Abstract
Background
Little evidence has been generated for how best to manage patients with non-small-cell lung cancer (nsclc) presenting with rarer clinical scenarios, including oligometastases, oligoprogression, and pseudoprogression. In each of those scenarios, oncologists have to consider how best to balance efficacy with quality of life, while maximizing the duration of each line of therapy and ensuring that patients are still eligible for later options, including clinical trial enrolment.
Methods
An expert panel was convened to define the clinical questions. Using case-based presentations, consensus practice recommendations for each clinical scenario were generated through focused, evidence-based discussions.
Results
Treatment strategies and best-practice or consensus recommendations are presented, with areas of consensus and areas of uncertainty identified.
Conclusions
In each situation, treatment has to be tailored to suit the individual patient, but with the intent of extending and maximizing the use of each line of treatment, while keeping treatment options in reserve for later lines of therapy. Patient participation in clinical trials examining these issues should be encouraged.
Keywords: Non-small-cell lung cancer, advanced; nsclc, advanced; oligometastatic disease; oligoprogression; pseudoprogression
BACKGROUND
Little evidence has been generated for how best to treat patients with non-small-cell lung cancer (nsclc) presenting with rarer clinical scenarios, including oligometastases, oligoprogression, and pseudoprogression. In each of those scenarios, oncologists have to consider how best to balance efficacy with quality of life, while maximizing the duration of each line of therapy and ensuring that patients are still eligible for later options, including clinical trial enrolment.
METHODS
An invited expert panel of thoracic oncology specialists in medical and radiation oncology and anatomic and molecular pathology was convened. Panellists were tasked to perform an evidence-based overview of specific topics related to oligometastatic and oligoprogressive nsclc and to pseudoprogression on immuno-oncology agents. Case presentations were used to illustrate typical examples of those rare clinical situations, and after an overview of the evidence by all attendees, evidence-informed recommendations for practice were developed. The guideline presented here was drafted by the first author with the assistance of a medical writer, and all authors provided feedback. The final guideline was approved by all authors and submitted for publication.
RESULTS
Non–Central Nervous System Oligometastatic and Oligopersistent Wild-Type NSCLC
Case Description
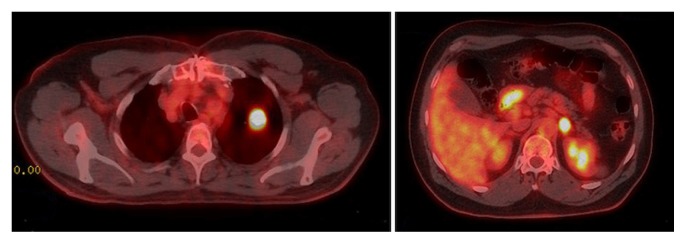
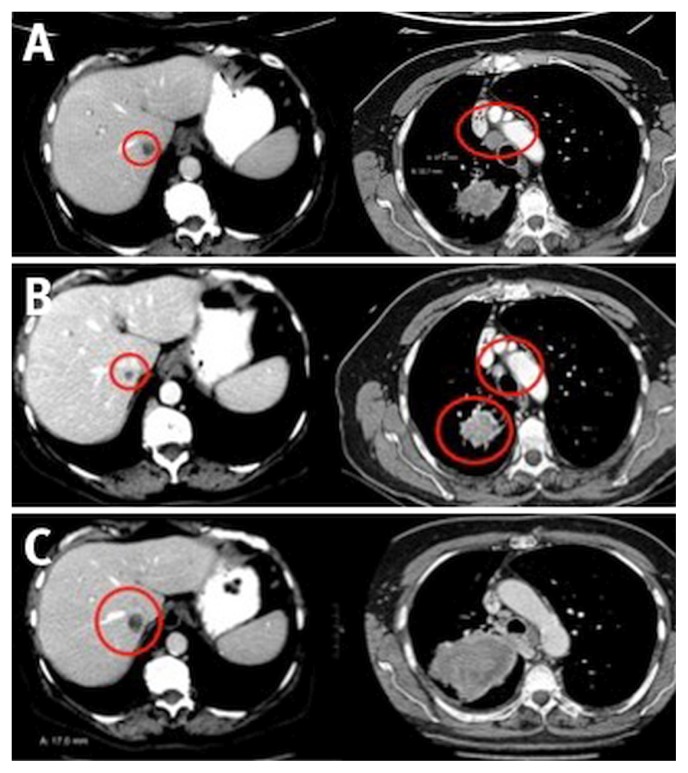
An incidental left upper lobe mass found in a 59-year-old male ex-smoker during a coronary angiogram was followed by serial computed tomography (ct) imaging until slight growth prompted investigations. Combined positron-emission tomography and ct imaging in March 2016 identified a 2.3 cm left upper lobe mass (standardized uptake value 13.6), and biopsy showed an adenocarcinoma, which was EGFR- and ALK-negative (Figure 1, left panel). A positron-emission tomography–positive 1.8 cm mass was also noted in the left adrenal gland (Figure 1, right panel), and although an adrenal biopsy in April 2016 showed only rare atypical cells, there was some concern that this location might represent a single site of metastatic disease.
FIGURE 1.
Combined positron-emission tomography–computed tomography imaging in March 2016 shows a 2.3 cm left upper lobe mass (left panel) and a positive (standardized uptake value 12.2) 1.8 cm mass in the left adrenal gland (right panel).
In June 2016, the patient had a left upper lobe lobectomy to remove a 2.3 cm node-negative invasive adenocarcinoma. Because the adrenal biopsy was non-diagnostic, benefit of the doubt led to planning for an adrenalectomy. However, before that surgery occurred, the patient developed a rapidly growing malignant right supraclavicular lymph node, and repeat imaging confirmed significant progression of his adrenal metastasis.
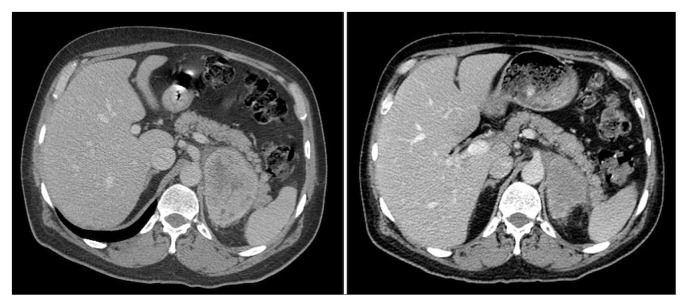
Carboplatin–gemcitabine chemotherapy was initiated, but by the 3rd cycle, the supraclavicular node had progressed, although the adrenal metastasis had shrunk to 6×5 cm from 8×7 cm. Because the patient was PD-L1 positive [≥50% tumour progression score by the Dako 28-8 pharmDx PD-L1 immunohistochemistry assay (Dako Corporation, Glostrup, Denmark)], he received 4 cycles of pembrolizumab. Initially, there appeared to be no clinical response, but after the 4th cycle, the patient experienced a rapid and excellent response such that the node in his neck was no longer palpable, and the adrenal metastasis had shrunk further to 2.9×1.5 cm by July 2017 (Figure 2). The patient continues on treatment and is doing well.
FIGURE 2.
Computed tomography imaging in October 2016, before administration of pembrolizumab (left panel), and in February 2017, after 4 cycles of pembrolizumab (right panel).
Panelist Presenters
Drs. J. Laskin and P. Cheung
Clinical Questions
■ What is oligometastatic nsclc, and proportionally, how many patients present in this fashion?
■ Is oligometastatic nsclc a distinct clinical entity?
■ Which patients warrant aggressive, localized ablative therapy of all sites of metastatic disease, either as initial therapy or after induction chemotherapy?
