Abstract
This study aimed to explore the risk factors for acute myocardial injury (AMI) caused by acute organophosphorus pesticide poisoning (AOPP).
The clinical data of 98 patients, who were treated in our hospital due to oral AOPP from April 2013 to April 2017, were retrospectively analyzed. These patients were divided into two groups: AMI group and control group. The incidence of AMI was analyzed. Furthermore, the dosage forms and dose of the pesticide, and the interval between pesticide taking and doctor visit were compared between these two groups. Moreover, their clinical symptoms were observed; the serum cholinesterase levels, myocardial injury, and heart failure markers were detected, and the occurrence of arrhythmia and the structure and function of the heart were investigated through continuous electrocardiographic monitoring and transthoracic echocardiography.
Among these 98 AOPP patients, 51 patients were complicated with AMI, and the incidence was 52.0%. The main manifestations of these 51 patients with AMI were as follows: the serum levels of myocardial injury markers (creatine kinase-Mb [CK-Mb] and cardiac troponin I [cTnI]) and heart failure markers (N-terminal pro B-type natriuretic peptide [NT-pro BNP]) were significantly higher, when compared with the control group (P < .001), and the incidence of arrhythmia (FVPB, P = .02; RAA, P = .03; RVA, P = .02; ST-T changes, P = .01) and heart failure (P = .04) was also significantly higher when compared with the control group. With regard to dosage forms of the pesticides, the number of patients taking the pesticides with solvents containing aromatic hydrocarbons was significantly higher in the AMI group than in the control group (P = .001). And the number of patients taking over 100 mL of pesticides was also significantly higher in the AMI group than in the control group (P < .001). Significantly more patients in the AMI group had an interval of over 1 h between pesticide taking and doctor visit than in the control group (P < .001).
Risk factors for AMI after AOPP may include the dose and dosage form of the pesticide, and the interval between pesticide taking and doctor visit.
Keywords: arrhythmia, heart failure, myocardial injury, organophosphorus pesticides poisoning, risk factors
1. Introduction
Organophosphorus pesticides are the most widely used insecticides, especially in rural or undeveloped areas of developing countries.[1] Annually approximately 200,000 persons die from organophosphorus pesticide poisoning, and the mortality rate is generally above 15%.[2] Acute organophosphorus pesticide poisoning (AOPP) is one of the most common drug poisonings in the Emergency Department of a large number of hospitals.
The pathogenesis of AOPP is clarified. Organophosphorus pesticides inhibit acetylcholinesterase by irreversibly binding with the zymolysis site of acetylcholinesterase and forming phosphorylated cholinesterase, causing the accumulation of acetylcholine. Thus, the cholinergic nerve is excited, and a series of symptoms appear.[3,4] Its main clinical manifestations include acute myocardial injury (AMI), acute respiratory failure, and acute liver and renal impairment or even failure,[5–8] impairment of the central nervous system, muscarinic and nicotine symptoms, and symptoms of the sympathetic nervous system.[9,10] It is also correlated with increased risk of Parkinson's disease.[11]
The clinical manifestations of damage caused by organophosphorus pesticides to the myocardium vary among patients, which include arrhythmia, heart failure, cardiogenic shock, and sudden death.[12–14] However, myocardial injuries caused by AOPP are rarely reported, especially myocardial infarction.[13] In fact, cardiac complications of AOPP are fatal, yet they are often overlooked.[15] In addition, the mechanism of damage caused by organophosphorus pesticides to the myocardium remains unclear.[16]
Thus, in this study, by analyzing the manifestations of AOPP in patients with or without AMI, we aimed to explore the risk factors for myocardial injury caused by AOPP, in order to provide suggestions for the early protection of myocardial functions and prevent various adverse cardiac events.
2. Material and methods
2.1. Patients
This study was conducted in accordance with the Declaration of Helsinki and approved by Ethics Committee of our hospital. Written informed consent was obtained from all participants.
From April 2013 to April 2017, 98 patients with oral organophosphorus poisoning were treated in our hospital, and their data were collected and retrospectively analyzed. Among these patients, 51 patients were complicated with AMI. All the original data in this study were obtained from the clinical data of 98 patients in Lianyungang Second People's Hospital, including the general data of the patients, the results of laboratory tests and imaging examinations. Inclusion criteria:
-
(1)
Patients who orally took organophosphorus pesticides and received standardized treatment within 6 h in our hospital;
-
(2)
Patients whose serum cholinesterase activity was determined and a definite clinical diagnosis was drawn, were included.
