Abstract
Background
Combined androgen blockade (cab) is a promising treatment modality for prostate cancer (pca). In the present meta-analysis, we compared the efficacy and safety of first-line cab using an antiandrogen (aa) with castration monotherapy in patients with advanced pca.
Methods
PubMed, embase, Cochrane, and Google Scholar were searched for randomized controlled trials (rcts) published through 12 December 2016. Hazard ratios (hrs) with 95% confidence intervals (cis) were determined for primary outcomes: overall survival (os) and progression-free survival (pfs). Subgroup analyses were performed for Western compared with Eastern patients and use of a nonsteroidal aa (nsaa) compared with a steroidal aa (saa).
Results
Compared with castration monotherapy, cab using an aa was associated with significantly improved os (n = 14; hr: 0.90; 95% ci: 0.84 to 0.97; p = 0.003) and pfs (n = 13; hr: 0.89; 95% ci: 0.80 to 1.00; p = 0.04). No significant difference in os (p = 0.71) and pfs (p = 0.49) was observed between the Western and Eastern patients. Compared with castration monotherapy, cab using a nsaa was associated with significantly improved os (hr: 0.88; 95% ci: 0.82 to 0.95; p = 0.0009) and pfs (hr: 0.85; 95% ci: 0.73 to 0.98; p = 0.007)—a result that was not achieved with cab using a saa. The safety profiles of cab and monotherapy were similar in terms of adverse events, including hot flushes, impotence, and grade 3 or 4 events, with the exception of risk of diarrhea and liver dysfunction or elevation in liver enzymes, which were statistically greater with cab using an aa.
Conclusions
Compared with castration monotherapy, first-line cab therapy with an aa, especially a nsaa, resulted in significantly improved os and pfs, and had an acceptable safety profile in patients with advanced pca.
Keywords: Prostate cancer, advanced; antiandrogens; androgen blockade, combined; castration monotherapy; overall survival; progression-free survival; safety
INTRODUCTION
After lung cancer, prostate cancer (pca) is the 2nd most common malignancy in men, with more than 1 million new cases estimated to be diagnosed annually worldwide1. Although the prevalence of pca is higher in Western populations than in Asian populations2, mortality rates have been declining in the United States, Canada, and Northern and Western Europe because of early diagnosis and improved treatment. In contrast, an alarming rise in mortality (attributed to growth in the economy) has been observed in some Asian countries such as the Republic of Korea, China (Hong Kong), and Kazakhstan3,4. A recent report about advanced pca in Asia showed a mortality-to-incidence ratio (40%) much higher than the global (25%), European (18%), and North American (10%) rates. Although detection of pca in China is improving, most patients are still diagnosed at an advanced stage4.
Androgen suppression with luteinizing hormone– releasing hormone analogs [lhrhas (medical castration)] or bilateral orchiectomy (surgical castration) was considered the standard treatment for metastatic pca5. The survival benefit of castration in combination with chemotherapy has been proved in randomized controlled trials (rcts) and has become a standard of care for men with metastatic hormone-sensitive pca who are eligible for chemotherapy. However, in real-world practice, because of factors such as advanced patient age, poor performance status, coexisting illnesses, and concerns about chemotherapy-related toxicity, not all newly diagnosed patients with metastatic disease are fit for, or will to be treated with, chemotherapy3. For such patients, optimal use of available drugs with a survival benefit and a considerable safety profile is practical and valuable. As evidence about the association of androgen synthesis with pca suggests6,7, complete inhibition of androgen might provide better treatment outcomes in such patients. That evidence led to introduction of combined androgen blockade (cab)—a combination of surgical or medical castration with an antiandrogen (aa)—as a promising treatment modality8. Evidence from previous meta-analyses comparing castration monotherapy (lhrha or orchiectomy) with cab using an aa are conflicting. A meta-analysis conducted by the Prostate Cancer Trialists’ Collaborative Group in 1995 combined the results of twenty-two rcts involving 5710 patients and showed no effect on survival for the administration of cab using an aa compared with monotherapy9. However, Caubet et al.10 demonstrated significantly favourable outcomes with cab using an aa. Another meta-analysis conducted by the Prostate Cancer Trialists’ Collaborative Group reported a 2%–3% improvement in 5-year survival with cab using a nonsteroidal aa (nsaa)11.
Previous studies comparing cab using an aa with castration monotherapies reported inconsistent findings in terms of efficacy for patients with advanced pca. Given that most of the rcts in Eastern populations were performed after the year 2000, evidence from those studies was not included in the various meta-analyses12,13. We therefore undertook this systemic review and meta-analysis comparing first-line cab using an aa with castration monotherapy (lhrha or orchiectomy) in terms of their efficacy and safety in adult men with advanced pca. Further, we evaluated differences in outcomes for patients by geography (Western vs. Eastern patients) and by the type of aa used [nsaa or steroidal aa (saa)] in cab.
METHODS
Search Strategy
This systematic review and meta-analysis was conducted in accordance with the prisma (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines14 and registered in the prospero international prospective register of systematic reviews (CRD42016054301, 2017).
The electronic databases medline (PubMed), embase, Cochrane Central Register of Controlled Trials (central), and Google Scholar were extensively searched for rcts published from inception to 12 December 2016. Specific search queries were formulated according to the pico framework (population, intervention, comparison, outcome) using specific keywords: “prostatic neoplasms,” “prostate cancer,” “prostatic carcinoma,” “prostatic adenocarcinoma,” “hormone replacement therapy,” “estrogen replacement therapy,” “androgen deprivation therapy,” and “androgen suppression therapy.” To cover the maximal time duration and include the maximal number of articles, no time limit was applied for the start date.
Selection Criteria
Initial screening of the articles was performed based on title and abstract. A full-text examination of the articles thus included was then conducted to assess relevance according to the defined inclusion and exclusion criteria. Articles were included in the analysis if they
■ were rcts involving patients with histologically confirmed pca.