Oligometastasis, a term first formally defined in 19951, refers to a minimal metastatic state in which patients have a low burden of metastatic disease with only a small number of metastatic sites at initial presentation of their illness. Given that metastatic burden is a continuum, some authors question the existence of the oligometastatic state2. However, many believe that it represents a distinct group of patients who might have a more favourable outcome and in whom more aggressive therapy might be warranted. There are data to suggest that, compared with patients having more diffuse disease, those with fewer sites of metastases might experience longer survival3–5. The recognition that different sites and numbers of metastases are associated with different prognoses has been integrated into the 8th edition of the staging system for nsclc, in which malignant effusions or isolated contralateral lung metastases are considered M1a, a single site of extrathoracic metastatic disease is considered M1b, and more extensive extrathoracic metastatic disease is considered M1c6. “Oligopersistent disease” is a closely related concept referring to an oligometastatic state that, after systemic therapy, either persists or is induced from a more widely metastatic state.
It is known that patients with a solitary site of metastatic disease (most commonly brain or adrenal gland) who undergo surgical resection of both their primary and the metastasis can occasionally experience long-term survival or cure, and that dual resection is a generally accepted treatment strategy for such patients7–9. Whether patients with wild-type nsclc and more than a solitary site of distant metastatic spread should be considered for more aggressive localized therapy was the topic for discussion.
The development of increasingly sophisticated radiotherapy techniques [stereotactic body radiation (sbrt), also called stereotactic ablative radiation (sabr)] allows for the delivery of radical doses of radiation safely in a very short treatment time to almost any body site10, thus making the local control option feasible for some patients with metastatic nsclc.
The rationale for the treatment of oligometastatic and oligopersistent disease arises from the fact that, rather than develop metastases at new sites, many patients with advanced nsclc treated with systemic therapy relapse at a site of pre-existing disease11–13. Hypothetically, those sites will harbour chemotherapy-resistant clones and can serve to seed other sites with metastases. There are data to suggest that the larger the tumour deposit, the greater the likelihood of residual resistant clones, and thus the greater the likelihood of benefit from local control of that lesion14. Thus, it might be possible to delay the onset of treatment resistance and the development of new sites of metastases by aggressive ablative local therapy to the oligometastatic sites.
The absolute number of metastatic sites that constitutes the upper limit of the oligometastatic state remains a subject of debate, ranging from 3 or fewer7 or 5 or fewer15,16 to 6 active extracranial lesions (3 each in liver and lung parenchyma)17. Estimates of its occurrence fall into the 26%–55% range3,15,18,19, with the variation likely representing definition differences. An individual patient metaanalysis of 757 patients having 1–5 either synchronous or metachronous nsclc metastases found that most oligometastases were either in brain (35.5%) or lung (33.6%), followed by adrenal gland (13.0%), bone (8.5%), other (7.8%), liver (2.4%), and lymph node (2.4%)16. The meta-analysis revealed that, in patients treated with ablation to all sites of disease, including the primary, median overall survival was 26 months, and survival at 1, 2, 5, and 8 years was 70.2%, 51.1%, 29.4%, and 23.4% respectively. The longest survival times were observed in patients with metachronous metastases and an absence of nodal disease, but the 5-year overall survival rate was still 13.8% in patients with synchronous metastases and N1–2 disease.
Data about whether the treatment of oligometastatic or oligopersistent disease alters the natural history of advanced nsclc are limited, given that most of the published literature consists of retrospective case series or single-arm phase II trials and are thus subject to selection bias. Data suggest that, for treated patients, progression-free survival (pfs) or even overall survival might be prolonged in comparisons with historical controls17,20. The optimal sequencing of systemic therapy and local ablative therapy (lat) remains unclear. Initial ablative treatment to all disease might delay the need for initiation of systemic therapy in selected patients. It might also be a useful strategy for those not felt to be suitable for systemic therapy because of poor performance status or comorbidities, or for patients who want to avoid the toxicities of systemic therapy. However, somewhat more data about the use of local ablative therapies as consolidation treatment after the use of systemic therapy are available.
In a small randomized phase II study, 49 patients with oligometastatic nsclc (≤3 sites of metastatic disease) who had received at least 4 cycles of chemotherapy or 3 months of an appropriate targeted therapy and who had not progressed were randomized to maintenance systemic therapy or to sbrt to all sites of disease, followed by maintenance therapy7. Most patients (88%) had wild-type EGFR. The trial was halted early because a significant improvement in pfs in favour of sbrt was observed (11.9 months vs. 3.9 months; hazard ratio: 0.35; p = 0.0054), with no significant toxicities.
Since the consensus meeting, a second small (29 patients) single-centre randomized phase II study, enrolling only patients with wild-type EGFR and up to 5 sites of metastatic disease in addition to the primary lesion, has been published. It also revealed increased pfs (9.7 months vs. 3.5 months, p = 0.01) with no significant increase in toxic effects21. In that study (NCT02045446 at http://ClinicalTrials.gov/), patients who experienced stable disease or a partial response [by the Response Evaluation Criteria in Solid Tumors (recist)] after 4–6 cycles of first-line platinum-based chemotherapy were randomized to sabr plus maintenance chemotherapy or to maintenance chemotherapy alone. The results satisfied the hypothesis that using sabr prevented local failure at the original disease sites—the most likely sites of first recurrence. Based on the findings of that study, the use of radiation therapy after chemotherapy is being evaluated in a phase III setting for patients with limited metastatic nsclc.
Consensus Statement
Overall, the current level of evidence does not support the routine use of lat as the initial treatment in oligometastatic disease, for which systemic therapy remains the standard of care. Local treatment approaches could be considered for patients not suitable for, or who refuse or want to delay, systemic therapy.
Some available data suggest that the use of consolidative local ablative radiotherapy (sbrt) to all sites of disease in patients without progression after first-line systemic therapy might lead to longer pfs without undue toxicity. Those data were obtained mostly in patients with EGFR wild-type nsclc. We encourage the enrolment of such patients into ongoing clinical trials [such as nrg-lu002 (NCT03137771 at http://ClinicalTrials.gov/)] that are examining this issue. Outside a clinical trial, such an approach could be considered in selected patients.
Non–Central Nervous System Oligoprogressive Oncogene-Driven NSCLC
Case Description—Oligoprogressive Oncogene-Driven NSCLC, ALK Rearrangement
A previously well 42-year-old male never-smoker first presented in 2009 with extensive pulmonary infiltrates, biopsy-proven to be adenocarcinoma. During the subsequent year, he received multiple therapies, including a platinum doublet, pemetrexed, and erlotinib.
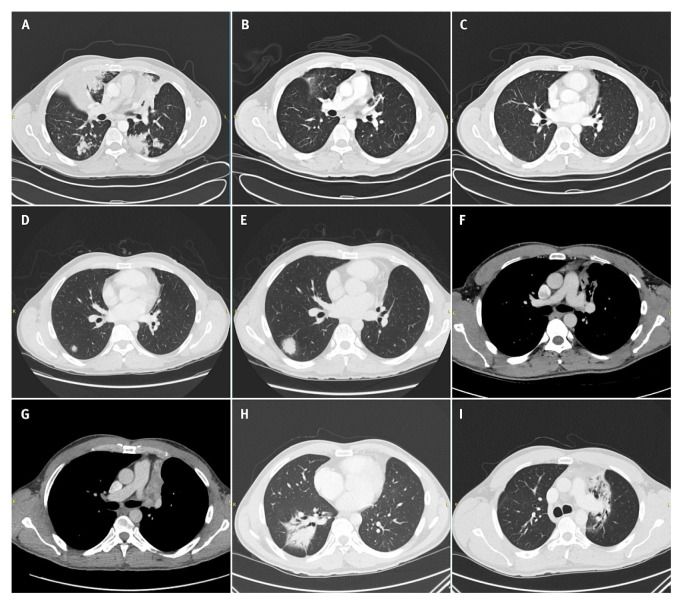
By mid-2010, the patient was very symptomatic with progressive disease, and results of fluorescence in situ hybridization testing revealed that he had an ALK rearrangement. He started treatment with crizotinib in October 2010 and experienced a dramatic response [Figure 3(A–C)]. He continued on crizotinib for several years. However, in March 2014, ct imaging showed a new nodule in the right lower lobe [Figure 3(D)]. Because the patient’s performance status was good and he remained asymptomatic, crizotinib was continued despite further progression in that nodule [Figure 3(E)].
FIGURE 3.
