Abstract
We aimed to investigate the association between carotid artery stenosis and peripheral artery disease (PAD) by screening carotid ultrasonography (CUS).
From January 2012 to December 2015, 231 consecutive patients who had undergone preoperative CUS for PAD were included in this study. A radiologist assessed the degree of internal carotid artery (ICA) stenosis by using the North American Symptomatic Carotid Endarterectomy Trial (NASCET). Severe (>70%) ICA stenosis was evaluated based on the type of vascular surgery, PAD lesion, and ankle-brachial index (ABI). Data were analyzed using multiple logistic regression analysis and the χ2 test.
Among 231 PAD patients, multilevel lesions revealed significantly higher incidence of severe ICA stenosis than iliac and infrainguinal lesion (22.5% vs 9.4% vs 8%: P = .016). Age (odds ratio [OR]: 1.05, 95% confidence interval [CI]: 1.00–1.12: P = .035), chronic kidney disease (CKD, OR: 6.19, 95% CI: 2.04–45.04: P = .013), and cerebral vascular disease (CVD, OR: 4.08, 95% CI: 1.13–16.46: P = .037) were significant risk factors of severe ICA stenosis in multivariate analysis. Prevalence of severe ICA stenosis according to ABI in PAD was not significant.
Preoperative screening by CUS provides valuable information onasymptomatic carotid artery stenosis (ACAS) that can identify severe ACAS patients who are at high risk of stroke and to consider more intensive management of carotid disease in PAD patients. CUS can be a useful noninvasive preoperative screening imaging tool for PAD patients with multilevel lesions, aged > 65 years old, with CKD and CVD.
Keywords: carotid artery stenosis, peripheral artery disease, ultrasound
1. Introduction
Cerebrovascular disease is a common risk factor for stroke, which is the second leading cause of death in the world.[1,2] Ischemic stroke is more frequent than hemorrhagic stroke.[2] Carotid endarterectomy has been recommended to prevent ischemic stroke in asymptomatic patients with severe carotid stenosis according to evidence from multicenter prospective randomized controlled trials.[3–5]
Carotid ultrasonography (CUS) is a proven noninvasive diagnostic tool to detect asymptomatic carotid artery stenosis (ACAS), and it can evaluate carotid structural alterations, as well as the severity of arterial damage by atherosclerotic changes.[6] Therefore, routine screening CUS prior to vascular surgery may be useful to detect ACAS in peripheral artery disease (PAD) patients to prevent the occurrence of stroke. Previous studies by Marek et al[7] recommended routine screening CUS because of a high prevalence of ACAS in PAD patients. Although, routine screening CUS examination prior to peripheral vascular surgery has been questioned by the multidisciplinary committee, which created the 2012 Appropriate Use Criteria for Peripheral Vascular Laboratory Testing,[8,9] detection of ACAS is still important and requires screening of patient with PAD, usually by CUS. Currently, the routine screening CUS is seldom undertaken and ACAS is probably often missed for PAD patients. Early detection of ACAS with screening CUS can decrease incidence of stroke in PAD patients.
The goal of this study was to investigate the association between carotid artery stenosis evaluated by routine screening CUS and PAD.
2. Material and methods
This is a cross-sectional study.
2.1. Study population
This retrospective study was approved by our institutional review board (Pusan National University Yangsan Hospital review board), which waived the requirement to obtain an informed consent. The database was provided by our institution for research purposes. Between January 2012 and December 2015, routine screening CUS was performed on 231 consecutive patients, who were diagnosed with PAD and underwent 1 or more elective endovascular treatments or bypass surgeries. Patients were excluded if they underwent emergency vascular operations due to acute limb ischemia and had a history of cerebrovascular symptoms or previous carotid surgery. Clinical characteristics, such as age, gender, diabetes mellitus (DM), hypertension, coronary artery disease (CAD), cerebral vascular disease (CVD), smoking history, chronic kidney disease (CKD), the ankle-brachial index (ABI), distribution of lesion, and the findings of CUS, were retrospectively reviewed.