■ enrolled patients with previously untreated advanced pca (including locally advanced and metastatic pca) that was then treated by cab using an aa or by castration monotherapy (lhrha or orchiectomy alone).
■ were published in English.
■ reported primary outcomes [overall survival (os) and progression-free survival (pfs), with presentation of hazard ratio (hr) or median survival time].
Articles were excluded if they
■ were not rcts (case reports, reviews, meta-analysis, etc.).
■ included patients undergoing second-line hormonal therapy (such as abiraterone and enzalutamide).
■ compared androgen deprivation therapy (adt) plus chemotherapy or adt plus radiotherapy with adt alone.
■ reported insufficient statistical data.
■ involved various castration treatments in the intervention and control groups (aa plus lhrha vs. orchiectomy, or aa plus orchiectomy vs. lhrha).
■ were published as separate articles (subgroup or post hoc analysis), but included the same set of patients (in this case, only the most recent complete publication was considered).
Literature Screening, Data Extraction, and Quality Assessment
All articles retrieved from the databases were screened to remove duplicates and were evaluated by 2 reviewers independently (YY, RC). Articles were included in the analysis if both reviewers agreed on their inclusion. Disagreements between reviewers were resolved by discussion and consensus. When consensus was not reached, the decision was made after consultation with a third reviewer.
The information extracted from the studies included study details (author name, year, sample size in the intervention and control groups, geography), patient demographics and treatment details (age, dose, and type of aa administered), efficacy (os and pfs duration), and safety [individual and grades 3 and 4 adverse events (aes)]. Quality assessment for the eligible studies was performed using the Jadad scale for reporting rcts, based on randomization, blinding, and accounting for all patients in each trial15. Each relevant article was scored as high-quality (4–5), medium-quality (3), or low-quality (0–2). Primary outcomes for the analysis were os and pfs. Safety was analyzed as a secondary outcome.
Statistical Analysis
The meta-analysis was performed using the R statistical software package (version 3.3.2: The R Foundation, Vienna, Austria). Baseline information is presented as descriptive statistics (that is, numbers) or qualitative values. If not provided, hrs with 95% confidence intervals (cis) were calculated for survival outcomes in all groups and are presented as forest plots. The hrs were calculated using these formulas16:
where M1 is median survival in control subjects, M2 is median survival in intervention subjects, E1 is the number of deaths in intervention subjects, and E2 is the number of deaths in control subjects.
Heterogeneity in the studies was determined using the Cochran Q test17 and I2 statistic18. A random-effects model was used for analysis when the p value for heterogeneity between studies was less than 0.05 or when I2 was greater than 50%. Otherwise, the analysis was performed using a fixed-effects model. Based on either of those models, the difference in efficacy outcomes between treatments was evaluated. Subgroup analyses were performed for all outcomes based on geography (Western vs. Eastern) and type of aa used for cab. Safety was determined in terms of event rates and risk ratios (rrs) with 95% cis. Western patients were defined as those included in studies performed in Europe, the Americas, and Africa; Eastern patients were defined as those included in studies from Asia.
RESULTS
Study Characteristics
Of 4863 studies from the selected databases that were screened, 3288 unique studies were selected. After further curation and application of the exclusion criteria, 16 relevant studies19–34 reporting median survival duration (os or pfs) or survival hr were included in the final analysis (Figure 1).
FIGURE 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow chart.
Table I summarizes the baseline characteristics of the patients. The included studies (12 from Western countries, 4 from Eastern countries; 11 using nsaas, 5 using saas) enrolled 6084 patients treated with either cab using an aa or castration monotherapy. Follow-up duration was 24–102 months. Five studies were rated as high-quality (Jadad score 4 or 5), three studies were rated as medium-quality (Jadad score 3), and the remaining eight studies were rated as low-quality (Jadad score 2).
TABLE I.
Characteristics of included studies
| Reference | Sample size | Intervention | Comparator | Geographic area | Score on the Jadad scale |
|---|---|---|---|---|---|
| Béland et al., 199020 | 194 | Nilutamide + bilateral orchiectomy | Bilateral orchiectomy | West | 5 |
| Crawford et al., 199021 | 603 | Flutamide + leuprolide | Leuprolide | West | 4 |
| Di Silverio et al., 199022 | 328 | CPA + goserelin | Goserelin | West | 2 |
| Benson et al., 199123 | 603 | Flutamide + leuprolide | Leuprolide | West | 3 |
| Jurincic et al., 199124 | 50 | Flutamide + goserelin | Goserelin | West | 2 |
| Tyrrell et al., 199125 | 589 | Flutamide + goserelin | Goserelin | West | 2 |
| Boccardo et al., 199326 | 373 | Flutamide + goserelin | Goserelin | West | 2 |
| Thorpe et al., 199627 | 350 | CPA + goserelin | Goserelin | West | 2 |
| Zalcberg et al., 199628 | 222 | Flutamide + bilateral orchiectomy | Bilateral orchiectomy | West | 4 |
| Dijkman et al., 199729 | 457 | Nilutamide + bilateral orchiectomy | Bilateral orchiectomy | West | 4 |
| de Voogt et al., 199830 | 224 | CPA + buserelin | Buserelin | West | 2 |
| Eisenberger et al., 199819 | 1387 | Flutamide + bilateral orchiectomy | Bilateral orchiectomy | West | 5 |
| Kotake et al., 199931 | 181 | CMA + goserelin | Goserelin | East | 2 |
| Akaza et al., 200332 | 151 | CMA + leuprolide | Leuprolide | East | 3 |
| Akaza et al., 200933 | 205 | Bicalutamide + goserelin–leuprolide | Goserelin–leuprolide | East | 2 |
| Kanetake et al., 201434 | 167 | Flutamide + goserelin–leuprolide | Goserelin–leuprolide | East | 3 |
CPA = cyproterone acetate; CMA = chlormadinone acetate.