Computed tomography imaging showing the course of illness: (A) Before administration of crizotinib, October 2010. (B) After crizotinib treatment, December 2010. (C) Continued response to crizotinib, January 2013. (D) A new metastasis in the right lung, March 2014. (E) Growth of the metastasis, February 2015. (F) An area of tumour growth in the left upper lung, January 2016. (G) An area of tumour growth in the left upper lung, May 2016. (H) Right lower lobe lesion after stereotactic body radiotherapy, April 2017. (I) Left upper lobe lesion after stereotactic body radiotherapy, April 2017.
One year later, in January 2016, imaging showed continued growth of the nodule in the right lung and a new area of tumour growth in the left upper lobe [Figure 3(F,G)]. Given a concern for the possible development of symptoms from the left lung tumour, treatment with sbrt was delivered to the right lung in June 2016 and to the left lung in August 2016. Follow-up ct imaging in April 2017 showed typical radiation-related changes in both lungs and ongoing disease control [Figure 3(H,I)]. The patient has experienced only those two isolated areas of progression. The bulk of his metastatic burden has remained under control, and he remains well while still taking crizotinib.
Panelist Presenters
Drs. J. Rothenstein, S. Brule, R. MacRae, S. Banerji, and D. Hao
Clinical Questions
■ What is oligoprogression, and how often does it occur?
■ When might treatment past progression with a tyrosine kinase inhibitor (tki) be considered for patients with extracranial progressive disease?
Compared with standard platinum-based chemotherapy, targeted therapy for oncogene-driven (EGFR-mutated and ALK-translocated) nsclc is associated with significantly improved outcomes22–33. Most patients treated with an appropriate targeted therapy will experience some degree of tumour shrinkage. However, treatment resistance remains an inevitable occurrence, and at some point, all patients on targeted therapy will progress. Acquired resistance to first-line tkis typically develops after 9–12 months on erlotinib, gefitinib, or afatinib22,24,25,28 and after approximately 11 months on crizotinib31.
Acquired resistance can be attributed to a number of different mechanisms (concisely described elsewhere34) that can either already exist at low frequency in subclones at diagnosis or that can develop under the selective pressure of drug exposure34,35. Three different patterns of resistance can be observed with tki therapy: isolated central nervous system (cns) progression without extra-cns progression (discussed in a later subsection), generalized disease progression requiring a change in therapy (extra-cns with or without cns progression), and oligoprogression36.
Oligoprogressive disease describes a situation in which a patient develops disease progression in one or a limited number of sites after a targeted therapy has resulted in either a period of stable disease or a partial or complete response34,37. Some definitions describe a specific numbers of lesions, such as “≤4 discrete lesions amenable to invasive/non-invasive ablation”38. The frequency of oligoprogression during tki treatment varies depending on the definition used and whether isolated cns progression is included; however, estimates range from 15% to 47%11,37,38. Oligoprogression is felt to arise as a consequence of tumour heterogeneity, and the development of an isolated resistant subclone at only 1 or a few metastatic sites39.
An increasingly common method for treating oligo-progression in nsclc patients with driver mutations is to continue the tki that is controlling the greater proportion of the disease, while using lat to eradicate the resistant clones in the area or areas of progression. Given its relatively few fractions and short treatment time, sbrt might be preferred to more extended radiation schedules or invasive surgery, both of which can be associated with longer interruptions of the tki. Retrospective studies suggest that aggressive local treatment can eradicate tki-resistant oligometastases and could have several theoretical benefits, including prevention or treatment of local symptoms and complications from a growing tumour; prevention of secondary seeding by the tki-resistant clone or clones; and potential for ongoing maintenance with the current tki, which might be providing overall clinical benefit despite oligoprogression. Retrospective data suggest that sbrt can permit continuation of the tki and delay the time to a change in therapy. For example, in a review of 18 patients with EGFR mutation–positive disease treated with lat, the median time to another progression event was 10 months, and the median time to a change in therapy was 22 months40. In another cohort of 46 patients, the median time to another progression event was 7 months41. Similar results were observed in a cohort of 33 patients with ALK-positive lung cancer who experienced progression while on crizotinib. In 14 patients with oligoprogression who were suitable for lat, the median total duration of crizotinib was 28 months, compared with 10 months in those who progressed and were not suitable for lat42. Data suggest that higher radiation doses might lead to better local control of the oligoprogressive sites, although it is unclear whether doses as used for curative intent are required in this setting34.
Retrospective studies such as the foregoing are inherently susceptible to selection bias, and the lack of a control arm precludes definitive conclusions about the true value of lat in this setting. However, based on limited data, guidelines from the U.S. National Comprehensive Cancer Network currently recommend this strategy9. Ongoing clinical trials conducted specifically in oligoprogressive oncogene-driven nsclc (summarized in Kim et al.43) will help to provide prospective evidence for the use of this strategy in this situation, and participation in such trials should be encouraged. Likewise, as in the case of EGFR wild-type nsclc, there is also a desire to evaluate the role of lat in oligopersistent disease after a period of time on a tki in oncogene-driven disease. Sites of residual tumour could be more likely to harbour resistant subclones that could lead to treatment failure. Ongoing trials are studying this strategy (see NCT02759835, NCT01941654, and NCT01573702 at http://ClinicalTrials.gov/).
Consensus Statement
Canadian oncologists believe that certain selected patients with limited extra-cns oligoprogression, who are otherwise experiencing clinical benefit and good tolerance of their targeted therapy, could be considered for an approach that combines lat with continuation of their current targeted therapy. Generally, to avoid prolonged interruption of the targeted therapy, and because it is safe and effective, sbrt or sabr is, when possible, preferred to more protracted radiation courses or surgery. Careful and close follow-up of treated patients is required, because additional progression events are expected.
Case Description—Baseline CNS Oligometastatic Disease, Both Wild-Type and Oncogene-Driven
In October 2014, an otherwise healthy 49-year-old male architect presented with an 18-month history of progressive fatigue; intermittent left chest pain; and retrosternal, back, and eye pain.
Staging investigations revealed a dominant left upper lobe mass, with widespread metastases to mediastinal lymph nodes, lung, pleura, and brain. Biopsy of the lung lesions confirmed adenocarcinoma. Molecular testing of the biopsy sample revealed the presence of the EGFR exon 21 L858R mutation.
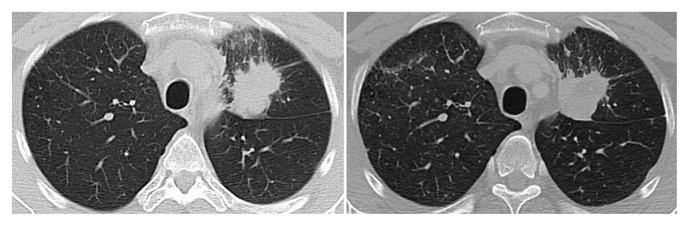
In November 2014, the patient received whole-brain radiotherapy (wbrt). He then started gefitinib. In December 2014, after 6 weeks of gefitinib, the patient demonstrated a slight interval decrease in the left upper lobe mass, stable small pulmonary nodules, and stable or improved brain metastases (Figure 4). The patient continued with gefitinib and continued to show a decrease in enhancing brain lesions and stable disease in the chest.
FIGURE 4.
Computed tomography imaging of chest at baseline (left panel) and after 6 weeks of gefitinib therapy (right panel), December 2015.
In January 2016, after 14 months taking gefitinib, the patient began to experience some headaches, and magnetic resonance imaging of the brain showed an interval increase in a cerebellar lesion. The remainder of his disease burden was stable, and so the cerebellar lesion was treated with stereotactic radiosurgery (srs), and the patient was continued on gefitinib.
In May 2016, after 18 months on gefitinib, the patient was still clinically well, but chest ct imaging suggested early progression, with slightly more prominent pulmonary nodules, suggestive of possible lymphangitic carcinomatosis. The patient continued gefitinib with closer monitoring.
