Abstract
Rationale:
Metastasis of T1N1 gastric cancer (GC) at early stage after curative gastrectomy is unusual. Reports on the diagnosis, treatment, and prognosis of peritoneal metastasis following curative gastrectomy for T1N1 GC are lacking.
Patient concerns:
A 54-year-old woman was admitted to our hospital with complaints of mild abdominal distension and failure to pass gas and stool for 2 days. She has a history of distal gastrectomy for T1N1 GC. About 1 year after surgery, she presented with persistent abdominal distension and underwent conservative managements.
Diagnoses:
Imaging tests failed to identify the apparent cause of intestinal obstruction. When conservative managements failed to relieve the symptoms, she underwent emergency laparotomy, which revealed extensive small bowel metastasis and peritoneal dissemination.
Interventions:
Peritoneal irrigation and drainage were performed with the consent of the patient's families.
Outcomes:
The patient abandoned further therapy and died 1 week later during the follow-up period.
Lessons:
Although the metastasis of T1N1 GC is rare, patients with high risk of metastasis after curative surgery should also be closely followed and be considered as candidates for more aggressive screening strategies. In addition, the use of more effective chemotherapeutic drugs as adjuvant chemotherapy after curative surgery in T1N1 patients may also need to be explored.
Keywords: distal gastrectomy, early gastric cancer, intestinal obstruction, metastasis, T1N1
1. Introduction
Gastric cancer (GC) remains the fourth most common malignant disease and the second most frequent cause of cancer death worldwide, with the highest incidence rates are in East Asia, East Europe, and South America.[1] In China, the incidence rate of GC remains relatively high, although mortality of GC has decreased significantly as the result of progress in diagnosis and therapy. For early gastric cancer (EGC), defined as gastric carcinomas confined to mucosa and/or submucosa irrespective of lymph node involvement, it is possible to achieve nearly 100% curability by radical surgery.[2] Furthermore, due to the improvement in various approaches and therapies, the 5-year survival for patients with EGC is reported to be greater than 90%.[3] Despite this relatively high survival rate, the outcome for patients who experience metastasis after curative surgery is poor, and the risk factors are still unclear.
Here, we describe a case of extensive small bowel metastasis and peritoneal dissemination 1 year following curative distal gastrectomy and S-1 postoperative adjuvant chemotherapy for T1N1 GC.
2. Case report
A 54-year-old woman was admitted in November 2016 because of EGC detected by screening gastrofiberscopy. The lesion was preoperatively diagnosed as a type 0-IIc adenocarcinoma, 1.0 × 0.8 cm in size, with an ulcer scar on the lesser curvature of the middle gastric body. Endoscopic ultrasound revealed the lesion to be confined to the submucosa. Abdominal computed tomography (CT) scan showed multiple small lymph nodes near the stomach. A standard open distal gastrectomy with D2 lymph node dissection was carried out. Precise pathological examination revealed poorly cohesive carcinoma, 1.0 × 1.0 × 0.8 cm in size, minute submucosal invasion depth (100 μm) with an ulcer and 2/24 regional lymph nodes metastasis, positive neural invasion but negative lymphovascular invasion as well as tumor-free margins, so it was determined that the operation was curative based on the standard pathological criteria. The immunohistochemical staining indicated that the positive rate of Ki67 was about 50%. After gastrectomy, she received 4 courses of S-1 monotherapy as postoperative adjuvant chemotherapy. Thus, she was followed up by abdominal CT scan at 3, 6, and 12 months, as well as endoscopy at 12 months after surgery. Every CT scan within the first postoperative year indicated no specific findings and no evidence of metastasis (Fig. 1). And the endoscopy only revealed remnant gastritis and anastomositis, while no evidence of local recurrence (Fig. 2).
Figure 1.