OS
A fixed-effects model was used for the os analysis because heterogeneity testing revealed a low level of heterogeneity for the studies (I2 = 9%, τ2 = 0.0017, p = 0.38). The os analysis included fourteen studies19–23,25,26,28–34. The analysis showed that os was significantly prolonged for patients receiving cab using an aa compared with those receiving castration monotherapy (hr: 0.90; 95% ci: 0.84 to 0.97; p = 0.003; Figure 2).
FIGURE 2.
Overall survival (OS), study cohort overall. HR = hazard ratio; CI = confidence interval.
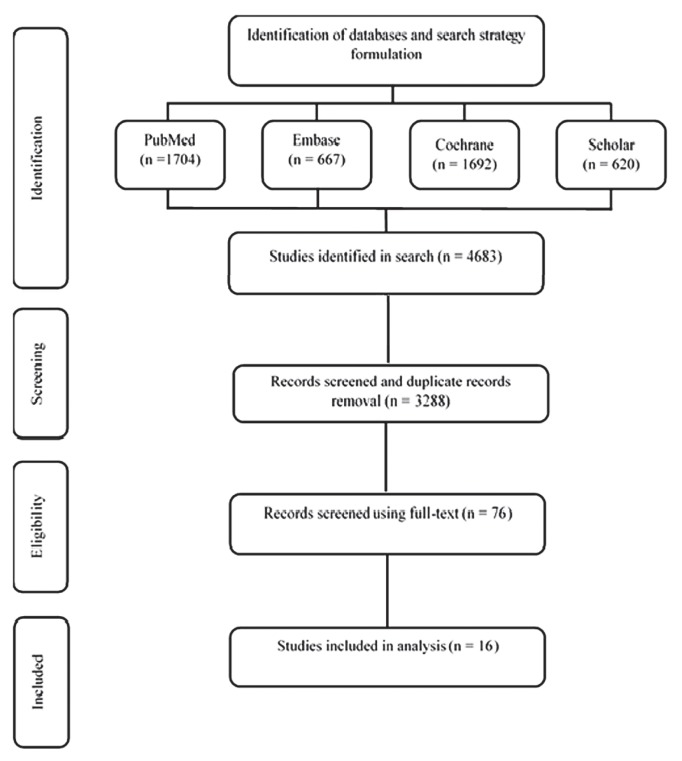
In the Western patients, os was significantly improved for those receiving cab using an aa than for those receiving castration monotherapy (hr: 0.90; 95% ci: 0.84 to 0.97; p = 0.0033). In contrast, no significant improvement in os was observed for Eastern patients (hr: 0.94; 95% ci: 0.75 to 1.19; p = 0.61). The nonsignificant improvement in os for the Eastern population is probably attributable to the smaller number of studies and the small sample size. No significant difference in os between the Western and Eastern patients was observed [p = 0.71, Figure 3(A)].
FIGURE 3.
Overall survival (OS), subgroup analysis. (A) Western patients compared with Eastern patients. (B) Nonsteroidal compared with steroidal antiandrogens. HR = hazard ratio; CI = confidence interval; Geo = geographic area.
Comparing the efficacy of cab using a nsaa with the efficacy of control treatments, os was significantly longer in the cab group than in the control group (hr: 0.88; 95% ci: 0.82 to 0.95, p = 0.0009). In contrast, os was nonsignificantly lower for cab using a saa than for control treatments [hr: 1.03; 95% ci: 0.86 to 1.25; p = 0.74; Figure 3(B)]. The difference in os for the different cab treatments was nonsignificant (p = 0.13).
PFS
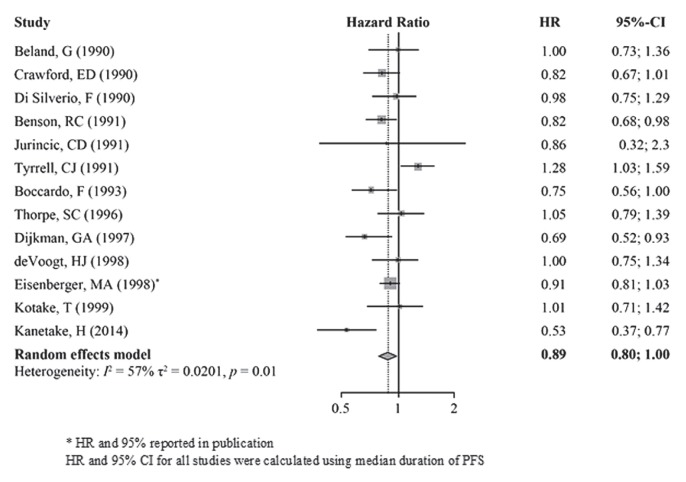
A random-effects model including thirteen studies19–27,29–31,34 (I2 = 57%, τ2 = 0.0201, p < 0.01) was used to compare PFS between CAB and monotherapy. The reported hr was significantly lower for cab than for castration monotherapy, indicating longer pfs (hr: 0.89; 95% ci: 0.80 to 1.00; p = 0.04; Figure 4).
FIGURE 4.
Progression-free survival (PFS), study cohort overall. HR = hazard ratio; CI = confidence interval.