2.2. Diagnoses of PAD and ACAS
The presence and severity of PAD in this study were quantified on the basis of the degree of ABI abnormality. Systolic blood pressure was measured in the bilateral brachial arteries and the bilateral posterior tibial or dorsalispedis artery. Bilateral ABI measurements were obtained by dividing the ankle systolic blood pressure by the highest arm blood pressure. PAD was defined by an ABI lower than 0.90 in either leg.[10] The lesion distribution of PAD was evaluated by contrast enhanced angiography.
Carotid artery stenosis was defined by the degree of internal carotid artery (ICA) stenosis according to the criteria of Society of Radiologists in Ultrasound Consensus Conference.[11] An ICA stenosis ≥70% was defined as severe stenosis. The degree of ICA stenosis was performed by a single radiologist who had 14 years of vascular ultrasonography experience using Philips IU 22 (Philips Healthcare, Amsterdam, The Netherlands) according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET).[12] The results were interpreted by 1 radiologist and 1 vascular surgeon, with 14 years and 8 years of vascular ultrasonography experience, respectively, with consensus. The carotid artery was examined by using B-mode imaging of the arterial wall, such as intima-media thickness, plaque, stenosis, obstruction, and color Doppler flow analysis. According to the findings of CUS, patients were divided into 4 groups: stenosis less than 50%, stenosis 50% to 69%, stenosis greater than 70% (Fig. 1), and unstable plaque.
Figure 1.
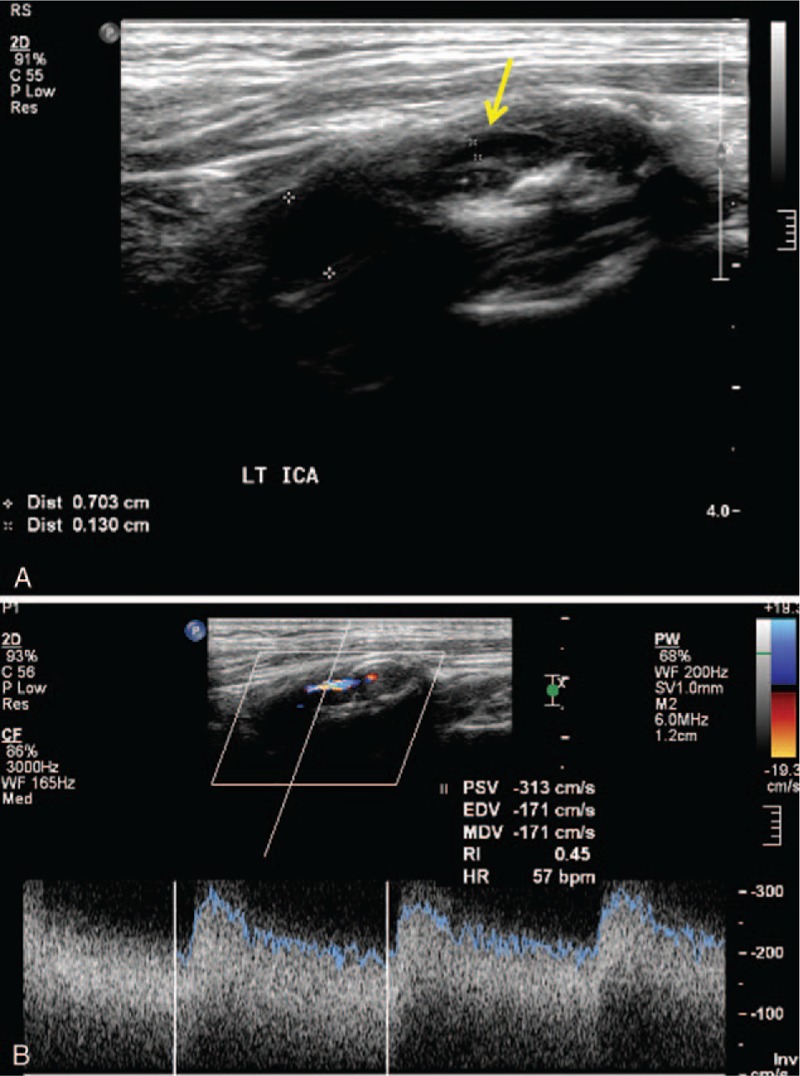
A, Gray scale ultrasound shows more than 70% stenosis by fibrous plaque in proximal internal carotid artery (arrow) for patient with multiple significant peripheral artery stenosis. B, Color Doppler and duplex spectral form ultrasound shows high peak systolic velocity in stenotic proximal internal carotid artery (corresponding to gray scale ultrasound).