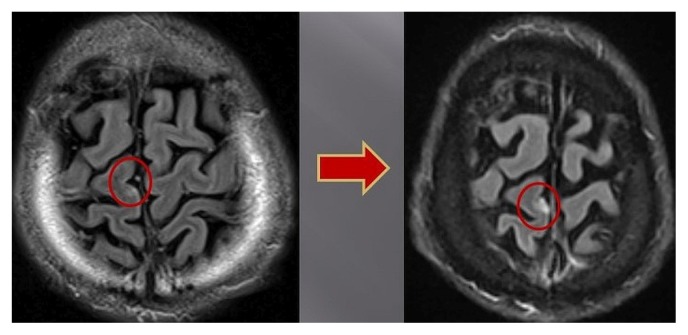
By the following month, June 2016, brain magnetic resonance imaging revealed that 6 enhancing lesions in the brain were starting to enlarge (Figure 5). The patient received further srs to all lesions and continued taking gefitinib.
FIGURE 5.
Magnetic resonance imaging of brain in June 2016 shows 1 of 6 enhancing lesions enlarging slightly.
In August 2016, the patient started to experience mild hemoptysis. Imaging by ct showed an increase in the size of the dominant left upper lobe mass, a bilateral increase in pulmonary nodules, appearance of new nodules, and worsening lymphangitic carcinomatosis [Figure 6(A,B)]. The molecular analysis of a biopsy sample from the left upper lobe mass revealed that the patient had an EGFR T790M resistance mutation. The gefitinib was therefore stopped after 21 months, and treatment with osimertinib was started.
FIGURE 6.
Computed tomography imaging (A) at baseline; (B) at August 2016, showing an increase in in the size of the dominant left upper lobe mass, an increase in bilateral pulmonary nodules, appearance of new nodules, and lymphangitic carcinomatosis; and (C) at December 2016, showing a slight decrease in the size of the left upper lobe mass with osimertinib treatment.
By September 2016, brain magnetic resonance imaging showed 2 enlarging brain metastases, which were treated by srs. The patient continued on osimertinib, and by December 2016, after 3 months on the drug, his best response was stable disease. Chest ct imaging showed a slight decrease in the size of the left upper lobe mass, with the remainder of his disease being stable [Figure 6(C)].
By March 2017, he had been treated with osimertinib for 7 months. A routine restaging ct showed that his pulmonary disease was completely stable, but a new paraaortic lymph node (1.8×2.2 cm) was visible. The nodule was irradiated (25 Gy in 5 fractions), and osimertinib treatment is ongoing.
Panelist Presenters
Drs. S. Brule, D. Roberge, V. Hirsh, N. Leighl, and D.J. Stewart
Clinical Questions
■ How should patients with brain oligometastases be treated?
Patients who present with symptomatic brain metastases require appropriate treatment with corticosteroids and consultations with radiation oncology or neurosurgery (or both). There has been a shift away from the use of wbrt, because it has become clear that this therapy is associated with short-term effects of asthenia and longer-term neurocognitive toxicity. Many authors advocate that its use be avoided or postponed whenever possible38. Thus, if radiation is felt to be required (either as definitive treatment or after resection), patients, whenever suitable, should be considered for srs. For patients with 1–4 metastases, srs is replacing wbrt, because srs is associated with improved cognitive outcomes44–46.
Multiple randomized trials have compared wbrt and srs with srs alone or wbrt alone44–51. In general, those studies have shown that overall survival is as good with srs alone as with strategies that use wbrt. At the time points studied, neurocognitive functioning has been better preserved with srs alone; however, cns recurrences are more common with srs alone than when wbrt is used. Strategies to decrease the neurocognitive effects of wbrt include the use of memantine, an N-methyl-d-aspartate receptor antagonist used in the treatment of dementia52, and the concept of hippocampal avoidance, which is being studied in randomized trials.
For patients with surgically resected metastases, adjuvant srs, compared with wbrt, provides inferior intracranial control at 12 months [40.7% (95% confidence interval: 25.9% to 64.2%) vs. 81.5% (95% confidence interval: 68.1% to 97.5%)]46. Although the randomized trial did not show that the use of local srs, compared with whole-brain treatment, is associated with an increased risk of leptomeningeal relapse, multiple published retrospective experiences show that such relapse can be an important pattern after srs alone53. Careful surveillance is a crucial component of any strategy, especially when wbrt is withheld, and the issues of neurocognitive impact and increased intracranial recurrence have to be discussed with patients.
In patients presenting with asymptomatic brain metastases that do not require corticosteroids and for whom urgent local therapy is not felt to be required, consideration could be given to initiating systemic therapy—particularly for patients with an oncogenic driver, in whom targeted agents have a high likelihood of controlling cns disease. Studies of gefitinib, erlotinib, and afatinib have revealed intracranial disease control rates of up to 89% in patients with nsclc showing common EGFR mutations54,55. In patients with measurable cns disease, response rates of up to 40% have been reported54,56,57. Similarly, in ALKtranslocated nsclc, crizotinib leads to intracranial disease control in 56% of patients with previously untreated brain metastases and in 62% of patients with previously treated brain metastases, with an objective response rate in the range of 18%–33% depending on whether the patient has received prior treatment58. Control of cns disease appears to be even higher with newer alk inhibitors. In the phase III ascend-4 trial comparing ceritinib with chemotherapy as first-line therapy32, ceritinib was associated with an intracranial response rate of 73% in patients with measurable disease, and at 24 weeks, a 70% rate of disease control in all 54 patients with brain metastasis (both measurable and non-measurable). In the more recently reported phase III alex trial59, which compared crizotinib with alectinib as first-line treatment, analysis of patients with measurable disease found that alectinib was associated with longer pfs overall and with an 81% intracranial response rate (38% complete response rate) compared with crizotinib’s 50% intracranial response rate (5% complete response rate), with a median duration of intracranial response of 17.3 months compared with 5.5 months. Furthermore, the risk of cns progression was markedly reduced with alectinib, even in patients with baseline cns metastases.
Standard platinum doublet chemotherapy has also been shown to have intracranial activity in ALK-positive nsclc. For example, in profile 1014, disease control rates with chemotherapy, while less than those with crizotinib, were approximately 40% at 12 weeks and 20% at 24 weeks in patients with previously treated brain metastases60. Similar results were seen for the chemotherapy arm of ascend-4, with an intracranial disease control rate of 55% at 24 weeks32. Fewer data about the value of chemotherapy in the treatment of EGFR mutation–positive brain metastases are available. In a pooled analysis of patients enrolled to lux-Lung 3 and 6, rates of cns progression with chemotherapy were similar to rates seen with afatinib in patients both with and without brain metastases at baseline55.
Newer tkis might result in even better outcomes for patients with brain metastases. Initial results of the phase III aura3 trial showed a longer duration of pfs for osimertinib compared with platinum therapy plus pemetrexed in the subgroup of patients with cns metastases61. Independent radiologic assessment of all intracranial metastases for that trial is ongoing, and so intracranial response and disease control rates are not yet available. Preliminary data from the flaura trial comparing osimertinib with erlotinib or gefitinib as first-line therapy in nsclc patients with common EGFR mutations were recently presented62, demonstrating that osimertinib shows durable cns control. Similarly, lorlatinib and brigatinib, next-generation ALK/ROS1 inhibitors, demonstrated substantial intracranial efficacy in phase II trials63,64.
Although data are limited, response rates to chemotherapy in previously untreated brain metastases from EGFR wild-type lung cancer appear to approach response rates in extracranial disease65—although response rates appear to be lower than those observed with targeted therapies in mutation-positive disease and could be lower than those observed with platinum-doublet chemotherapy in ALK-positive nsclc, given data suggesting that ALKpositive cancers might be more susceptible to pemetrexed66,67. Thus, any patient with wild-type nsclc for whom local brain-directed therapy is delayed in favour of a trial of systemic therapy will have to be monitored very closely for cns progression.