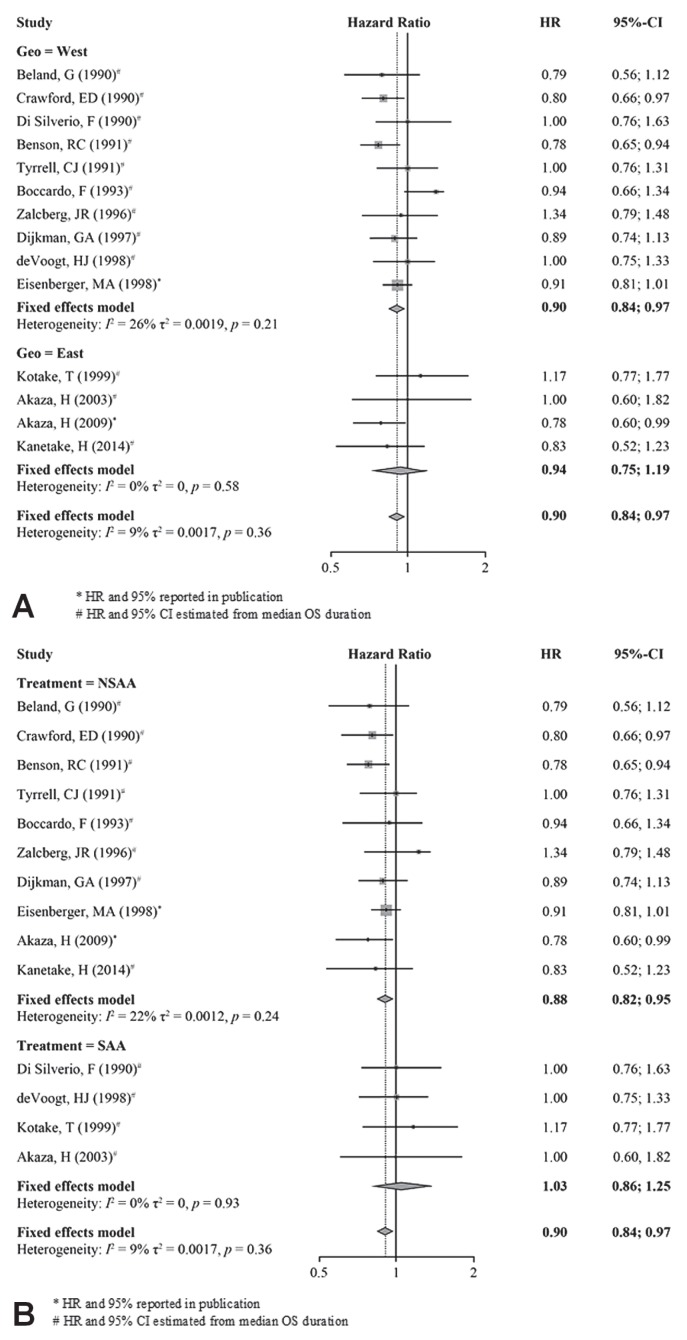
In the geographic subgroups, pfs was nonsignificantly longer with cab treatment than with control treatments [Western hr: 0.92; 95% ci: 0.85 to 1.01; p = 0.09; Eastern hr: 0.73; 95% ci: 0.39 to 1.38; p = 0.33; Figure 5(A)], with no significant different between the Western and Eastern populations [p = 0.49, Figure 5(A)].
FIGURE 5.
Progression-free survival (PFS), subgroup analysis. (A) Western patients compared with Eastern patients. (B) Nonsteroidal compared with steroidal antiandrogen. HR = hazard ratio; CI = confidence interval; Geo = geographic area.
Compared with patients receiving castration monotherapy, those receiving cab using a nsaa experienced significantly longer pfs (hr: 0.85; 95% ci: 0.73 to 0.98; p = 0.007). In contrast, treatment with cab using a saa and with castration monotherapy demonstrated similar pfs durations [hr: 1.01; 95% ci: 0.87 to 1.17; p = 0.74; Figure 5(B)]. Improvement in pfs was nonsignificantly greater for cab using a nsaa than for cab using a saa (p = 0.09).
Safety Evaluation
Safety events were reported in ten studies (eight Western, two Eastern)19,22,24–28,30,31,34. Table II presents the overall occurrence risk for all aes reported in the included studies. Impotence, decreased libido, and hot flushes were the aes most commonly reported in both groups (>10%); however, although nonsignificant, impotence (rr: 0.92; 95% ci: 0.39 to 2.48; p = 0.49), decreased libido (rr: 0.92; 95% ci: 0.76 to 1.14; p = 0.46), and hot flushes (rr: 0.87; 95% ci: 0.74 to 1.03; p = 0.12) occurred at lower rates in the cab group. The risks of the more frequent aes were similar for patients receiving cab using an aa and for those receiving castration monotherapy, except that risks of diarrhea (rr: 4.25; 95% ci: 2.72 to 6.65; p < 0.01) and liver dysfunction or elevated liver enzymes (rr: 2.64; 95% ci: 1.83 to 3.81; p < 0.01) were greater in the cab group. Grade 3 or 4 aes were reported in only one of the ten studies (in Western patients). In that study, 32 patients treated with cab and 28 patients treated with a control intervention experienced grade 3 or 4 aes (rr: 1.22; 95% ci: 0.78 to 1.90; p = 0.38).
TABLE II.
Safety events
| Adverse event | Studies (n) | Patients (n) | Events (%) | RR | 95% CI | p Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| CAB | Control | With CAB | With LHRHa or orchiectomy | |||||||
|
|
|
|||||||||
| With events | All | With events | All | |||||||
| Hot flushes | Seven19,22,25,27,28,30,31 | 212 | 1641 | 240 | 1623 | 13.00 | 15.00 | 0.87 | 0.74 to 1.03 | 0.12 |
|
| ||||||||||
| Diarrhea | Six19,24–28 | 99 | 1484 | 23 | 1466 | 6.67 | 1.56 | 4.25 | 2.72 to 6.65 | <0.01 |
|
| ||||||||||
| Liver dysfunction or elevated liver enzymes | Five19,25,26,28,34 | 108 | 1390 | 37 | 1320 | 7.77 | 2.80 | 2.64 | 1.83 to 3.81 | <0.01 |
|
| ||||||||||
| Gynecomastia | Four22,25,26,31 | 77 | 733 | 66 | 724 | 10.50 | 9.11 | 1.15 | 0.84 to 1.57 | 0.37 |
|
| ||||||||||
| Nausea to vomiting | Three19,24,25 | 48 | 1122 | 32 | 1105 | 4.28 | 2.90 | 1.48 | 0.95 to 2.29 | 0.08 |
|
| ||||||||||
| Rash | Three25–27 | 9 | 649 | 9 | 645 | 1.39 | 1.40 | 0.99 | 0.39 to 2.48 | 0.98 |
|
| ||||||||||
| Impotence | Three22,28,31 | 112 | 370 | 122 | 364 | 30.27 | 33.52 | 0.92 | 0.74 to 1.15 | 0.49 |
|
| ||||||||||
| Decreased libido | Three22,25,28 | 136 | 563 | 145 | 557 | 24.15 | 26.03 | 0.92 | 0.76 to 1.14 | 0.46 |
|
| ||||||||||
| Cardiovascular events | Three25,27,31 | 7 | 551 | 9 | 550 | 1.27 | 1.64 | 0.77 | 0.29 to 2.07 | 0.61 |
|
| ||||||||||
| Peripheral edema | Two25,27 | 6 | 462 | 5 | 459 | 1.30 | 1.09 | 1.19 | 0.72 to 2.39 | 0.77 |
|
| ||||||||||
| Any grade 3 or 4 event | One28 | 32 | 112 | 26 | 110 | 29.00 | 24.00 | 1.22 | 0.78 to 1.90 | 0.38 |
CAB = combined androgen blockade; LHRHa = luteinizing hormone–releasing hormone analog; RR = relative risk; CI = confidence interval.