2.3. Statistics
Clinical characteristics are presented as mean ± standard deviation for continuous variables and proportions for categorical variables. Data were presented as frequencies and percentages according to type of data. χ2 test was used for comparing categorical variables. Multivariable logistic regression models were used to evaluate the association between severity of internal carotid artery stenosis and PAD. Multivariable models were adjusted for age, sex, diabetes, hypertension, smoking, CAD, CVD, and CKD. Odds ratios (ORs) and 95 percentage confidence intervals (CIs) were represented to examine the strength of the associations. All statistical calculations were carried out using Statistical Package for the Social Sciences (SPSS, version 22 for Microsoft Windows; SPSS Inc, Chicago, IL) and performed by a biostatistician. P values less than.05 were considered statistically significant.
3. Results
3.1. Clinical characteristics and demographics
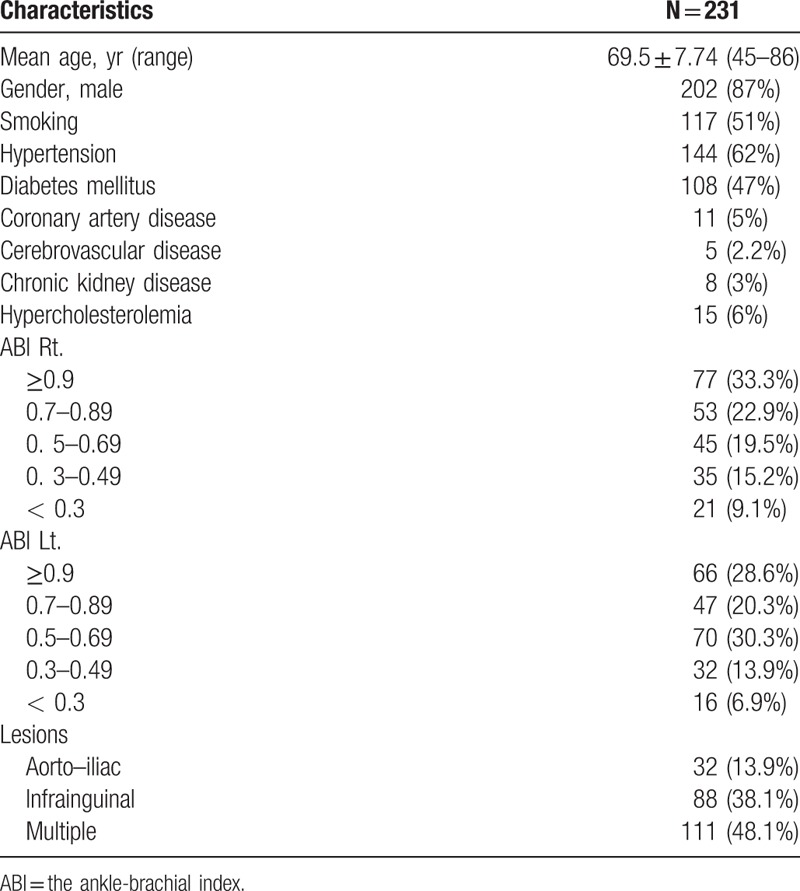
Among 231 PAD patients included in the analysis, the mean age was 69.5 ± 7.74 years (ranging from 45 to 86 years old), and 202 patients (87%) were male. Thirty-two patients (13.9%) presented with aorto-iliac arterial disease, 88 patients (38.1%) showed infrainguinal disease, and 111 patients (48.1%) had combined multiple lesions. Clinical characteristics and demographics are presented in Table 1.
Table 1.
Clinical characteristics and demographics.