Exclusion criteria:
-
(1)
Patients without oral organophosphorus poisoning;
-
(2)
Patients who were transferred to our hospital from other hospitals, and the time from pesticide taking to admission was more than 6 h;
-
(3)
Patients with previous serious heart and liver diseases were excluded.
2.2. Management of poisoning
All patients received gastrolavage with 30°C warm water (approximately 10–30 liters), until the water became colorless and odorless.[17] Atropine (5–20 mg) was administrated during the whole treatment period;[18] its dose was decided by observing the status and vital signs of the patients, including consciousness, heart rate, pupil, and respiration rate. For patients with mild intoxication, 0.5–1 mg of atropine was administrated via intramuscular injection, and frequency was b.i.d or t.i.d. For those with moderate intoxication, atropine was given at a dose of 1–2 mg via intramuscular or intravenous (IV) injection, with a frequency of once every 0.5–2 hours. For those with severe intoxication or even in coma, atropine was given at a dose of 1–3 mg via IV injection, with a frequency of once every 15–30 minutes, until the relief of the sever intoxication symptoms or “atropinization.”
In addition to general routine treatments (including acid suppression, stomach protection, liver protection, anti-infection poison excretion through the catharsis and enema), the following measures were concurrently given:
-
(1)
Placement of gastric tube and decompression of the stomach and intestines, as well as feeding or nasal fluid feeding was started after 48–72 hours.
-
(2)
All patients were treated with two sessions of hemoperfusion: one performed within 6 h, and the other performed on the next day within 24 hours.
-
(3)
0.5–1.0 mg of atropine was intravenously injected, q1h to q4h; 1 mg of penehyclidine was intramuscularly injected, q12h to q8h; pralidoxime chloride was given at 1.0 g, q1h for three times, and q2h to q6h;
-
(4)
Eight patients developed acute respiratory distress syndrome (ARDS), and mechanical ventilation was performed with tracheal catheter connecting to a ventilator.[19,20] The mode was volume-controlled synchronized intermittent mandatory ventilation (SIMV), low tidal volume (6 mL/kg) was adopted for all eight patients, and appropriate positive end-expiratory pressure (PEEP) and pressure support ventilation (PSV) were performed.
2.3. Outcomes and data collection
For all patients, continuous electrocardiographic monitoring and pulse oxygen saturation monitoring were performed, and the following biochemical indexes were regularly detected: routine blood indexes, coagulation function, liver and kidney function, cholinesterase, creatine kinase-Mb (CK-Mb), cardiac troponin I (cTnI), and N-terminal pro B-type natriuretic peptide (NT-pro BNP). Transthoracic echocardiography (TTE) was performed after the condition of the patient was stabilized, to evaluate and understand the structure and function of the heart. X-ray or computed tomography (CT) was performed to evaluate the lungs and chest as well.
All data of the patients were collected from Electronic Medical Record in our Hospital Information System.
2.4. Statistical analysis
Data were analyzed using statistical software SPSS 18.0. Age and the result of serum cholinesterase, myocardial necrosis markers, and heart failure markers were continuous variables; thus they were compared using independent sample t-test. All the continuous variables were verified to be normally distributed by Kolmogorov–Smirnov test, and independency was verified by Durbin–Watson test, and homogeneity of variance was verified by Levene's test. The proportion of gender, complications, heart failure Killip classification, ECG changes, UCG, dosage forms of the pesticides, the amount of pesticides, and the interval from pesticide taking to doctor visit were count data; so they were compared using X2-test and Fisher's Exact test, and odds ratio (OR) supplemented with 95% confidence interval (CI) was also calculated. P < .05 was considered statistically significant.
3. Results
3.1. Basic data
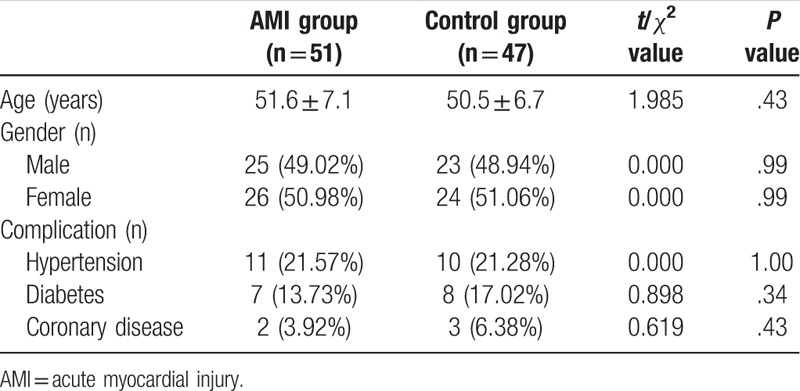
As shown in Table 1, difference in age of the patients in the two groups was not statistically significant (P > .05), so did gender (P > .05) and combined disease (P > .05). The result shows that basic conditions of the two groups of patients were similar, and the results of the two groups were thus suitable for further analysis.