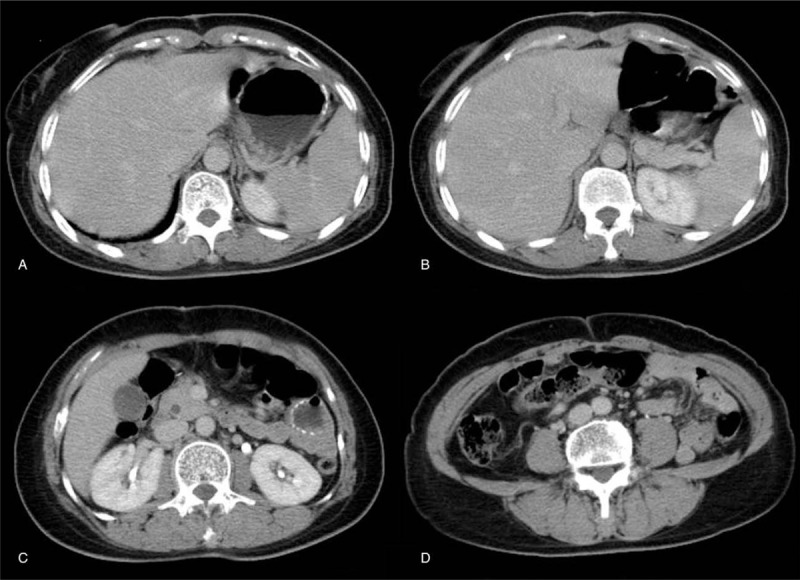
Postoperative abdominal CT scan. (A–D) Postoperative abdominal CT indicated no specific findings and no evidence of metastasis. CT = computed tomography.
Figure 2.
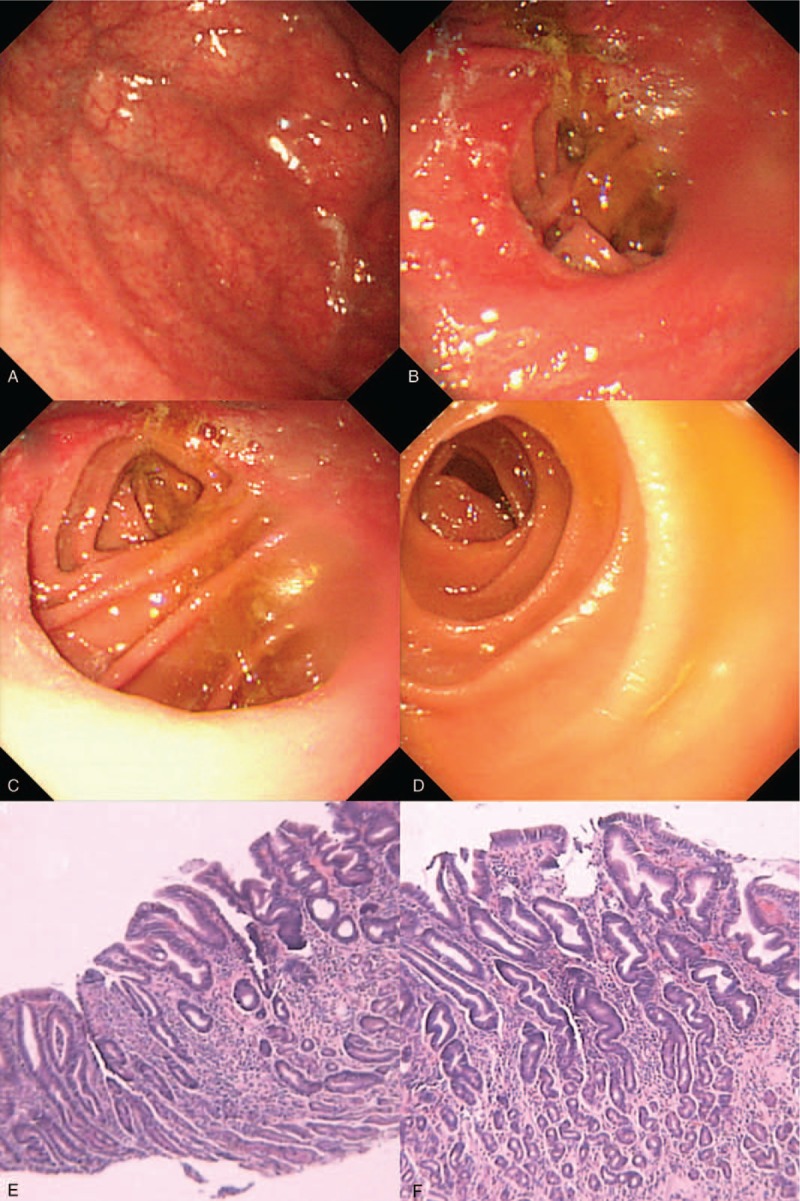
Postoperative endoscopy and pathological examination. (A) Postoperative endoscopy revealed remnant gastritis. (B) Postoperative endoscopy showed anastomositis. No obvious abnormalities were identified in afferent loop (C) and efferent loop (D). (E, F) No evidence of local recurrence was detected.