Overall, the analyses indicated that, for most aes (except diarrhea and liver enzymes), safety profiles were comparable for cab and for castration monotherapy.
DISCUSSION
The present systematic review and meta-analysis demonstrates that, for patients with advanced pca treated with cab, os and pfs are longer (os hr: 0.90; 95% ci: 0.84 to 0.97; p = 0.003; and pfs hr: 0.89; 95% ci: 0.80 to 1.00; p = 0.04), and the treatment safety profile is acceptable. To the best of our knowledge, our meta-analysis is the first to include rcts comparing cab with castration monotherapy in Eastern patients and to present outcome comparisons by geographic location (Western vs. Eastern). In addition, given the trend toward the use of nsaas in recent years (because of their acceptable efficacy and better tolerability compared those for saas), we also assessed how the different types of cab (cab using nsaas vs. cab using saas) compared with control treatments.
Most of the included studies reported a nonsignificantly longer median os with cab than with castration monotherapy19,20,23– 26,28,30–32,34. In the study by Crawford et al.21, patients treated with cab, compared with those treated with lhrha (leuprolide) alone, experienced significantly longer os (35.0 months vs. 27.9 months, p = 0.03) and lower all-cause mortality (173 vs. 199 deaths). Compared with another lhrha (goserelin), cab was also associated with lower overall mortality (26 vs. 38 deaths) and a significantly improved median os (p = 0.05)32. Similar results were obtained when cab treatment was compared with orchiectomy in advanced pca (27.3 months vs. 23.6 months, p = 0.03)29.
Our analysis demonstrated significantly greater efficacy for cab than for castration monotherapy in terms of prolonging os in men with advanced pca. This clinical benefit of cab using an aa—and especially a nsaa—in our analysis was probably attributable to increased patient numbers. Therefore, cab using an aa might be preferred as a first-line treatment option for patients with advanced pca. Other rcts also concluded that patients treated with cab live longer and have a higher survival rate35–38, supporting the efficacy of first-line cab using an aa in advanced pca. However, the latter rcts were excluded from our analysis because they did not report hrs or median survival (os, pfs) for the treatments of interest, or because patients received other treatments.
Evidence from the included studies19,21–24,26,29,34 suggests that pfs is longer with cab than with castration monotherapy. A few studies also reported that pfs is similar20,30 or shorter25,27,31 in patients receiving cab using an aa and in those receiving castration monotherapy—a result that was possibly a result of patient deaths or loss to follow-up.
Results from our analysis accord with the results from most rcts, showing a significant overall increase in pfs for patients treated with cab compared with those treated with castration monotherapy in the first line (hr: 0.89; 95% ci: 0.80 to 1.00; p = 0.04). Our results are supported by findings in other studies in which a higher pfs was reported with cab than with orchiectomy alone35–37. In the recently concluded latitude study39, which compared adt–abiraterone–prednisolone with adt alone in patients with high-risk metastatic castration-sensitive pca, os and pfs were reported to be significantly longer in the experimental group (not reached vs. 34.7 months; hr: 0.62; 95% ci: 0.51 to 0.76; p < 0.001; and 33.0 months vs. 14.8 months; hr: 0.47; 95% ci: 0.39 to 0.55; p < 0.001 respectively). Moreover, in the stampede study of patients with advanced pca, the number of deaths (184 vs. 262, p < 0.001) and the incidence of treatment failure (262 vs. 535, p < 0.001) were significantly lower with the adt–abiraterone–prednisolone combination than with adt alone40. However, safety events occurred more frequently in the combination group than in the adt-only group39,40. Findings in both studies indicated that adt (castration) alone is not sufficient for the first-line treatment of patients with advanced pca, validating our results as well. However, in developing countries, long-term use of abiraterone will be an economic hurdle for most patients, and the cost-effectiveness of treatment will be an important factor in the treatment decision. Treatment with cab using an aa—and especially a nsaa—has proved to be more cost-effective than castration monotherapy41, which makes cab a compelling treatment regimen, especially in developing countries.
Our comparative evaluation of cab by geographic area observed that os (p = 0.33) and pfs (p = 0.71) were similar in Western and Eastern groups of patients. The Western world has already adopted the practice of medical castration (lhrha) in preference to surgical castration42, and that practice is also becoming prevalent in the Eastern world43. With the results of the present study showing a greater response to cab than to to either lhrha monotherapy or orchiectomy, the use of cab as first-line therapy could further scale up. In addition, most aes in our analysis were nonsignificantly higher in patients receiving cab than in patients treated with castration monotherapy, which accords with previously published findings41. Our meta-analysis is the first to include rcts involving Eastern patients and to assess any differences in treatment outcomes on the basis of geography—unlike previous analyses, which included only Western patients8–13,44.