3.2. Prevalence of ACAS based on PAD
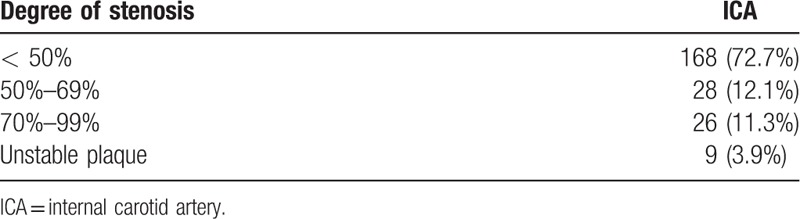
One hundred sixty-eight patients (72.7%) were found to have less than 50% ICA stenosis, 28 patients (12.1%) had 50% to 69% ICA stenosis, 26 patients (11.3%) had greater than 70% ICA stenosis, and 9 patients (3.9%) had unstable plaque. Thirty-five patients (15.2%) were found with severe ICA stenosis (≥70%) and unstable plaque (Table 2). Of them, 9 patients with unstable plaque underwent carotid endarterectomy prior to vascular surgery, while the rest of severe stenosis cases were treated with medical therapy after vascular surgery.
Table 2.
Degree of internal carotid artery stenosis (N = 231 patients).
3.3. Relation of ICA stenosis to the anatomical site of involved lesion
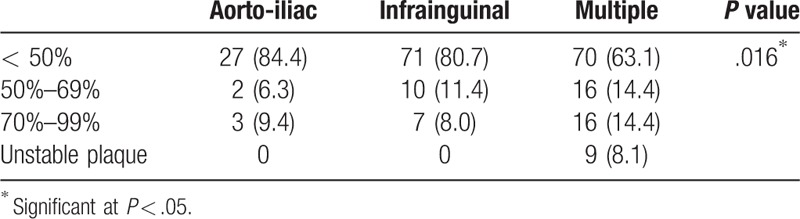
The combined multiple lesions involving both iliac and infrainguinal arteries presented a higher incidence of severe ICA (Table 3).
Table 3.
Relation of internal carotid artery stenosis to lesion.
3.4. Univariate and multivariate analysis of associated risk factors for the severity of ICA stenosis in PAD patients
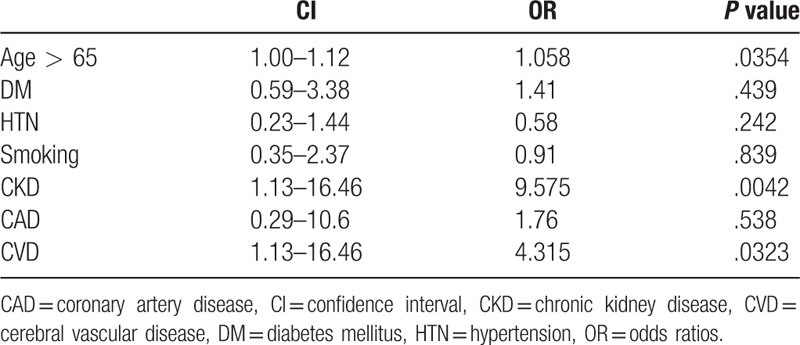
On univariate logistic regression analysis, CVD (P = .0368), CKD (P = .0129), right ABI less than 0.3 (P = .0425), left ABI (P = .0454), and multiple lesions combining iliac and infrainguinal vessels (P = .0076) were significant factors affecting the severity of ICA stenosis in PAD patients. By multivariate logistic regression analysis, the risk factors of ACAS patients with PAD were age > 65 years (OR: 1.058, 95% CI 1.00–1.12, P = .0354), CVD (OR: 4.315, 95% CI 1.13–16.46, P = .0323), and CKD (OR: 9.575, 95% CI 2.04–45.04, P = .0042) (Table 4).
Table 4.
Multivariate analysis of risk factor of affecting the severity of internal carotid artery stenosis in patients with peripheral artery disease.