In December 2017, however, the patient complained of mild abdominal distension and failure to pass gas and stool for 2 days. On admission, her vital signs were stable with body temperature of 36.5 °C, blood pressure of 122/73 mmHg, and a pulse rate of 71 beats per minute. The abdomen was slightly distended without abdominal tenderness or rebounded pain, the borborygmus was decreased. The abdominal plain X-ray revealed small bowel obstruction with several gas-fluid levels formation (Fig. 3). The patient was hospitalized after initial examination. Conservative treatments with fasting, anti-dehydration, anti-infection, and gastrointestinal decompression were applied, but there was no clear improvement.
Figure 3.
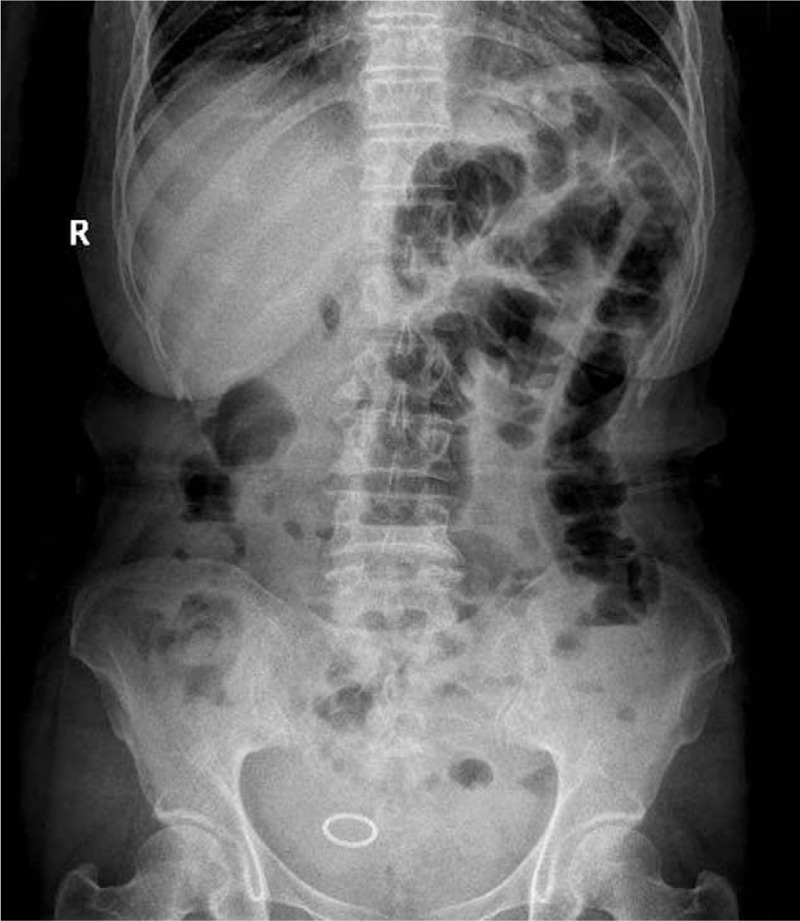
Abdominal plain X-ray examination. Plain X-ray showed small bowel obstruction with local bowel tube to accumulate the gases and extend, and several gas-fluid levels formation.
On the fourth day of admission, the patient complained of persistent abdominal distension and mild abdominal pain. We suspected that it must be the aggravation of intestinal obstruction. As such, emergency abdominal CT was conducted immediately, which demonstrated massive seroperitoneum and severe intestinal dilation (Fig. 4). Emergency laparotomy was performed. At operation, however, the patient was found to be no clear obstruction lesions while the small intestine and peritoneum were full of metastatic nodules (Fig. 5). These findings were suggestive for extensive small bowel metastasis and peritoneal dissemination, considered to be unresectable. Peritoneal irrigation and drainage were performed with the consent of the patient's families. The patient abandoned further therapy and died 1 week later during the follow-up period.
Figure 4.