Table 1.
The comparison of basic data between the two groups.
3.2. Clinical manifestations of the patients
Among the 98 AOPP patients, 51 patients presented with AMI, and the incidence was 52.0%. Among these patients, three patients died in the AMI group, of which two patients had heart failure complicated with respiratory failure, and the other one died of severe pancreatitis complicated with respiratory failure, but no heart failure occurred. No cases of death were found in the control group. Among the 98 patients, 8 patients developed ARDS, who all presented with varying degrees of pulmonary edema and pleural effusion, and all were treated with mechanical ventilation. Moreover, among the eight patients who developed ARDS, five patients were diagnosed with heart failure, one patient with severe pancreatitis, one patient with severe pneumonia, and the other one patient with capillary leakage syndrome, secondary pulmonary edema, and pneumonia.
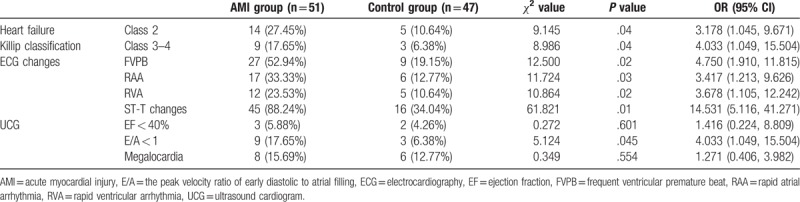
The proportion of patients with clinical symptoms of heart failure was significantly higher in the AMI group than in the control group (OR = 3.178, 95% CI [1.045, 9.671], P = .04). Among changes in the electrocardiogram results, the most common manifestations included frequent ectopic beats (OR = 4.750, 95% CI [1.910, 11.815], P = .02), rapid atrial arrhythmia (OR = 3.417, 95% CI [1.213, 9.626], P = .03), rapid ventricular arrhythmia (OR = 3.678, 95% CI [1.105, 12.242], P = .02) and ST-T changes (OR = 14.531, 95% CI [5.116, 41.271], P = .01), and the incidences were all significantly higher in the AMI group than in the control group. In the AMI group, 12 patients had ventricular tachycardia/ventricular fibrillation. Among these patients, two patients died. Cardiac diastolic function was lower in the AMI group than in the control group (OR = 4.033, 95% CI [1.049, 15.504], P = .04), while the differences in cardiac systolic function and structure between these two groups were not statistically significant (OR = 1.416, 95% CI [0.224, 8.809], P = .60, and OR = 1.271, 95% CI [0.406, 3.982], P = .55, respectively, Table 2).
Table 2.
The comparison of clinical symptoms of heart failure and imaging results between the two groups.
3.3. Comparison of the detection results of serum cholinesterase, myocardial necrosis markers, and heart failure markers between the two groups
The serum cholinesterase level was significantly lower in the AMI group than in the control group (P < .001). Furthermore, the serum levels of CK-Mb, cTnI, and NT-pro BNP were significantly higher in the AMI group than in the control group (P < .001, Table 3).
Table 3.
The comparison of the detection results of serum cholinesterase, myocardial necrosis markers, and heart failure markers between the two groups.

3.4. Comparison and analysis of dosage forms of the pesticides (whether the solvents contained aromatic hydrocarbons), the amount of pesticides (>100 mL), and the interval between pesticide taking and doctor visit (>1 h) between the two groups
The dosage forms of the pesticides (whether the solvents contained aromatic hydrocarbons), the amount of pesticides (>100 mL), and the interval between pesticide taking and doctor visit (>1 h) between the two groups were compared, and the differences were statistically significant (OR = 4.024, 95% CI [1.693, 9.562], P = .001, and OR = 6.108, 95% CI [2.368, 15.754], P < .001, and OR = 2.468, 95% CI [1.018, 5.984], P < .001, respectively, Table 4).