4. Discussion
Atherosclerosis is a systemic disease of the large and medium-sized arteries that may involve multiple vascular segments and bloodstreams, such as carotid, coronary, and peripheral arteries, and is the globally leading cause of death.[13] Atherosclerotic changes in one arterial territory strongly affect changes in the other territories, leading to vascular diseases, which increase the risk of vascular events in the cerebrovascular, coronary, and peripheral arteries.[14–16] These concomitant atherosclerotic diseases from different vascular territories cause an adverse prognosis.[17] The strong association between carotid artery disease and PAD is well known.[18] The prevalence and severity of carotid artery stenosis have been shown to significantly increase in subjects with PAD regardless of the clinical symptoms.[19] Perioperative or postoperative stroke remains one of the major complications of peripheral artery vascular surgery leading to higher mortality, prolonged hospitalization, and increased hospital costs.[20] CUS is a valuable screening imaging tool to identify significant carotid stenosis. Screening of asymptomatic patients for more than 70% critical stenosis by duplex ultrasound can increase the quality-adjusted life years and can be cost-effective.[21] The prevalence of severe ACAS in the general population was reported to range from 0% to 3.1%, and its prevalence increases with age and with risk factor levels.[22] However, there are few reports about the prevalence of ACAS in patients with PAD comparing the association between PAD and CAD, and some studies showed that the prevalence of carotid stenosis in PAD patients ranged from 5% to 24%.[23–25] Ahmed and Al-Khaffaf[26] reported a meta-analysis of 19 studies presenting a high prevalence of ACAS patients with PAD. A prevalence of 25% to 28% for > 50%, whereas a prevalence of 14% was seen for >70% ACAS. Our data also showed 15.2% of severe (>70%) ACAS in PAD patients. The prevalence of ACAS is much higher in PAD patients than in the general population.
Regarding the risk factors of ACAS, previous studies reported that carotid bruit, rest pain, age>65, and ABI < 0.7 were associated with >50% stenosis.[14,15] In our study, we defined severe stenosis as >70% stenosis or unstable plaque and obtained similar outcomes to the previous studies, demonstrating that age > 65, CVD, and CKD were significant risk factors for ACAS in PAD patients. However, CAD, DM, hypertension, and smoking were not associated with ACAS in PAD patients in this study. Sen et al[27] reported that the ABI can be an appropriate measure for screening patients with stroke or transient ischemic attack at a high risk for peripheral vascular events. As the ABI represents the severity of stenosis or occlusion in the peripheral artery, we assumed that lower ABI reveals more severe stenosis of the carotid artery. However, the severity of ABI was not associated with that of ACAS in this study. However, CVD showed a statistically significant association with ACAS in PAD. Cina et al[28] reported that carotid stenosis greater than 50% was associated with a history of stroke. Patients with severe PAD must be closely monitored to avoid cerebrovascular events whether ACAS was found or not, and in the presence or absence of carotid bruit.[29] 15.2% of severe internal carotid artery stenosis was detected by routine screening CUS in our study. Our results implicate 15.2% PAD patients with severe internal carotid artery stenosis can be protected from stroke by carotid endarterectomy prior to vascular operation. The major limitation of our study is the retrospective design, was interpreted by single radiologist and 1 vascular surgeon with consensus and has the relatively short follow-up period. As this study was designed only for patients who underwent preoperative vascular surgery including open surgery and endovascular treatment, patients of pharmaceutical treatment were not involved because screening CUS was not routinely performed for this group. Further prospective studies including all PAD patients will be necessary.
5. Conclusion
PAD is strongly associated with carotid artery stenosis. Preoperative screening CUS provides valuable information on ACAS and identifies severe ACAS patients who are at high risk of stroke to consider more intensive management of carotid disease in PAD patients. Consequently, it can provide the opportunity for a better quality of life and longer life expectancy in PAD patients. Elderly patients with CKD and CVD in PAD prior to vascular surgery should consider undergoing CUS for carotid artery disease evaluation.
Author contributions
Conceptualization: Ki Seok Choo.
Data curation: Hyun Yul Kim.
Formal analysis: Hyuk Jae Jung, Ki Seok Choo.
Investigation: Byung Soo Park, Dong Il Kim.
Methodology: Kyoung Jin Nam.
Resources: Sang Su Lee, Ji Eun Roh.
Visualization: Ki Seok Choo.
Writing – original draft: Hyuk Jae Jung.
Footnotes
Abbreviations: ABI = the ankle-brachial index, ACAS = asymptomatic carotid artery stenosis, CAD = coronary artery disease, CIS = confidence intervals, CKD = chronic kidney disease, CUS = carotid ultrasonography, CVD = cerebral vascular disease, DM = diabetes mellitus, ICA = internal carotid artery, NASCET = North American Symptomatic Carotid Endarterectomy, ORS = odds ratios, PAD = peripheral artery disease.