In our analysis, significantly longer survival durations were associated with cab using a nsaa than with castration monotherapy; cab using a saa did not achieve the same result. Although the difference between cab using a nsaa and cab using a saa was nonsignificant, the higher survival duration with cab using a nsaa suggests its greater clinical benefit compared with cab using a saa. Our study findings correlate with findings from the meta-analysis by the Prostate Cancer Trialists’ Collaborative Group11, in which mortality rates were lower for cab using a nsaa than for castration monotherapy (nilutamide: 69.5% vs. 73.4%; flutamide: 71.1% vs. 73.3%). However, mortality rates were higher for treatment with cab using a saa than for castration monotherapy (69.2% vs. 68.6%). Our results are further supported by findings from a previously concluded study, in which nsaas as first-line therapy for advanced pca were associated with improvements in survival45, indicating that nsaas are a more suitable choice from among the aas.
In terms of safety, for the aes most commonly reported with castration (10%—including impotence, decreased libido, and hot flushes), incidence rates in our analysis were similar in the intervention and control groups. Moreover, grades 3 and 4 aes were not significantly greater in patients treated with cab using an aa. Furthermore, saas are typically found to be associated with a greater incidence of aes such as decreased libido and increased edema and cardiovascular events46, also making nsaas a more suitable choice from among the aas. In our analysis, cab using an aa, compared with castration monotherapy, was associated with significantly greater incidences of diarrhea and elevation in liver enzymes. In our analysis, most studies reporting a higher risk of diarrhea and elevation in liver enzymes used flutamide. Our findings are consistent with reported evidence that the incidences of diarrhea and of elevation of liver enzymes are significantly greater with the use of cab with flutamide in both white and Japanese patients41,47,48. Studies have demonstrated advantages in tolerability for bicalutamide compared with flutamide as a component of cab, specifically with respect to abnormal hepatic function and diarrhea, providing a compelling rationale for using bicalutamide as a component of cab49,50. Therefore, given the significantly greater clinical benefits in terms of survival prolongation and clinically acceptable safety profile, cab using an aa (preferably bicalutamide, a nsaa) could be used as primary treatment for advanced pca after a proper risk evaluation in the patient.
The present systematic review and meta-analysis has a number of strengths. In addition to its overall comparison of cab with lhrha or orchiectomy, we compared the effects of cab by geographic are (Western, Eastern) to evaluate any differences in outcome related to ethnicity, and we compared cab using a saa or a nsaa with castration monotherapy to determine the better choice of an aa for cab.
Our study reports a similar safety profile for cab using an aa and for castration monotherapy, indicating a clinically acceptable safety profile for cab using an aa. Furthermore, we used robust methods and an extensive literature search for the systematic review of rcts to ensure the inclusion of the most relevant and high-quality articles. Our study also has a few limitations. First, because of unavailability of data in most of the included studies, we could not compare parameters such as time to treatment failure and quality of life. Second, only four studies included Eastern patients. Third, a few studies were not included in the analysis because of the unavailability of relevant survival data—for example, those in which median survival time, odds ratios, or hrs with 95% cis were not presented. Finally, we could not separately analyze patients undergoing cytoreductive prostatectomy because of the scant number of studies (two of sixteen) and low number of patients (n = 276).
CONCLUSIONS
The present meta-analysis confirms the better efficacy, in terms of survival for patients with advanced pca, of first-line cab using an aa compared with castration monotherapy. No significant difference was observed for patients coming from different geographic locations. The overall risk of aes, including grade 3 or 4 aes, was similar for patients receiving cab and for patients receiving castration monotherapy. Considering the significant improvement in survival and acceptable safety profile associated with cab using an aa (and especially a nsaa), cab might be a reasonable option for first-line therapy in patients with advanced pca.
ACKNOWLEDGMENTS
The authors acknowledge Karan Sharma and Dr. Amit Bhat (Indegene Pvt. Ltd., Bangalore, India) for providing statistical and medical writing support and technical assistance in the development of this manuscript.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none. The study was funded by AstraZeneca Pharmaceutical Co., Ltd.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87– 108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Marugame T, Katanoda K. International comparisons of cumulative risk of breast and prostate cancer, from Cancer Incidence in Five Continents. Vol. VIII. Jpn J Clin Oncol. 2006;36:399–400. doi: 10.1093/jjco/hyl049. [DOI] [PubMed] [Google Scholar]
- 3.Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–92. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 4.Chen R, Ren S, Yiu MK, et al. on behalf of the Chinese Prostate Cancer Consortium. Prostate cancer in Asia: a collaborative report. Asian J Urol. 2014;1:15–29. doi: 10.1016/j.ajur.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denis L, Murphy GP. Overview of phase III trials on combined androgen treatment in patients with metastatic prostate cancer. Cancer. 1993;72(suppl):3888–95. doi: 10.1002/1097-0142(19931215)72:12+<3888::AID-CNCR2820721726>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 6.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22:232–40. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad I, Sansom OJ, Leung HY. Advances in mouse models of prostate cancer. Expert Rev Mol Med. 2008;10:e16. doi: 10.1017/S1462399408000689. [DOI] [PubMed] [Google Scholar]