Oligoprogression in the CNS in Patients Without Baseline CNS Metastases and With Oncogene-Driven NSCLC
In some patients, the cns will be the sole site of progression, while extracranial disease remains completely or mostly controlled; in others, cns progression will be just one component of more generalized disease progression. In nsclc, cns metastases are a frequent occurrence, affecting 21%–64% of patients12,68, with one third of patients presenting with brain metastasis at baseline58. The rates of cns progression with first-line tki are 20%–46% for nsclc patients with ALK-positive translocations38,58 and 22%–52% for nsclc patients with EGFR mutations11,38,40,69. Treatment decisions will depend on the degree of progression both intracranially and extracranially, and on the systemic treatment options remaining for the patient. Regardless, patients with symptomatic cns metastases require the approach described earlier: corticosteroids and a referral to radiation oncology or neurosurgery (or both).
In patients with oncogene-driven asymptomatic cns progression in the setting of more widespread progression, a change of systemic therapy is usually warranted. If another targeted agent with cns activity is available, treatment with that agent and careful cns surveillance can be considered. In patients with cns oligoprogression alone, consideration could be given to local brain treatment and continuation of the current targeted therapy that is controlling the extra-cns disease. Alternatively, if another targeted agent is available that has cns activity, withholding local therapy and switching to the other targeted agent, with careful brain surveillance, could also be considered.
In pat ients with ALK-posit ive nsclc in whom oligoprogressive cns disease develops during crizotinib treatment, some data suggest that both ceritinib and alectinib (both available in Canada) can lead to an intracranial response, at rates of 45% and 52%–67% respectively70– 72. Osimertinib has been approved by Health Canada for patients with an acquired EGFR T790M resistance mutation after first- or second-generation tkis. A subset analysis of the aura 3 trial demonstrated that patients with 1 or more measurable or non-measurable cns metastases on baseline brain imaging experienced a significantly longer median pfs with osimertinib than with chemotherapy: 11.7 months compared with 5.6 months (p = 0.004). The cns objective response rate was 70% with osimertinib and 31% with chemotherapy for those with evaluable disease in the brain73.
Asymptomatic Oligoprogression in the CNS in Wild-Type NSCLC Without Baseline CNS Metastases
In this situation, greater consideration should be given to local therapy (srs, if feasible), given that data about the role of systemic therapies, either further cytotoxic chemotherapy or immunotherapy, are limited. Responses in the cns with immunotherapy have been reported74,75, but the relevant data are too preliminary to routinely support the use of immunotherapy specifically for the treatment of cns metastases. If a local therapy is deferred in this situation, very careful cns monitoring is recommended.
Consensus Statement
If clinically appropriate and possible, wbrt should be deferred in favour of srs. With small-volume asymptomatic brain metastases, consideration might be given to starting with systemic therapy, particularly if the patient has an oncogenic driver. Careful brain surveillance is vital to ensure disease control in the cns.
Pseudoprogression with Immuno-oncology Agents in NSCLC
Case Description
In February 2016, a fit 80-year-old woman with EGFR- and ALK-negative adenocarcinoma of the lung was started on therapy with a PD-1 inhibitor. Originally diagnosed with metastatic nsclc in 2013, her previous treatments had included carboplatin doublet chemotherapy and single-agent pemetrexed in addition to stereotactic radiation to liver and brain.
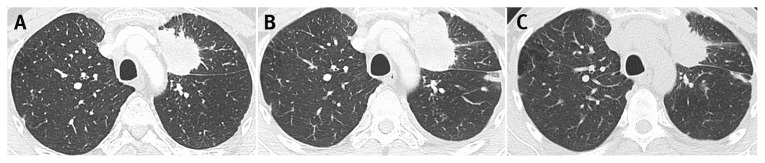
At restaging in June 2016 after several months of a PD-1 inhibitor, the patient’s liver lesions had grown in size, as had her paratracheal lymph node [Figure 7(A)]. Because she remained fit, with an Eastern Cooperative Oncology Group performance status of 0 and good tolerance to treatment save for a mild rash and hypothyroidism, the patient was continued on the PD-1 inhibitor. After 4 more doses, she was restaged, with clear evidence of improvement [Figure 7(B)], thus demonstrating that the original imaging was indicative of pseudoprogression. The patient was therefore continued on therapy. By February 2017, she was experiencing clinical deterioration, with increased pain and worsening performance status. At that point, imaging confirmed significant disease progression [Figure 7(C)] and immunotherapy was discontinued.
FIGURE 7.
Changes in computed tomography imaging over time. (A) In June 2016, compared with an earlier image, liver metastases have increased to 2.0 cm and 1.4 cm; the paratracheal node has increased to 1.2 cm; the right upper lung mass measures 3.7 cm (stable), but has changed in shape and decreased in attenuation. (B) In August 2016, liver metastases have decreased to 0.9 cm and 0.9 cm; the paratracheal node has decreased to 6 mm; the right upper lung mass has decreased to 2.6 cm. (C) In February 2017, the liver metastasis has increased to 1.7 cm; the paratracheal node is stable; and the right upper lung mass has increased to 9.3 cm, with invasion of the mediastinum.
Panelist Presenters
Drs. G. Nicholas, P.K. Cheema, N. Daaboul, M.S. Tsao, R. Juergens, and N. Blais
Clinical Questions
■ What is pseudoprogression?
■ How is it determined?
■ How often is it observed in nsclc?
■ How should a patient who manifests radiologic disease progression while on an immuno-oncology agent be treated?
Immunotherapy can be effective in nsclc: approximately 20% of patients with advanced disease experience durable responses with immune checkpoint inhibitor therapy76. Multiple anti–PD-1 and –PD-L1 antibodies have become standard therapies in the first- or second-line treatment of patients with advanced nsclc74,77–80, and still others are in various phases of clinical development. In contrast to cytotoxic chemotherapy and targeted agents, immunotherapy is associated with a number of response patterns, including delayed response to therapy, disease regression that continues even after therapy is discontinued, and pseudoprogression. Pseudoprogression has to be differentiated from hyperprogression—very rapid disease progression associated with clinical deterioration that has been described with immuno-oncology agents81 and which is not discussed here.
Definitions of pseudoprogression vary, but generally describe apparent tumour growth arising from a treatment effect rather than true disease progression. Distinguishing between pseudo and true disease progression is crucial to avoid prematurely discontinuing an effective therapy, while at the same time not continuing an ineffective, costly, and potentially toxic treatment and losing an opportunity to move on to another therapy if the patient deteriorates clinically from true disease progression.
In the initial clinical trials of immuno-oncology agents, it was clear that patients were occasionally manifesting radiologic disease progression that met recist 1.1 criteria for progressive disease, but were felt to be clinically benefiting from treatment. Those patients were continued on study therapy, and some subsequently went on to experience either a radiologic response or more prolonged stabilization of disease after the initial progression. After this “atypical” or “unconventional” response pattern was recognized, attempts were made to standardize its measurement using either international consensus (for example, immune-related response criteria82) or agent- and protocol-specific criteria (for example, in clinical trials of nivolumab74,78). More recently, the immunotherapy recist (irecist) criteria, based on recist 1.183, have been published84. The irecist framework standardizes tumour response evaluation in clinical trials of immunotherapy agents, but it requires further validation and is not meant for decision-making in routine clinical practice. However, aspects of irecist are helpful in defining pseudoprogression.
Pseudoprogression might manifest as temporary tumour growth or development of new lesions (or both) on imaging, subsequently followed by disease stabilization or a disease response85. The cause of the apparent tumour growth is unclear, but might possibly be related to infiltration of the tumour with immune effector cells and inflammation.
Table I summarizes differences between disease progression and pseudoprogression. Although changes in tumour size over time as measured on serial radiologic imaging will eventually distinguish the two, the patient’s clinical status at any given time is crucial. With true disease progression, the patient is more likely to experience a deterioration in performance status and worsening of disease-related symptoms. With pseudoprogression, the patient’s performance status usually remains stable or improves, systemic symptoms might or might not improve, and symptoms of tumour enlargement might or might not be present.
TABLE I.
