. The episode of hyperglycemia that occurred 26 days before admission for diabetic ketoacidosis made it possible for us to observe the course of insulin secretion exhaustion probably induced by nivolumab. Initially, we did not diagnose t1dm, let alone an acute-onset type, because her insulin secretion was preserved during her first hyperglycemic event. However, her insulin secretion continuously declined and finally reached exhaustion (Figure 1, Table II). Her clinical course suggests that nivolumab-induced t1dm can present as a rapid decline in insulin secretion leading to exhaustion in approximately a month, which is slower than fulminant t1dm16.
In the 35 published cases of nivolumab-induced t1dm, islet autoantibodies have been reported to be positive in 17 patients7,10,15,17, negative in 175–7,9–14,18,19, and not measured in 1. As in the present case, rapidly progressive t1dm was observed in 8 patients without any detectable autoimmune islet antibodies6,7,10,13,18,19; however, those patients were hla-type-positive, which resulted in sensitivity to acute-onset t1dm (supplemental Table 1). Nivolumab probably induces rapidly progressive t1dm through an autoimmune mechanism independent of conventional pancreatic islet cell–related autoantibodies.
Of the 20 pat ients who were evaluated for hla typing, 17 showed the t1dm-sensitive type for their ethnicity6,7,10,13,18,19 (supplemental Table 1). Previous studies have shown that t1dm develops in people with specific hla types. Acute-onset t1dm that is anti-glutamic acid decarboxylase antibody–positive and fulminant t1dm that is anti-glutamic acid decarboxylase antibody–negative show distinct correlations with specific hla types4. The DRB1*09:01 type has been reported to be related to acute-onset t1dm. However, in the present case, we did not observe any elevation of t1dm-related autoantibodies, although the patient experienced acute-onset t1dm and had a sensitive hla type. In that context, PD-1 inhibition possibly induced an autoimmune diabetes, especially given that the patient had a t1dm-susceptible background (supplemental Table 1), although the pathologic mechanisms might differ from those observed in conventional clinical categories. Hence, through hla typing, the risk of immune checkpoint inhibitor–induced t1dm might be predictable.
Although the precise mechanisms of nivolumabinduced t1dm are not fully understood, activated CD8+ T cells have been speculated to evoke autoimmunity against and to destroy beta cells, resulting in insulin exhaustion3,20. Low-dose glucocorticoid (<10 mg daily) is sometimes used as monotherapy for immediate amelioration of inflammation in rheumatoid arthritis21. A prednisolone dose of 5 mg daily has been reported to be the cure for autoimmune pancreatitis22. However, prednisolone 5 mg daily, a dose sufficient to suppress adrenocortical function, could not prevent the development and progression of nivolumab-induced t1dm in our patient with her t1dm-sensitive genetic background. Glucocorticoids suppress the activity of the regulatory T cells, just as nivolumab does, which might be an important factor in preventing t1dm. The hla type and glucocorticoid dose also play a role in the extent of such suppression3,23. Our patient developed t1dm and showed exhaustion of insulin secretion despite daily glucocorticoid treatment. In that context, glucocorticoid could not prevent t1dm caused by the autoimmune-related beta-cell destruction.
Supplemental Table 1 presents 7 cases in which glucocorticoids were administered for various reasons after t1dm developed. In 6 patients, glucocorticoid was reportedly ineffective in reversing t1dm9–12,15,17; in the remaining 1 patient, dexamethasone administration transiently improved insulin insufficiency18. It is worth noting that, in the latter case, the patient did not present with a t1dm-sensitive hla type and was originally diagnosed with pancreatic diabetes, with incomplete decline in insulin secretion. Thus, the increase in plasma insulin observed in our patient might have been a result of dexamethasone-induced insulin resistance24 and not an improvement in nivolumabinduced t1dm. In our patient, nivolumab-induced t1dm was possibly glucocorticoid-resistant, although glucocorticoid therapies have been reported to be effective in relieving several iraes2 induced by nivolumab without disrupting its antitumour effect25. Elucidation of the exact mechanisms of immune-related t1dm will surely provide a novel means for preventing this critical irae, leading to safe management of several malignancies with this promising drug.
SUMMARY
We report a case of acute-onset t1dm probably induced by nivolumab-evoked autoimmunity despite continuous glucocorticoid administration. The patient showed a gradual but continuous decline in insulin secretion leading to exhaustion as part of the course of her disease. Nivolumab possibly induced t1dm because of the patient’s t1dmsensitive hla type, which might have acted as a sensitizing factor (among several probable causes). Physicians should be aware that nivolumab can induce t1dm and should not overlook this critical irae.