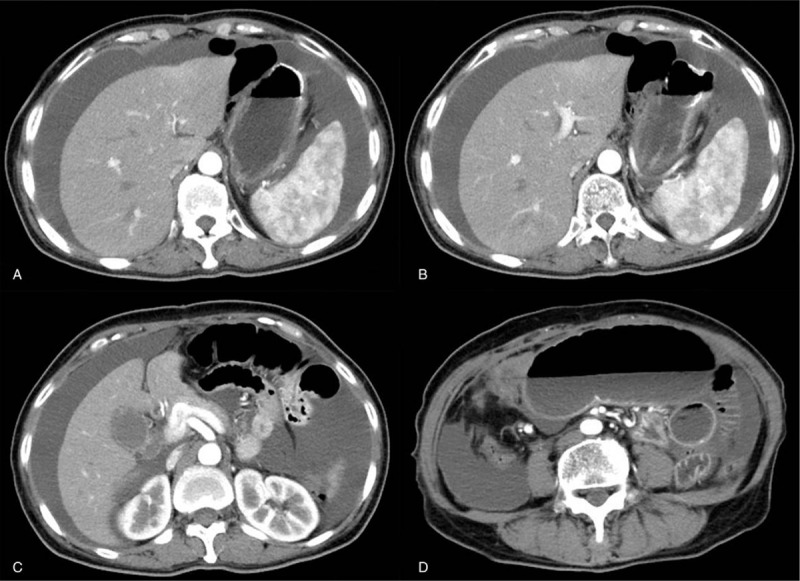
Emergency CT scan. (A–D) Emergency abdominal CT demonstrated massive seroperitoneum and severe intestinal dilation. CT = computed tomography.
Figure 5.
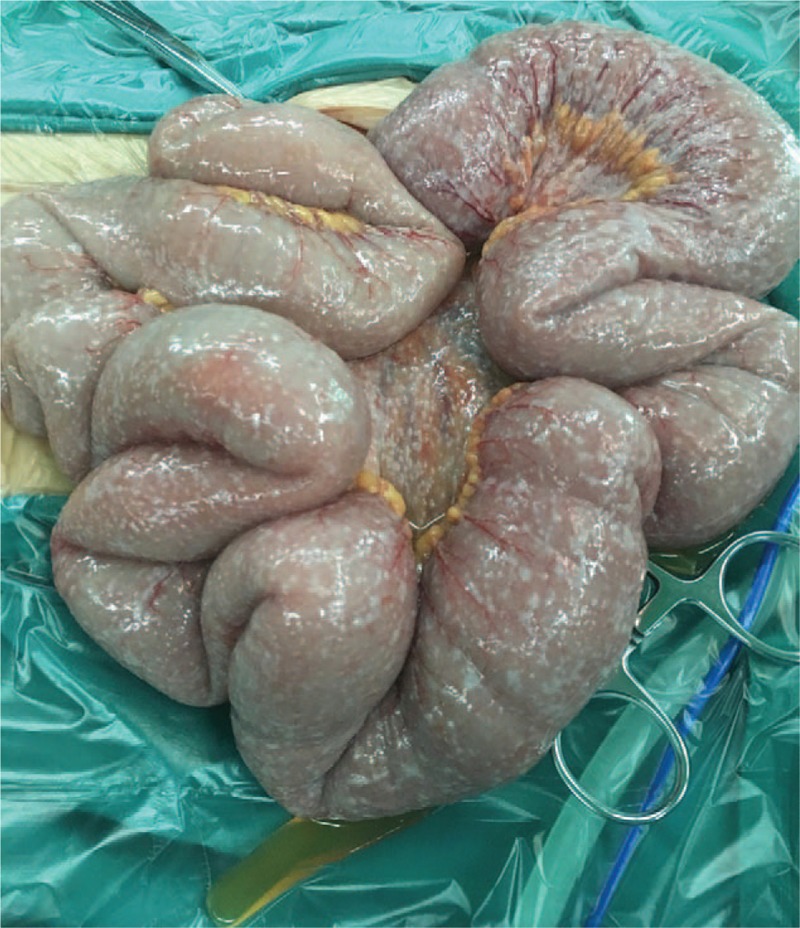
Intraoperative findings of the small bowel. The small bowel was found full of metastatic nodules during the operation.