- 8.Moul JW, Wu H, Sun L, et al. Early versus delayed hormonal therapy for prostate specific antigen only recurrence of prostate cancer after radical prostatectomy. J Urol. 2008;179(suppl):S53–9. doi: 10.1016/j.juro.2008.03.138. [DOI] [PubMed] [Google Scholar]
- 9.Prostate Cancer Trialists’ Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of 22 randomised trials with 3283 deaths in 5710 patients. Lancet. 1995;346:265–9. doi: 10.1016/S0140-6736(95)92163-X. [DOI] [PubMed] [Google Scholar]
- 10.Caubet JF, Tosteson TD, Dong EW, et al. Maximum androgen blockade in advanced prostate cancer: a meta-analysis of published randomized controlled trials using nonsteroidal antiandrogens. Urology. 1997;49:71–8. doi: 10.1016/S0090-4295(96)00325-1. [DOI] [PubMed] [Google Scholar]
- 11.Prostate Cancer Trialists’ Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet. 2000;355:1491–8. doi: 10.1016/S0140-6736(00)02163-2. [DOI] [PubMed] [Google Scholar]
- 12.Samson DJ, Seidenfeld J, Schmitt B, et al. Systematic review and meta-analysis of monotherapy compared with combined androgen blockade for patients with advanced prostate carcinoma. Cancer. 2002;95:361–76. doi: 10.1002/cncr.10647. [DOI] [PubMed] [Google Scholar]
- 13.Bennett CL, Tosteson TD, Schmitt B, Weinberg PD, Ernstoff MS, Ross SD. Maximum androgen-blockade with medical or surgical castration in advanced prostate cancer: a metaanalysis of nine published randomized controlled trials and 4128 patients using flutamide. Prostate Cancer Prostatic Dis. 1999;2:4–8. doi: 10.1038/sj.pcan.4500265. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Shamseer L, Clarke M, et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (prisma-p) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiehna EN, Starke RM, Pouratian N, Dumont AS. Standards for reporting randomized controlled trials in neurosurgery. J Neurosurg. 2011;114:280–5. doi: 10.3171/2010.8.JNS091770. [DOI] [PubMed] [Google Scholar]
- 16.Hackshaw AK. A Concise Guide to Clinical Trials. Chichester, UK: Wiley–Blackwell; 2009. [DOI] [Google Scholar]
- 17.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–6. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenberger MA, Blumenstein BA, Crawford ED, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339:1036–42. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 20.Béland G, Elhilali M, Fradet Y, et al. A controlled trial of castration with and without nilutamide in metastatic prostatic carcinoma. Cancer. 1990;66(suppl):1074–9. doi: 10.1002/cncr.1990.66.s5.1074. [DOI] [PubMed] [Google Scholar]
- 21.Crawford ED, Blumenstein BA, Goodman PJ, et al. Leuprolide with and without flutamide in advanced prostate cancer. Cancer. 1990;66(suppl):1039–44. doi: 10.1002/cncr.1990.66.s5.1039. [DOI] [PubMed] [Google Scholar]
- 22.Di Silverio F, Serio M, D’Eramo G, Sciarra F. Zoladex vs. Zoladex plus cyproterone acetate in the treatment of advanced prostatic cancer: a multicenter Italian study. Eur Urol. 1990;18(suppl 3):54–61. doi: 10.1159/000463982. [DOI] [PubMed] [Google Scholar]
- 23.Benson RC, Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Dorr FA. National Cancer Institute study of luteinizing hormone–releasing hormone plus flutamide versus luteinizing hormone–releasing hormone plus placebo. Semin Oncol. 1991;18(suppl 6):9–12. [PubMed] [Google Scholar]
- 24.Jurincic CD, Horlbeck R, Klippel KF. Combined treatment (goserelin plus flutamide) versus monotherapy (goserelin alone) in advanced prostate cancer: a randomized study. Semin Oncol. 1991;18(suppl 6):21–5. [PubMed] [Google Scholar]
- 25.Tyrrell CJ, Altwein JE, Klippel F, et al. A multicenter randomized trial comparing the luteinizing hormone–releasing hormone analogue goserelin acetate alone and with flutamide in the treatment of advanced prostate cancer. The International Prostate Cancer Study Group. J Urol. 1991;146:1321–6. doi: 10.1016/S0022-5347(17)38080-1. [DOI] [PubMed] [Google Scholar]
- 26.Boccardo F, Pace M, Rubagotti A, et al. Goserelin acetate with or without flutamide in the treatment of patients with locally advanced or metastatic prostate cancer. The Italian Prostatic Cancer Project (poncap) study group. Eur J Cancer. 1993;29A:1088–93. doi: 10.1016/S0959-8049(05)80293-X. [DOI] [PubMed] [Google Scholar]
- 27.Thorpe SC, Azmatullah S, Fellows GJ, Gingell JC, O’Boyle PJ. A prospective, randomised study to compare goserelin acetate (Zoladex) versus cyproterone acetate (Cyprostat) versus a combination of the two in the treatment of metastatic prostatic carcinoma. Eur Urol. 1996;29:47–54. doi: 10.1159/000473717. [DOI] [PubMed] [Google Scholar]
- 28.Zalcberg JR, Raghaven D, Marshall V, Thompson PJ. Bilateral orchidectomy and flutamide versus orchidectomy alone in newly diagnosed patients with metastatic carcinoma of the prostate—an Australian multicentre trial. Br J Urol. 1996;77:865–9. doi: 10.1046/j.1464-410X.1996.01517.x. [DOI] [PubMed] [Google Scholar]
- 29.Dijkman GA, Janknegt RA, De Reijke TM, Debruyne FM. Long-term efficacy and safety of nilutamide plus castration in advanced prostate cancer, and the significance of early prostate specific antigen normalization. International Anandron Study Group. J Urol. 1997;158:160–3. doi: 10.1097/00005392-199707000-00051. [DOI] [PubMed] [Google Scholar]