Table 4.
The comparison of dosage forms of the pesticides, the amount of pesticides, and the interval from pesticide taking to doctor visit between the two groups.

4. Discussion
According to the acute oral median lethal dose (LD50) of rats, the toxicity of organophosphorus pesticides can be divided into four grades: rank poison, high poison, medium poison, and low poison. However, the present study revealed that the damage induced by organophosphorus pesticides to the human myocardium is also correlated to many other factors, such as the dosage forms and dose of the pesticides, and the interval between pesticide taking and doctor visit. These 51 patients with organophosphate poisoning and myocardial injury were retrospectively analyzed. Among these patients, 39 patients had oral organophosphorus pesticides, in which the solvents contained toluene or xylene. Relevant information[21] revealed that toluene and xylene have direct toxic effects on the skin mucosa, respiratory system, and nervous system. The present study revealed that the solvents of these organophosphorus pesticides may also induce significant damage to the myocardium, and these were significantly higher in the AMI group than in the control group.
The clinical manifestations of myocardial injury induced by AOPP were mainly arrhythmia and heart failure. This study found that diastolic heart failure, that is, heart failure with preserved ejection fraction (HF-PEF) was more common in the heart failure induced by AOPP. Left ventricular ejection fraction (LVEF) in 40%–49% was called HF-mrEF, and LVEF > 50% could be diagnosed with HF-PEF. This study took LVEF > 40% as the diagnostic standard of diastolic heart failure. Its clinical manifestations were arrhythmia and heart failure. Echocardiographic results revealed that cardiac diastolic function was significantly lower in the AMI group than in the control group, but the differences in cardiac systolic function and heart morphologic structure between these two groups were not statistically significant. Both the dose of the pesticide and the interval between pesticide taking and doctor visit were closely correlated to myocardial injury. Among the 51 patients with AMI, 38 patients had oral doses of more than 100 mL. Among these patients, 22 patients orally took dichlorvos, 11 patients orally took parathion, 9 patients orally took methamidophos, 4 patients orally took phoxim, and 5 patients orally took other organophosphorus pesticides. The intervals between pesticide taking and doctor visit of these patients were all more than one hour. Among these patients, eight patients were complicated with acute respiratory failure and treated with mechanical ventilation, while three patients died. In addition, the amount of poison in the 12 patients with ventricular tachycardia/ventricular fibrillation was all more than 100 mL, and the interval between pesticide taking and doctor visit was more than one hour. Among the three patients who died, two patients orally took more than 200 mL of dichlorvos and visited a doctor after five hours. The other one patient orally took 350 mL of phoxim and visited a doctor after four hours.
There were also some following limitations in this study.
-
(1)
This study had a small sample size (we only analyzed 98 patients) and needed more clinical studies to verify the conclusion.
-
(2)
The biggest limitation is that we did not analyze the correlation of the risk factors to AMI after AOPP, nor did we figure out the main independent risk factors. This will be done in the future with more samples.
-
(3)
This study was limited to explore the risk factors of AOPP complicated with myocardial injury, and the protective effects and mechanisms of positive and effective measures and medicines on AOPP should be further investigated.
In summary, the effect of organophosphorus pesticides on human myocardial injury was closely correlated to the dosage forms and dose of the pesticides, and the interval between pesticide taking and doctor visit. In patients with AMI, compared with the control group, there were significantly more patients that took >100 ml of pesticides. In addition, there were also significantly more patients that were sent to hospital for treatment at >1 h after pesticide taking, compared with the control group, which indicates that the main risk factors that help develop AMI could probably be the large amount of pesticide intake and the delay in doctor visit. Assessment and judgment, and strengthening myocardial protection can reduce the incidence of adverse cardiac events.[22,23] So we put forward the following suggestions.