3. Discussion
T1N1 GC is classified as stage Ib according to the third English edition of the Japanese Classification of Gastric Carcinoma. Due to the advances in diagnosis and therapy, the prognosis of T1N1 patients is excellent, with 5-year survival about 90.2%.[3] Curative surgery alone has been defined as the standard treatment for T1N1 GC; however, a few researchers have demonstrated a certain proportion of patients might recur or metastasize even after curative surgery. Once recurrence or metastasis has developed, the prognosis is limited and is up to 1 year.[4,5] Moreover, several previous studies have suggested that adjuvant chemotherapy is needed to reduce recurrence and metastasis.[6,7]
We experienced for the first time a fatal case of extensive small bowel metastasis and peritoneal dissemination 1 year following curative distal gastrectomy and postoperative adjuvant chemotherapy for T1N1 GC. One possible explanation was that there might be high-risk factors of abdominal and peritoneal metastasis for T1N1 GC. Ichiyoshi et al previously demonstrated that positive lymph node metastasis, and invasion of lymphatic and venous vessels were strongest prognostic factors for unfavorable outcomes of EGC.[8] Review of the literature showed the fact that there were several risk factors for the unfavorable outcome of our patient, including a poorly cohesive carcinoma, ulceration, positive lymph nodes metastasis and neural invasion.[8,9] In addition, micrometastasis apart from the margin of the initial resected specimen might be another risk factor. This growth pattern was not common but it might contribute to the development of peritoneal metastasis. Thus, while curative surgery involving systematic lymph node dissection was performed in our case, we still recommended 4 courses of S-1 monotherapy as postoperative adjuvant chemotherapy. Unfortunately, we failed to prevent metastasis and improve the prognosis of our patient. Combined with the present researches, we assumed the reasons for the metastasis in our patient were closely associated with histological type, ulcerative findings, positive lymph nodes metastasis, and neural invasion.
Given the fact that we performed careful follow-up examinations, we failed to make an early recognition of metastasis through current diagnostic strategies. To overcome these shortages, more efficient and accurate methods like routine exploration by laparoscopy might be needed for patients with high risk for metastasis. Actually, based on this report, researches of exploring prognostic factors, appropriate adjuvant chemotherapy protocols, as well as alternative follow-up examinations in patients with EGC, is ongoing in our center. We will report the results in the future.
4. Conclusion
Our case highlights the fact that although the metastasis of T1N1 GC is rare, patients with high risk of metastasis after curative surgery should also be closely followed and be considered as candidates for more aggressive screening strategies. Moreover, the use of more effective chemotherapeutic drugs as adjuvant chemotherapy after curative surgery in T1N1 patients may also need to be explored.
Author contributions
Conceptualization: Shengxian Fan.
Resources: Shengxian Fan, Min Feng.
Writing – original draft: Shengxian Fan, Min Feng.
Writing – review & editing: Meng Wang, Wenxian Guan.
Footnotes
Abbreviations: CT = computed tomography, EGC = early gastric cancer, GC = gastric cancer.
The authors have no conflicts of interest to disclose.
A written informed consent was obtained from the patient and her families for submitting the manuscript.
References
- [1].Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- [2].Kim JS, Kang SH, Moon HS, et al. Clinical outcome after endoscopic submucosal dissection for early gastric cancer of absolute and expanded indication. Medicine (Baltimore) 2017;96:e6710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Shin KY, Jeon SW, Cho KB, et al. Clinical outcomes of the endoscopic submucosal dissection of early gastric cancer are comparable between absolute and new expanded criteria. Gut Liver 2015;9:181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lee HJ, Kim YH, Kim WH, et al. Clinicopathological analysis for recurrence of early gastric cancer. Jpn J Clin Oncol 2003;33:209–14. [DOI] [PubMed] [Google Scholar]
- [5].Boku N, Yamamoto S, Fukuda H, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol 2009;10:1063–9. [DOI] [PubMed] [Google Scholar]
- [6].Aoyama T, Yoshikawa T, Fujikawa H, et al. Prognostic factors in stage IB gastric cancer. World J Gastroenterol 2014;20:6580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ogawa N, Inokuchi M, Takagi Y, et al. Clinical significance of platelet derived growth factor-C and -D in gastric cancer. Oncol Lett 2015;10:3495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ichiyoshi Y, Toda T, Minamisono Y, et al. Recurrence in early gastric cancer. Surgery 1990;107:489–95. [PubMed] [Google Scholar]
- [9].Kunisaki C, Shimada H, Nomura M, et al. Therapeutic strategy for patients with pN0 gastric carcinoma. J Surg Oncol 2006;94:212–9. [DOI] [PubMed] [Google Scholar]