- 30.de Voogt HJ, Studer U, Schröder FH, Klijn JG, de Pauw M, Sylvester R. Maximum androgen blockade using lhrh agonist buserelin in combination with short-term (two weeks) or long-term (continuous) cyproterone acetate is not superior to standard androgen deprivation in the treatment of advanced prostate cancer. Final analysis of eortc gu group trial 30843; European Organization for Research and Treatment of Cancer (erotc) Genito-urinary Tract Cancer Cooperative Group. Eur Urol. 1998;33:152–8. doi: 10.1159/000019547. [DOI] [PubMed] [Google Scholar]
- 31.Kotake T, Usami M, Akaza H, et al. Goserelin acetate with or without antiandrogen or estrogen in the treatment of patients with advanced prostate cancer: a multicenter, randomized, controlled trial in Japan. Zoladex Study Group. Jpn J Clin Oncol. 1999;29:562–70. doi: 10.1093/jjco/29.11.562. [DOI] [PubMed] [Google Scholar]
- 32.Akaza H, Homma Y, Okada K, et al. A prospective and randomized study of primary hormonal therapy for patients with localized or locally advanced prostate cancer unsuitable for radical prostatectomy: results of the 5-year follow-up. BJU Int. 2003;91:33–6. doi: 10.1046/j.1464-410X.2003.04014.x. [DOI] [PubMed] [Google Scholar]
- 33.Akaza H, Hinotsu S, Usami M, et al. on behalf of the Study Group for the Combined Androgen Blockade Therapy of Prostate Cancer. Combined androgen blockade with bicalutamide for advanced prostate cancer: long-term follow-up of a phase 3, double-blind, randomized study for survival. Cancer. 2009;115:3437–45. doi: 10.1002/cncr.24395. [DOI] [PubMed] [Google Scholar]
- 34.Kanetake H, Usami M, Ohashi Y, Ijima T, Akaza H. Efficacy of flutamide-combined androgen blockade therapy in advanced prostate cancer patients: a phase III randomized, comparative trial. Gan To Kagaku Ryoho. 2014;41:2591–7. [PubMed] [Google Scholar]
- 35.Ansari MS, Gupta NP, Hemal AK, Dogra PN, Seth A. Combined androgen blockade in the management of advanced prostate cancer: a sensible or ostensible approach. Int J Urol. 2004;11:1092–6. doi: 10.1111/j.1442-2042.2004.00953.x. [DOI] [PubMed] [Google Scholar]
- 36.Bono AV, DiSilverio F, Robustelli della Cuna G, et al. Complete androgen blockade versus chemical castration in advanced prostatic cancer: analysis of an Italian multicentre study. Italian Leuprorelin Group. Urol Int. 1998;60(suppl 1):18–24. doi: 10.1159/000056541. [DOI] [PubMed] [Google Scholar]
- 37.Ferrari P, Castagnetti G, Ferrari G, Baisi B, Dotti A. Combination treatment versus lhrh alone in advanced prostatic cancer. Urol Int. 1996;56(suppl 1):13–17. doi: 10.1159/000282863. [DOI] [PubMed] [Google Scholar]
- 38.Robinson MR, Smith PH, Richards B, Newling DW, de Pauw M, Sylvester R. The final analysis of the eortc Genito-urinary Tract Cancer Co-operative Group phase III clinical trial (protocol 30805) comparing orchidectomy, orchidectomy plus cyproterone acetate and low dose stilboestrol in the management of metastatic carcinoma of the prostate. Eur Urol. 1995;28:273–83. doi: 10.1159/000475067. [DOI] [PubMed] [Google Scholar]
- 39.Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–60. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 40.James ND, de Bono JS, Spears MR, et al. on behalf of the stampede investigators. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338–51. doi: 10.1056/NEJMoa1702900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akaza H. Combined androgen blockade for prostate cancer: review of efficacy, safety and cost-effectiveness. Cancer Sci. 2011;102:51–6. doi: 10.1111/j.1349-7006.2010.01774.x. [DOI] [PubMed] [Google Scholar]
- 42.Connolly RM, Carducci MA, Antonarakis ES. Use of androgen deprivation therapy in prostate cancer: indications and prevalence. Asian J Androl. 2012;14:177–86. doi: 10.1038/aja.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akaza H. Asian trends in primary androgen depletion therapy on prostate cancer. Cancer Biol Med. 2013;10:187–91. doi: 10.7497/j.issn.2095-3941.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lukka H, Waldron T, Klotz L, Winquist E, Trachtenberg J on behalf of the Genitourinary Cancer Disease Site Group and the Cancer Care Ontario Program in Evidence-Based Care. Maximal androgen blockade for the treatment of metastatic prostate cancer—a systematic review. Curr Oncol. 2006;13:81–93. doi: 10.3747/co.v13i3.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benson RC. A rationale for the use of non-steroidal anti-androgens in the management of prostate cancer. Prostate Suppl. 1992;21:85–90. doi: 10.1002/pros.2990210513. [DOI] [PubMed] [Google Scholar]
- 46.McLeod DG. Tolerability of nonsteroidal antiandrogens in the treatment of advanced prostate cancer. Oncologist. 1997;2:18–27. [PubMed] [Google Scholar]
- 47.Aso Y, Akaza H, Koiso K, et al. Clinical evaluation of flutamide, a pure antiandrogen, in prostatic cancer phase II dose-finding study [Japanese] Hinyokika Kiyo. 1993;39:391–403. [PubMed] [Google Scholar]
- 48.Botrel TEA, Clark O, Lima Pompeo AC, et al. Efficacy and safety of combined androgen deprivation therapy (adt) and docetaxel compared with adt alone for metastatic hormone-naïve prostate cancer: a systematic review and meta-analysis. PLoS One. 2016;11:e0157660. doi: 10.1371/journal.pone.0157660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schellhammer P, Sharifi R, Block N, et al. Maximal androgen blockade for patients with metastatic prostate cancer: outcome of a controlled trial of bicalutamide versus flutamide, each in combination with luteinizing hormone–releasing hormone analogue therapy. Casodex Combination Study Group. Urology. 1996;47(suppl):54–60. doi: 10.1016/S0090-4295(96)80010-0. [DOI] [PubMed] [Google Scholar]
- 50.Schellhammer PF, Sharifi R, Block NL, et al. Clinical benefits of bicalutamide compared with flutamide in combined androgen blockade for patients with advanced prostatic carcinoma: final report of a double-blind, randomized, multicenter trial. Casodex Combination Study Group. Urology. 1997;50:330–6. doi: 10.1016/S0090-4295(97)00279-3. [DOI] [PubMed] [Google Scholar]