-
(1)
Implementation of hemoperfusion. Although there were some controversies in the treatment of AOPP with hemoperfusion, we have achieved a good effect in the routine treatment of AOPP, which can quickly recover the activity of cholinesterase and reduce myocardial injury;
-
(2)
Reduce the dosage of atropine. Up to now, there was no medical guideline to confirm the therapeutic dose of atropine. We suggested that penehyclidine hydrochloride combined with small dose of atropine were more effective. Certainly, large dose of atropine would increase the myocardial injury, so reducing its dose could also protect the myocardium;
-
(3)
Application of traditional Chinese medicine, such as Xuebijing injection (50 mL, IV infusion, b.i.d), which can inhibit severe inflammatory reactions,[24,25] and Shenfu injection (50–100 mL, IV infusion, b.i.d), which contains Aconitum alkaloids and thus can be used as cardiotonic, has been reported to have a certain myocardial protective effect.[26–28] In addition, Spirulina platensis and thymoquinone, two natural antioxidants, could effectively alleviate the OP-induced cardiotoxicity via its antioxidant and anti-inflammatory activities.[29,30]
Acknowledgments
The authors would like to thank the entire medical team and the nursing team in the Intensive Care Unit (ICU) of the Emergency department for their hard work, and to thank the central laboratory and the medical imaging department for their strong technical support. More importantly, however, the authors are indebted to the patients for their effort and enthusiastic cooperation throughout the study.
Author contributions
Conceptualization: Kai-Xiang Chen, Xin-Hua Zhou.
Data curation: Cheng-Ai Sun, Pei-Xia Yan.
Formal analysis: Cheng-Ai Sun, Pei-Xia Yan.
Methodology: Cheng-Ai Sun, Pei-Xia Yan.
Project administration: Kai-Xiang Chen, Xin-Hua Zhou.
Software: Cheng-Ai Sun, Pei-Xia Yan.
Supervision: Xin-Hua Zhou.
Writing – original draft: Kai-Xiang Chen, Xin-Hua Zhou.
Writing – review & editing: Kai-Xiang Chen, Xin-Hua Zhou.
Footnotes
Abbreviations: AMI = acute myocardial injury, AOPP = acute organophosphorus pesticide poisoning, ARDS = acute respiratory distress syndrome, CK-Mb = creatine kinase-Mb, CT = computed tomography, cTnI = cardiac troponin I, HF-PEF = heart failure with preserved ejection fraction, ICU = intensive care unit, LVEF = left ventricular ejection fraction, NT-pro BNP = N-terminal pro B-type natriuretic peptide, PEEP = positive end-expiratory pressure, PSV = pressure support ventilation, SIMV = synchronized intermittent mandatory ventilation, TTE = transthoracic echocardiography.
The authors have no conflicts of interest to disclose.
References
- [1].International Programme on Chemical Safety, World Health Organization (WHO). Epidemiology of Pesticide Poisoning: Harmonized Collection of Data on Human Pesticide Exposure in Selected Countries. Geneva, Switzerland: WHO Press; 2004. [Google Scholar]
- [2].Eddleston M, Buckley NA, Eyer P, et al. Management of acute organo-phosphorus pesticide poisoning. Lancet 2008;371:597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hiremath P, Rangappa P, Jacob I, et al. Pseudocholinesterase as a predictor of mortality and morbidity in organophosphorus poisoning. Indian J Crit Care Med 2016;20:601–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vijayakumar HN, Kannan S, Tejasvi C, et al. Study of effect of magnesium sulphate in management of acute organophosphorus pesticide poisoning. Anesth Essays Res 2017;11:192–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Acikalin A, Dişel NR, Matyar S, et al. Prognostic factors determining morbidity and mortality in organophosphate poisoning. Pak J Med Sci 2017;33:534–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hulse EJ, Davies JO, Simpson AJ, et al. Respiratory complications of organophosphorus nerve agent and insecticide poisoning. Implications for respiratory and critical care. Am J Respir Crit Care Med 2014;190:1342–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Maheshwari M, Chaudhary S. Acute atrial fibrillation complicating organophosphorus poisoning. Heart Views 2017;18:96–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Thakur DS, Khot R, Joshi PP, et al. Glyphosate poisoning with acute pulmonary edema. Toxicol Int 2014;21:328–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Thunga G, Sam KG, Khera K, et al. Profile of acute mixed organophosphorus poisoning. Am J Emerg Med 2009;27:628e1–e3. [DOI] [PubMed] [Google Scholar]
- [10].Paudyal BP. Organophosphorus poisoning. JNMA J Nepal Med Assoc 2008;47:251–8. [PubMed] [Google Scholar]