Differences between disease progression and pseudoprogression82
| Characteristic | Pseudoprogression | Disease progression |
|---|---|---|
| Performance status | Improves or is stable | Deteriorates |
| Systemic symptoms | Might or might not improve | Worsens |
| Symptoms of tumour enlargement | Might or might not be present | Is present |
| Baseline tumour burden | Initial increase, followed by response or stabilization | Increase |
| New lesions | Appear, then remain stable or subsequently respond | Appear and increase in size |
| Biopsy outcomes | Evidence of immune-cell infiltration | No evidence of immune infiltration |
In clinical trials, some cancer patients treated beyond recist disease progression with immuno-oncology agents appear to do well86. However, in nsclc, pseudoprogression is uncommon; data from clinical trials in nsclc reveal that such “unconventional” responses occur much less than 10% of the time (Table II). Thus, although many patients have been treated past response in the clinical trials of immune agents, many received only a few additional cycles of therapy, suggesting that the greatest proportion of those patients were experiencing true disease progression (Table III). Those observations highlight the rarity of pseudoprogression and the rarity of long-term benefit resulting from treating past progression. Given current uncertainty about the role of PD-L1 status in selecting patients for immuno-oncology therapy, no data of which we are aware correlate baseline PD-L1 status and the likelihood of experiencing pseudoprogression. Given that genuine pseudoprogression is uncommon, the panel recommends close follow-up imaging in patients who continue treatment beyond progression to ascertain whether ongoing benefit is being achieved.
TABLE II.
Patients with non-small-cell lung cancer in trials of immuno-oncology therapy demonstrating pseudoprogression (PP)
| Reference (trial name) | Drug | Criteria used for progressive disease | PP (n/N) | Treatment past progression (% PP only) |
|---|---|---|---|---|
| Borghaei et al., 201578 (CheckMate 057, nonsquamous) | Nivolumab | RECIST 1.1 | 16/292 | 5.5 (range: 0.1 to 20.5+) at 5.56 months |
| Brahmer et al., 201574 (CheckMate 017, squamous) | Nivolumab | RECIST 1.1 | 9/135 | 6 (range: 0.8 to 6.5) at 3 months |
| Nishino et al., 201687 | Nivolumab | RECIST 1.1, irRECIST | 0/56 | 0 |
| Fehrenbacher et al., 201679, and Artal-Cortes et al., 201788 (POPLAR) | Atezolizumab | RECIST 1.1 | 5/144 | 3 |
| Kazandjian et al., 201789 (3 pooled trials, unidentified) | Anti–PD-1 | RECIST 1.1 | 10/535 | 1.9 |
RECIST = Response Evaluation Criteria in Solid Tumors; irRECIST = immune-related Response Evaluation Criteria in Solid Tumors.
TABLE III.
Characteristics of treatment past progression with immunotherapy
| Reference (trial name) | Treatment past progression | ||||
|---|---|---|---|---|---|
| Pts treated | Median weeks | Unconventional responses | |||
| (n/N) | (%) | (n) | (n/N) | (%) | |
| Borghaei et al., 201578 (CheckMate 057, nonsquamous) | 71/287 | 24.7 | 11 Doses (average) or 5.5 months | 16/292 | 5.6 |
| Brahmer et al., 201574 (CheckMate 017, squamous) | 28/135 | 21 | 5.8 Doses (average) or 3 months | 9/135 | 6 |
| Garon et al., 201590, and Hui et al., 201791 (KEYNOTE-001) | 32/101 | 4–9 Weeks 24 Days (range: 2–592 days) | |||
| Fehrenbacher et al., 201679 (POPLAR) | 5/144 | ||||
| Kazandjian et al., 201789 (3 spooled trials, unidentified) | 121/535 | 23 | Not stated | 10/535 | 1.9 |
| Rittmeyer et al., 201780 (OAK) | 243/609 | 40 | 3 Cycles (range: 1–34 cycles) | Not published | |
Pts = patients.
Consensus Statement
Canadian oncologists emphasize that pseudoprogression is uncommon in patients with nsclc treated with PD-1/ PD-L1 inhibitors. In patients showing clinical evidence of deterioration at radiologic progression, therapy should be discontinued, and the patient should be switched to another line of therapy, if appropriate. When selecting patients to continue on immunotherapy past recist progression, oncologists should use clinical judgment and take the patient’s clinical characteristics and burden of illness into consideration. Patients who continue immunotherapy must be in stable clinical condition, have no signs of rapid disease progression, and be tolerating the treatment. Patients who continue immunotherapy have to be continually monitored and re-imaged by ct at 4–8 weeks to ensure that they are not on a trajectory of true disease progression. No biomarkers are currently available to delineate between pseudo and true disease progression.
ACKNOWLEDGMENTS
This initiative was funded by an unrestricted educational grant from Pfizer Canada. Pfizer did not have representation at meetings, did not provide input into the content at any point, and did not review the consensus document before submission.
The authors thank Chrystal Palaty phd, from Metaphase Health Research Consulting Inc., for assistance with manuscript writing and submission.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: SAL has received honoraria from Pfizer, AstraZeneca, Boehringer Ingelheim, and Novartis; SB has served on advisory boards for AstraZeneca, Boehringer Ingelheim, Bristol–Myers Squibb, Eli Lilly, Novartis, Pfizer, and Roche, and has received research funding from AstraZeneca and Merck; NB has served on advisory boards for Amgen, AstraZeneca, Merck, Bayer, Boehringer Ingelheim, Bristol–Myers Squibb, Celgene, Eli Lilly, Novartis, Pfizer, Sanofi, and Roche; SB has received honoraria from AstraZeneca, Boehringer Ingelheim, Bristol–Myers Squibb, Eisai, and Merck; PKC has served as an advisor for AstraZeneca, Boehringer Ingelheim, Hoffmann–La Roche, Pfizer, Novartis, Takeda, Eli Lilly, and Bristol–Myers Squibb, and has received research funding from Boehringer Ingelheim and Hoffmann–La Roche; PC has received grants for investigator-initiated research projects from AbbVie, Pfizer, and Sanofi–Aventis; DH has served on advisory boards for AstraZeneca, Boehringer Ingelheim, Bristol–Myers Squibb, and Merck, and has received honoraria from Pfizer; VH has participated on advisory boards for AbbVie, AstraZeneca, Boehringer Ingelheim, Bristol–Myers Squibb, Merck, Pfizer, and Roche; RJ has served as an advisor or consultant for AstraZeneca/MedImmune, Bristol–Myers Squibb, Merck, Pfizer/emd Serono, and Roche, has received honoraria from AstraZeneca, Bristol–Myers Squibb, Merck, Pfizer/emd Serono, and Roche, and has received research funding from AstraZeneca, Bristol–Myers Squibb, and Merck; JL has received honoraria for talks from AstraZeneca, Boehringer Ingelheim, Pfizer, and Roche, and her institution has received grant funding for research trials from AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Roche; in the last 2 years, NL has received honoraria for independent unrelated continuing medical education from AstraZeneca, Bristol–Myers Squibb, and Merck; DR has received honoraria and support from Accuray, BrainLab, Elekta, Pfizer/emd Serono, Siemens Healthineers, and Varian Medical Systems; JR has received speaker and advisory board fees and institutional research support for clinical trials funded by Astra-Zeneca, Bristol–Myers Squibb, Merck, Pfizer, and Roche; DJS has received honoraria (2015–2018) from Roche Canada, Boehringer Ingelheim Canada, Novartis Canada, Merck Canada, AstraZeneca Canada, Bristol–Myers Squibb Canada, Exactis Innovation, and Pfizer Canada, and has received institutional research support from Boehringer Ingelheim, AstraZeneca, Novartis, Bristol– Myers Squibb, and Celgene; MST has received honoraria from AstraZeneca, Bristol–Myers Squibb, Merck, and Roche/Ventana, and a research grant from Merck. The remaining authors have no conflicts to disclose.