Supplementary Information
ACKNOWLEDGMENTS
We thank Editage (https://www.editage.jp/) for English-language editing.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
Supplemental material available at http://www.current-oncology.com
REFERENCES
- 1.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boutros C, Tarhini A, Routier E, et al. Safety profiles of anti-ctla-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13:473–86. doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 3.Sznol M, Postow MA, Davies MJ, et al. Endocrine-related adverse events associated with immune checkpoint blockade and expert insights on their management. Cancer Treat Rev. 2017;58:70–6. doi: 10.1016/j.ctrv.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Kawabata Y, Ikegami H, Awata T, et al. on behalf of the Committee on Type 1 Diabetes, Japan Diabetes Society. Differential association of hla with three subtypes of type 1 diabetes: fulminant, slowly progressive and acute-onset. Diabetologia. 2009;52:2513–21. doi: 10.1007/s00125-009-1539-9. [DOI] [PubMed] [Google Scholar]
- 5.Munakata W, Ohashi K, Yamauchi N, Tobinai K. Fulminant type I diabetes mellitus associated with nivolumab in a patient with relapsed classical Hodgkin lymphoma. Int J Hematol. 2017;105:383–6. doi: 10.1007/s12185-016-2101-4. [DOI] [PubMed] [Google Scholar]
- 6.Okamoto M, Okamoto M, Gotoh K, et al. Fulminant type 1 diabetes mellitus with anti–programmed cell death-1 therapy. J Diabetes Investig. 2016;7:915–18. doi: 10.1111/jdi.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Usui Y, Udagawa H, Matsumoto S, et al. Association of serum anti-gad antibody and hla haplotypes with type 1 diabetes mellitus triggered by nivolumab in patients with non–small cell lung cancer. J Thorac Oncol. 2017;12:e41–3. doi: 10.1016/j.jtho.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Faber OK, Binder C. C-Peptide response to glucagon. A test for the residual beta-cell function in diabetes mellitus. Diabetes. 1977;26:605–10. doi: 10.2337/diab.26.7.605. [DOI] [PubMed] [Google Scholar]
- 9.Lowe JR, Perry DJ, Salama AK, Mathews CE, Moss LG, Hanks BA. Genetic risk analysis of a patient with fulminant auto-immune type 1 diabetes mellitus secondary to combination ipilimumab and nivolumab immunotherapy. J Immunother Cancer. 2016;4:89. doi: 10.1186/s40425-016-0196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes J, Vudattu N, Sznol M, et al. Precipitation of autoimmune diabetes with anti–PD-1 immunotherapy. Diabetes Care. 2015;38:e55–7. doi: 10.2337/dc14-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Changizzadeh PN, Mukkamalla SKR, Armenio VA. Combined checkpoint inhibitor therapy causing diabetic ketoacidosis in metastatic melanoma. J Immunother Cancer. 2017;5:97. doi: 10.1186/s40425-017-0303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aleksova J, Lau PK, Soldatos G, McArthur G. Glucocorticoids did not reverse type 1 diabetes mellitus secondary to pembrolizumab in a patient with metastatic melanoma. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2016-217454. pii:bcr2016217454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickmott L, De La Peña H, Turner H, et al. Anti–PD-L1 atezolizumab-induced autoimmune diabetes: a case report and review of the literature. Target Oncol. 2017;12:235–41. doi: 10.1007/s11523-017-0480-y. [DOI] [PubMed] [Google Scholar]
- 14.Miyoshi Y, Ogawa O, Oyama Y. Nivolumab, an ant i–programmed cell death-1 antibody, induces fulminant type 1 diabetes. Tohoku J Exp Med. 2016;239:155–8. doi: 10.1620/tjem.239.155. [DOI] [PubMed] [Google Scholar]
- 15.Smith-Cohn MA, Gill D, Voorhies BN, Agarwal N, Garrido-Laguna I. Case report: pembrolizumab-induced type 1 diabetes in a patient with metastatic cholangiocarcinoma. Immunotherapy. 2017;9:797–804. doi: 10.2217/imt-2017-0042. [DOI] [PubMed] [Google Scholar]
- 16.Imagawa A, Hanafusa T, Awata T, et al. Report of the Committee of the Japan Diabetes Society on the research of fulminant and acute-onset type 1 diabetes mellitus: new diagnostic criteria of fulminant type 1 diabetes mellitus (2012) J Diabetes Investig. 2012;3:536–9. doi: 10.1111/jdi.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chae YK, Chiec L, Mohindra N, Gentzler R, Patel J, Giles F. A case of pembrolizumab-induced type-1 diabetes mellitus and discussion of immune checkpoint inhibitor–induced type 1 diabetes. Cancer Immunol Immunother. 2017;66:25–32. doi: 10.1007/s00262-016-1913-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumura K, Nagasawa K, Oshima Y, et al. Aggravation of diabetes, and incompletely deficient insulin secretion in a case with type 1 diabetes-resistant human leukocyte antigen DRB1*15:02 treated with nivolumab. J Diabetes Investig. 2018;9:438–41. doi: 10.1111/jdi.12679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumagai R, Muramatsu A, Nakajima R, et al. Acute-onset type 1 diabetes mellitus caused by nivolumab in a patient with advanced pulmonary adenocarcinoma. J Diabetes Investig. 2017;8:798–9. doi: 10.1111/jdi.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibbons Johnson RM, Dong H. Functional expression of programmed death–ligand 1 (B7-H1) by immune cells and tumor cells. Front Immunol. 2017;8:961. doi: 10.3389/fimmu.2017.00961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Everdingen AA, Jacobs JW, Siewertsz Van Reesema DR, Bijlsma JW. Low-dose prednisone therapy for patients with early active rheumatoid arthritis: clinical efficacy, disease-modifying properties, and side effects: a randomized, double-blind, placebo-controlled clinical trial. Ann Intern Med. 2002;136:1–12. doi: 10.7326/0003-4819-136-1-200201010-00006. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe M, Yamaguchi K, Kobayashi K, et al. Autoimmune pancreatitis diagnosed after pancreatoduodenectomy and successfully treated with low-dose steroid. J Hepatobiliary Pancreat Surg. 2007;14:397–400. doi: 10.1007/s00534-006-1179-0. [DOI] [PubMed] [Google Scholar]
- 23.Segaert S, Shear NH, Chiricozzi A, et al. Optimizing anti-inflammatory and immunomodulatory effects of corticosteroid and vitamin D analogue fixed-dose combination therapy. Dermatol Ther (Heidelb) 2017;7:265–79. doi: 10.1007/s13555-017-0196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogawa A, Johnson JH, Ohneda M, et al. Roles of insulin resistance and beta-cell dysfunction in dexamethasone-induced diabetes. J Clin Invest. 1992;90:497–504. doi: 10.1172/JCI115886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785–92. doi: 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.