The Editor
Current Oncology
11 December 2018
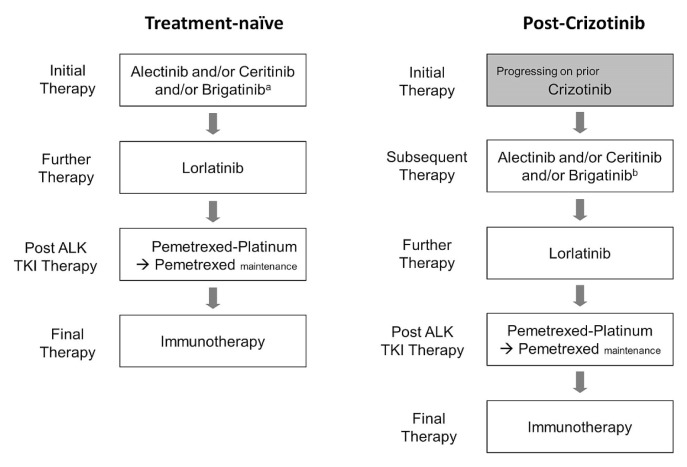
We recently published an article in Current Oncology titled “Canadian perspectives: update on inhibition of ALK-positive tumours in advanced non-small-cell lung cancer”1. Since that article was submitted to Current Oncology, new phase III data for brigatinib from the alta-1L study were presented at the International Association for the Study of Lung Cancer’s 19th World Conference on Lung Cancer (September 2018) and published in the New England Journal of Medicine2. Those data have changed our evidence-based perspective concerning the place of brigatinib as a treatment option in Canada (Figure 1).
FIGURE 1.
Revised treatment algorithms for ALK-positive non-smallcell lung cancer. aNot yet approved by Health Canada for first-line therapy. bHas received a Notice of Compliance with conditions from Health Canada based on the phase II ALTA study (full approval pending Health Canada review of the phase III ALTA-1L study). TKI = tyrosine kinase inhibitor.
The phase III alta-1L study compared brigatinib (180 mg once daily) with crizotinib (250 mg twice daily) in ALK-positive patients with non-small-cell lung cancer who had not previously received an alk inhibitor (n = 275). The primary endpoint was progression-free survival as assessed by an independent review committee. At a median follow-up of 11.0 months (range: 0–20.0 months) for brigatinib and 9.3 months (range: 0–20.9 months) for crizotinib, the results from the first pre-specified interim analysis showed a hazard ratio of 0.49 [95% confidence interval (ci): 0.33 to 0.74; p < 0.001] for disease progression or death favouring brigatinib2. The objective response rate (orr) was 71% (95% ci: 62% to 78%) for brigatinib and 60% (95% ci: 51% to 68%) for crizotinib. The intracranial orr was 78% (95% ci: 52% to 94%) for brigatinib and 29% (95% ci: 11% to 52%) for crizotinib. The cumulative incidence of intracranial disease progression was 9% (12 of 137 patients) for brigatinib and 19% (26 of 138 patients) for crizotinib, with a hazard ratio for time to progression of intracranial disease of 0.30 (95% ci: 0.15 to 0.60), with relatively short follow-up.
The phase II alta study randomized 222 crizotinib-refractory patients 1:1 to receive either brigatinib 90 mg once daily (n = 112) or brigatinib 180 mg once daily (n = 110)3. Patients could have received chemotherapy before receiving crizotinib, and in the 180 mg arm, 74% of the patients had received at least 1 prior line of chemotherapy. The primary endpoint was investigator-assessed confirmed orr, and the key secondary endpoints were progression-free survival and independent review committee–assessed intracranial orr. The study met its primary endpoint, and at the latest data cut-point4, it showed confirmed orrs of 56% (97.5% ci: 45% to 67%) and 46% (97.5% ci: 35% to 57%). The independent review committee–assessed median progression-free survival was 16.7 months (95% ci: 11.6 months to 21.4 months) in the 180 mg arm and 9.2 months (95% ci: 7.4 months to 12.8 months) in the 90 mg arm. The median overall survival for patients in the 180 mg arm was 34.1 months (95% ci: 27.7 months to not reached). Brigatinib was approved by the European Commission on 27 November 2018 for patients previously treated with crizotinib5, and it received a Notice of Approval with conditions from Health Canada on 30 July 20186.
The phase III data from alta-1L and the phase II data from alta provide a sound rationale for recommending brigatinib as a treatment option both for alk-inhibitor, treatment-naïve patients and for patients previously treated with crizotinib.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: BM serves on advisory boards for Novartis, Pfizer, and Roche; GL serves on advisory boards for and has received honoraria from AstraZeneca, Novartis, Pfizer, Roche, Merck, AbbVie, Bristol–Myers Squibb, and Takeda, and has received grants from AstraZeneca and Roche; PC has no conflicts of interest to disclose.
REFERENCES
- 1.Melosky B, Cheema P, Agulnik J, et al. Canadian perspectives: update on inhibition of ALK-positive tumours in advanced non-small-cell lung cancer. Curr Oncol. 2018;25:317–28. doi: 10.3747/co.25.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med. 2018;379:2027–39. doi: 10.1056/NEJMoa1810171. [DOI] [PubMed] [Google Scholar]
- 3.Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase–positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017;35:2490–8. doi: 10.1200/JCO.2016.71.5904. [DOI] [PubMed] [Google Scholar]
- 4.Huber RM, Kim DW, Ahn MJ, et al. Brigatinib (brg) in crizotinib (crz)–refractory ALK+ non–small cell lung cancer (nsclc): efficacy updates and exploratory analysis of cns orr and overall orr by baseline (bl) brain lesion status [abstract 9061] J Clin Oncol. 2018;36 doi: 10.1200/JCO.2018.36.15_suppl.9061. [Available online at: https://meetinglibrary.asco.org/record/160914/abstract; cited 18 December 2018] [DOI] [Google Scholar]
- 5.Columbus G. Brigatinib Approved in Europe for ALK+ NSCLC [Web news article] Cranbury, NJ: Intellisphere, LLC; 2018. [Available at: https://www.onclive.com/web-exclusives/brigatinib-approved-in-europe-for-alk-nsclc; cited 5 December 2018] [Google Scholar]
- 6.Takeda Canada. New Treatment Option for Canadians with Metastatic Lung Cancer [Web news release] Oakville, ON: Takeda Canada; 2018. [Available at: https://www.takeda.com/en-ca/newsroom/news-releases/2018/new-treatmentoption-for-canadians-with-metastatic-lung-cancer/; cited 5 December 2018] [Google Scholar]