REFERENCES
- 1.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Palma DA, Salama JK, Lo SS, et al. The oligometastatic state—separating truth from wishful thinking. Nat Rev Clin Oncol. 2014;11:549–57. doi: 10.1038/nrclinonc.2014.96. [DOI] [PubMed] [Google Scholar]
- 3.Parikh RB, Cronin AM, Kozono DE, et al. Definitive primary therapy in patients presenting with oligometastatic non–small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;89:880–7. doi: 10.1016/j.ijrobp.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol. 1991;9:1618–26. doi: 10.1200/JCO.1991.9.9.1618. [DOI] [PubMed] [Google Scholar]
- 5.Torok JA, Gu L, Tandberg DJ, et al. Patterns of distant metastases after surgical management of non-small-cell lung cancer. Clin Lung Cancer. 2017;18:e57–70. doi: 10.1016/j.cllc.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Brierley JD, Gospodarowicz MK, Wittekind C, editors. The TNM Classification of Malignant Tumours. 8th ed. Chichester, UK: Wiley–Blackwell; 2017. [Google Scholar]
- 7.Gomez DR, Blumenschein GR, Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17:1672–82. doi: 10.1016/S1470-2045(16)30532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salah S, Tanvetyanon T, Abbasi S. Metastasectomy for extracranial extra-adrenal non-small cell lung cancer solitary metastases: systematic review and analysis of reported cases. Lung Cancer. 2012;7:9–14. doi: 10.1016/j.lungcan.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Non–Small Cell Lung Cancer. Ver. 2.2018. Fort Washington, PA: nccn; 2018. [Current version available online at: https://www.nccn.org/professionals/physician_gls/pdf/nscl_blocks.pdf (free registration required); cited 6 November 2018] [Google Scholar]
- 10.Timmerman RD, Herman J, Cho LC. Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. J Clin Oncol. 2014;32:2847–54. doi: 10.1200/JCO.2014.55.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Halabi H, Sayegh K, Digamurthy SR, et al. Pattern of failure analysis in metastatic EGFR-mutant lung cancer treated with tyrosine kinase inhibitors to identify candidates for consolidation stereotactic body radiation therapy. J Thorac Oncol. 2015;10:1601–7. doi: 10.1097/JTO.0000000000000648. [DOI] [PubMed] [Google Scholar]
- 12.Patel SH, Rimner A, Foster A, et al. Patterns of initial and intracranial failure in metastatic EGFR-mutant non–small cell lung cancer treated with erlotinib. Lung Cancer. 2017;108:109–14. doi: 10.1016/j.lungcan.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rusthoven KE, Hammerman SF, Kavanagh BD, Birtwhistle MJ, Stares M, Camidge DR. Is there a role for consolidative stereotactic body radiation therapy following first-line systemic therapy for metastatic lung cancer? A patterns-of-failure analysis. Acta Oncol. 2009;48:578–83. doi: 10.1080/02841860802662722. [DOI] [PubMed] [Google Scholar]
- 14.Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Fløtten O, Aasebø U. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2013;109:1467–75. doi: 10.1038/bjc.2013.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milano MT, Katz AW, Muhs AG, et al. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer. 2008;112:650–8. doi: 10.1002/cncr.23209. [DOI] [PubMed] [Google Scholar]
- 16.Ashworth AB, Senan S, Palma DA, et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin Lung Cancer. 2014;15:346–55. doi: 10.1016/j.cllc.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Iyengar P, Kavanagh BD, Wardak Z, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-smallcell lung cancer. J Clin Oncol. 2014;32:3824–30. doi: 10.1200/JCO.2014.56.7412. [DOI] [PubMed] [Google Scholar]
- 18.Mehta N, Mauer AM, Hellman S, et al. Analysis of further disease progression in metastatic non–small cell lung cancer: implications for locoregional treatment. Int J Oncol. 2004;25:1677–83. [PubMed] [Google Scholar]
- 19.Yano T, Okamoto T, Haro A, et al. Local treatment of oligometastatic recurrence in patients with resected non–small cell lung cancer. Lung Cancer. 2013;82:431–5. doi: 10.1016/j.lungcan.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Sheu T, Heymach JV, Swisher SG, et al. Propensity score-matched analysis of comprehensive local therapy for oligometastatic non–small cell lung cancer that did not progress after front-line chemotherapy. Int J Radiat Oncol Biol Phys. 2014;90:850–7. doi: 10.1016/j.ijrobp.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Iyengar P, Wardak Z, Gerber D, et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer—a phase 2 randomized clinical trial. JAMA Oncol. 2017;4:e173501. doi: 10.1001/jamaoncol.2017.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosell R, Carcereny E, Gervais R, et al. on behalf of the Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (eurtac): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 23.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (optimal, ctong-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 24.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 25.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (wjtog3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 26.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 27.Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non–small-cell lung cancer in Asia (ipass) J Clin Oncol. 2011;29:2866–74. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 28.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–34. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 29.Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (lux-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–22. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 30.Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (lux-Lung 3 and lux-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141–51. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 31.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–77. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 32.Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ascend-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917–29. doi: 10.1016/S0140-6736(17)30123-X. [DOI] [PubMed] [Google Scholar]
- 33.Shaw AT, Kim TM, Crinò L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ascend-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017;18:874–86. doi: 10.1016/S1470-2045(17)30339-X. [DOI] [PubMed] [Google Scholar]
- 34.Campo M, Al-Halabi H, Khandekar M, Shaw AT, Sequist LV, Willers H. Integration of stereotactic body radiation therapy with tyrosine kinase inhibitors in stage IV oncogene-driven lung cancer. Oncologist. 2016;21:964–73. doi: 10.1634/theoncologist.2015-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oxnard GR. The cellular origins of drug resistance in cancer. Nat Med. 2016;22:232–4. doi: 10.1038/nm.4058. [DOI] [PubMed] [Google Scholar]
- 36.Gandara DR, Li T, Lara PN, et al. Acquired resistance to targeted therapies against oncogene-driven non-small-cell lung cancer: approach to subtyping progressive disease and clinical implications. Clin Lung Cancer. 2014;15:1–6. doi: 10.1016/j.cllc.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basler L, Kroeze SG, Guckenberger M. sbrt for oligoprogressive oncogene addicted nsclc. Lung Cancer. 2017;106:50–7. doi: 10.1016/j.lungcan.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene addicted non–small cell lung cancer. J Thorac Oncol. 2012;7:1807–14. doi: 10.1097/JTO.0b013e3182745948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–92. doi: 10.1056/NEJMoa1113205. [Erratum in: N Engl J Med 2012;367:976] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu HA, Sima CS, Huang J, et al. Local therapy with continued egfr tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to egfr tyrosine kinase inhibitors. J Thorac Oncol. 2013;8:346–51. doi: 10.1097/JTO.0b013e31827e1f83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu B, Liang Y, Li Q, et al. Local therapy for oligoprogressive disease in patients with advanced stage non-small-cell lung cancer harboring epidermal growth factor receptor mutation. Clin Lung Cancer. 2017;18:e369–73. doi: 10.1016/j.cllc.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Gan GN, Weickhardt AJ, Scheier B, et al. Stereotactic radiotherapy can safely and durably control sites of extra-cns oligoprogressive disease in ALK-positive lung cancer patients on crizotinib. Int J Radiat Oncol Biol Phys. 2014;88:892–8. doi: 10.1016/j.ijrobp.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim C, Hoang CD, Kesarwala AH, Schrump DS, Guha U, Rajan A. Role of local ablative therapy in patients with oligometastatic and oligoprogressive non–small cell lung cancer. J Thorac Oncol. 2017;12:179–93. doi: 10.1016/j.jtho.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 44.Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316:401–9. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–44. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 46.Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (ncctg n107c/cec.3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1049–60. doi: 10.1016/S1470-2045(17)30441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the rtog 9508 randomised trial. Lancet. 2004;363:1665–72. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 48.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant wholebrain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the eortc 22952-26001 study. J Clin Oncol. 