- [11].Ahmed H, Abushouk AI, Gabr M, et al. Parkinson's disease and pesticides: a meta-analysis of disease connection and genetic alterations. Biomed Pharmacother 2017;90:638–49. [DOI] [PubMed] [Google Scholar]
- [12].Karasu-Minareci E, Gunay N, Minareci K, et al. What may be happen after an organophosphate exposure: acute myocardial infarction? J Forensic Leg Med 2012;19:94–6. [DOI] [PubMed] [Google Scholar]
- [13].Joshi P, Manoria P, Joseph D, et al. Acute myocardial infarction: can it be a complication of acute organophosphorus compound poisoning? J Postgrad Med 2013;59:142–4. [DOI] [PubMed] [Google Scholar]
- [14].Karki P, Ansari JA, Bhandary S, et al. Cardiac and electrocardiographical manifestations of acute organophosphate poisoning. Singapore Med J 2004;45:385–9. [PubMed] [Google Scholar]
- [15].Monika M, Shreekant C. Acute atrial fibrillation complicating organophosphorus poisoning. Heart Views 2017;18:96–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cha YS, Kim H, Go J, et al. Features of myocardial injury in severe organophosphate poisoning. Clin Toxicol (Phila) 2014;52:873–9. [DOI] [PubMed] [Google Scholar]
- [17].Chinese Medical doctor Association Emergency Medical Branch, Chinese Society of Toxicology Poisoning and Treatment of Specialized Committee. Chinese expert consensus on diagnosis and treatment of acute poisoning. Chin J Crit Care Med 2016;36:961–74. [Google Scholar]
- [18].Chinese Society of Toxicology Poisoning and Treatment of Specialized Committee. Clinical guideline for the diagnosis and treatment of acute organophosphorus pesticide poisoning. Chin J Crit Care Med 2016;36:1057–65. [Google Scholar]
- [19].Jokanović M. Medical treatment of acute poisoning with organophosphorus and carbamate pesticides. Toxicol Lett 2009;190:107–15. [DOI] [PubMed] [Google Scholar]
- [20].Yilmaz M, Sebe A, Ay MO, et al. Effectiveness of therapeutic plasma exchange in patients with intermediate syndrome due to organophosphate intoxication. Am J Emerg Med 2013;31:953–7. [DOI] [PubMed] [Google Scholar]
- [21].Moebus S, Boedeker W. Frequency and trends of hospital treated pesticide poisonings in Germany 2000–2014. Ger Med Sci 2017;15:Doc13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Patil G, Murthy N, Nikhil M. Contributing factors for morbidity and mortality in patients with organophosphate poisoning on mechanical ventilation: a retrospective study in a teaching hospital. J Clin Diagn Res 2016;10:UC18–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Banday TH, Tathineni B, Desai MS, et al. Predictors of morbidity and mortality in organophosphorus poisoning: a case study in rural hospital in Karnataka, India. N Am J Med Sci 2015;7:259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cheng C, Lin JZ, Li L, et al. Pharmacokinetics and disposition of monoterpene glycosides derived from Paeonia lactiflora roots (Chishao) after intravenous dosing of antiseptic XueBiJing injection in human subjects and rats. Acta Pharmacol Sin 2016;37:530–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shi XF, Zhang Y, Wang YQ. Impact of Xuebijing and ulinastatin as assistance for hemoperfusion in treating acute paraquat poisoning. Int J Clin Exp Med 2015;8:14018–23. [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang Q, Li C, Shao F, et al. Efficacy and safety of combination therapy of Shenfu injection and postresuscitation bundle in patients with return of spontaneous circulation after in-hospital cardiac arrest: a randomized, assessor-blinded, controlled trial. Crit Care Med 2017;45:1587–95. [DOI] [PubMed] [Google Scholar]
- [27].Li Y, Zhang XC, Lin PH, et al. Effects of Shenfu injection in the treatment of septic shock patients: a multicenter, controlled, randomized, open-label trial. Evid Based Complement Alternat Med 2016;2016:2565169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang Q, Li C. The roles of traditional Chinese medicine: Shen-Fu injection on the postresuscitation care bundle. Evid Based Complement Alternat Med 2013;2013:319092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Abdel-Daim MM, Abushouk AI, Alkhalf MI, et al. Antagonistic effects of Spirulina platensis on diazinon-induced hemato-biochemical alterations and oxidative stress in rats. Environ Sci Pollut Res Int 2018;25:27463–70. [DOI] [PubMed] [Google Scholar]
- [30].Danaei GH, Memar B, Ataee R, et al. Protective effect of thymoquinone, the main component of Nigella sativa, against diazinon cardio-toxicity in rats. Drug Chem Toxicol 2018;12:1–7. [DOI] [PubMed] [Google Scholar]