2011;29:134–41. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aoyama H, Tago M, Shirato H. Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: secondary analysis of the jrosg 99-1 randomized clinical trial. JAMA Oncol. 2015;1:457–64. doi: 10.1001/jamaoncol.2015.1145. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (jlgk0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15:387–95. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 51.Sperduto PW, Wang M, Robins HI, et al. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus wbrt and srs with temozolomide or erlotinib for non–small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int J Radiat Oncol Biol Phys. 2013;85:1312–18. doi: 10.1016/j.ijrobp.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown PD, Pugh S, Laack NN, et al. on behalf of the Radiation Therapy Oncology Group. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15:1429–37. doi: 10.1093/neuonc/not114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lamba N, Muskens IS, DiRisio AC, et al. Stereotactic radiosurgery versus whole-brain radiotherapy after intracranial metastasis resection: a systematic review and meta-analysis. Radiat Oncol. 2017;12:106. doi: 10.1186/s13014-017-0840-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bai H, Xiong L, Han B. The effectiveness of egfr-tkis against brain metastases in EGFR mutation-positive non-small-cell lung cancer. Onco Targets Ther. 2017;10:2335–40. doi: 10.2147/OTT.S129809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schuler M, Wu YL, Hirsh V, et al. First-line afatinib versus chemotherapy in patients with non–small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J Thorac Oncol. 2016;11:380–90. doi: 10.1016/j.jtho.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 56.Iuchi T, Shingyoji M, Sakaida T, et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer. 2013;82:282–7. doi: 10.1016/j.lungcan.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 57.Porta R, Sanchez-Torres JM, Paz-Ares L, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J. 2011;37:624–31. doi: 10.1183/09031936.00195609. [DOI] [PubMed] [Google Scholar]
- 58.Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33:1881–8. doi: 10.1200/JCO.2014.59.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peters S, Camidge DR, Shaw AT, et al. on behalf of the alex trial investigators. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–38. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 60.Solomon BJ, Cappuzzo F, Felip E, et al. Intracranial efficacy of crizotinib versus chemotherapy in patients with advanced ALK-positive non-small-cell lung cancer: results from profile 1014. J Clin Oncol. 2016;34:2858–65. doi: 10.1200/JCO.2015.63.5888. [DOI] [PubMed] [Google Scholar]
- 61.Mok TS, Wu YL, Ahn MJ, et al. on behalf of the aura3 investigators. Osimertinib or platinum–pemetrexed in EGFR T790M–positive lung cancer. N Engl J Med. 2017;376:629–40. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vansteenkiste J, Reungwetwattana T, Nakagawa K, et al. cns response to osimertinib vs standard of care (soc) egfr-tki as first-line therapy in patients (pts) with egfr-tki sensitising mutation (egfrm)–positive advanced non–small cell lung cancer (nsclc): data from the flaura study [abstract LBA5] Ann Oncol. 2017;28(suppl 10) [Google Scholar]
- 63.Solomon B, Shaw A, Ou S, et al. Phase 2 study of lorlatinib in patients with advanced ALK+/ROS1+ non-small-cell lung cancer [abstract OA 05.06] J Thorac Oncol. 2017;12(suppl 2):S1756. doi: 10.1016/j.jtho.2017.09.351. [DOI] [Google Scholar]
- 64.Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase–positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017;35:2490–8. doi: 10.1200/JCO.2016.71.5904. [DOI] [PubMed] [Google Scholar]
- 65.Oh Y, Stewart DJ. Systemic therapy for lung cancer brain metastases: a rationale for clinical trials. Oncology (Williston Park) 2008;22:168–78. [PubMed] [Google Scholar]
- 66.Park S, Park TS, Choi CM, et al. Survival benefit of pemetrexed in lung adenocarcinoma patients with anaplastic lymphoma kinase gene rearrangements. Clin Lung Cancer. 2015;16:e83–9. doi: 10.1016/j.cllc.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 67.Camidge DR, Kono SA, Lu X, et al. Anaplastic lymphoma kinase gene rearrangements in non–small cell lung cancer are associated with prolonged progression-free survival on pemetrexed. J Thorac Oncol. 2011;6:774–80. doi: 10.1097/JTO.0b013e31820cf053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nguyen T, Deangelis LM. Treatment of brain metastases. J Support Oncol. 2004;2:405–10. [PubMed] [Google Scholar]
- 69.Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of brain metastases in tyrosine kinase inhibitor–naïve epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol. 2017;35:1070–7. doi: 10.1200/JCO.2016.69.7144. [DOI] [PubMed] [Google Scholar]
- 70.Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (af-002jg): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15:1119–28. doi: 10.1016/S1470-2045(14)70362-6. [DOI] [PubMed] [Google Scholar]
- 71.Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17:234–42. doi: 10.1016/S1470-2045(15)00488-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Crinò L, Ahn MJ, De Marinis F, et al. Multicenter phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: results from ascend-2. J Clin Oncol. 2016;34:2866–73. doi: 10.1200/JCO.2015.65.5936. [DOI] [PubMed] [Google Scholar]
- 73.Mok T, Ahn MJ, Han JY, et al. cns response to osimertinib in patients (pts) with T790M-positive advanced nsclc: data from a randomized phase III trial (aura3) [abstract 9005] J Clin Oncol. 2017;35 doi: 10.1200/JCO.2017.35.15_suppl.9005. [Available online at: http://ascopubs.org/doi/abs/10.1200/JCO.2017.35.15_suppl.9005; cited 7 November 2018] [DOI] [PubMed] [Google Scholar]
- 74.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Eng J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17:976–83. doi: 10.1016/S1470-2045(16)30053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Melosky B, Chu Q, Juergens R, Leighl N, McLeod D, Hirsh V. Pointed progress in second-line advanced non-small-cell lung cancer: the rapidly evolving field of checkpoint inhibition. J Clin Oncol. 2016;34:1676–88. doi: 10.1200/JCO.2015.63.8049. [DOI] [PubMed] [Google Scholar]
- 77.Reck M, Rodríguez-Abreu D, Robinson AG, et al. on behalf of the keynote-024 investigators. Pembrolizumab versus chemotherapy for PD-L1–positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 78.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Eng J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fehrenbacher L, Spira A, Ballinger M, et al. on behalf of the poplar study group. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (poplar): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–46. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 80.Rittmeyer A, Barlesi F, Waterkamp D, et al. on behalf of the oak study group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (oak): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–65. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23:1920–8. doi: 10.1158/1078-0432.CCR-16-1741. [DOI] [PubMed] [Google Scholar]
- 82.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 83.Eisenhauer EA, Therasse P, Bogaerts J, et al. New Response Evaluation Criteria in Solid Tumours: revised recist guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 84.Seymour L, Bogaerts J, Perrone A, et al. irecist: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–52. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol. 2015;33:3541–3. doi: 10.1200/JCO.2015.61.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kurra V, Sullivan RJ, Gainor JF, et al. Pseudoprogression in cancer immunotherapy: rates, time course and patient outcomes [abstract 6580] J Clin Oncol. 2016;34 doi: 10.1200/JCO.2016.34.15_suppl.6580. [Available online at: http://ascopubs.org/doi/abs/10.1200/JCO.2016.34.15_suppl.6580; cited 7 November 2018] [DOI] [Google Scholar]
- 87.Nishino M, Ramaiya NH, Chambers ES, et al. Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. J Immunother Cancer. 2016;4:84. doi: 10.1186/s40425-016-0193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Artal-Cortes A, Mazieres J, Fehrenbacher L, et al. Evaluation of non-classical response by immune-modified recist and efficacy of atezolizumab beyond disease progression in advanced nsclc: results from the randomized phase II study poplar [abstract 96PD_PR] Ann Oncol. 2017;28(suppl 2) doi: 10.1093/annonc/mdx091.016. [DOI] [Google Scholar]
- 89.Kazandjian D, Keegan P, Suzman DL, Pazdur R, Blumenthal GM. Characterization of outcomes in patients with metastatic non–small cell lung cancer treated with programmed cell death protein 1 inhibitors past recist version 1.1–defined disease progression in clinical trials. Semin Oncol. 2017;44:3–7. doi: 10.1053/j.seminoncol.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 90.Garon EB, Rizvi NA, Hui R, et al. on behalf of the keynote-001 investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 91.Hui R, Garon EB, Goldman JW, et al. Pembrolizumab as first-line therapy for patients with PD-L1-positive advanced non–small cell lung cancer: a phase 1 trial. Ann Oncol. 2017;28:874–81. doi: 10.1093/annonc/mdx008. [DOI] [PMC free article] [PubMed] [Google Scholar]