The authors have no conflicts of interest to disclose.
References
- [1].Smith EE, Saposnik G, Biessels GJ, et al. Prevention of stroke in patients with silent cerebrovascular disease: a scientific statement for healthcare professionals from the American heart association/American stroke association. Stroke 2017;48:e44–71. [DOI] [PubMed] [Google Scholar]
- [2].Katan M, Luft A. Global burden of stroke. Semin Neurol 2018;38:208–11. [DOI] [PubMed] [Google Scholar]
- [3].Brott TG, Howard G, Roubin GS, et al. Long-term results of stenting versus endarterectomy for carotid-artery stenosis. N Engl J Med 2016;374:1021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brott TG, Hobson RW, 2nd, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010;373:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rosenfield K, Matsumura JS, Chaturvedi S, et al. Randomized trial of stent versus surgery for asymptomatic carotid stenosis. N Engl J Med 2016;374:1011–20. [DOI] [PubMed] [Google Scholar]
- [6].Baroncini LA, de Oliveira A, Vidal EA, et al. Appropriateness of carotid plaque and intima-media thickness assessment in routine clinical practice. Cardiovasc Ultrasound 2008;6:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Marek J, Mills JL, Harvich J, et al. Utility of routine carotid duplex screening in patients who have claudication. J Vasc Surg 1996;24:572–7. [DOI] [PubMed] [Google Scholar]
- [8].Mohler ER, Gornik HL, Gerhard-Herman M, et al. Appropriate use criteria for pheripheral vascular ultrasound and physiological testing part I: arterial ultrasound and physiological testing. J Am Coll Cardiol 2012;60:242–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Barvalia M, Silber D, Divita M, et al. Utility of carotid duplex ultrasonography in a general inner-city hospital. Cardiovascular Ultrasound 2014;12:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation 2012;126:2890–909. [DOI] [PubMed] [Google Scholar]
- [11].Grant EG, Benson CB, Moneta GL, et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis-Society of Radiologists in Ultrasound Consensus Conference. Radiology 2003;229:340–6. [DOI] [PubMed] [Google Scholar]
- [12].Ferguson GG, Eliasziw M, Barr HWK, et al. The North American Symptomatic Carotid EndarterectomyTrial: surgical results in 1415 patients. Stroke 1999;30:1751–8. [DOI] [PubMed] [Google Scholar]
- [13].Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- [14].Lin JC, Kabbani LS, Peterson EL, et al. Clinical utility of carotid duplex ultrasound prior to cardiac surgery. J Vasc Surg 2016;63:710–4. [DOI] [PubMed] [Google Scholar]
- [15].Chun LJ, Tsai J, Tam M, et al. Screening carotid artery duplex in patients undergoing cardiac surgery. Ann Vasc Surg 2014;28:1178–85. [DOI] [PubMed] [Google Scholar]
- [16].Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006;295:180–9. [DOI] [PubMed] [Google Scholar]
- [17].Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation 2016;114:688–99. [DOI] [PubMed] [Google Scholar]
- [18].Razzouk L, Rockman CB, Patel MR, et al. Co-existence of vascular disease in different arterial beds: peripheral artery disease and carotid artery stenosis-Data from Life Line Screening(®). Atherosclerosis 2015;241:684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gentile AT, Taylor LM, Jr, Moneta GL, et al. Prevalence of asymptomatic carotid stenosis in patients undergoing infrainguinal bypass surgery. Arch Surg 1995;130:900–4. [DOI] [PubMed] [Google Scholar]
- [20].Banerjee A, Fowkes G, Rothwell PM. Associations between peripheral artery disease and ischemic stroke: implications for primary and secondary prevention. Stroke 2010;41:2102–7. [DOI] [PubMed] [Google Scholar]
- [21].Yin D, Carpenter JP. Cost-effectiveness of screening for asymptomatic carotid stenosis. J Vasc Surg 1998;27:245–55. [DOI] [PubMed] [Google Scholar]
- [22].de Weerd M, Breving JP, Hedblad B, et al. Prevalence of asymptomatic carotid artery stenosis in the general population. An individual participant data meta-analysis. Stroke 2010;41:1294–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pilcher JM, Danaher J, Khaw KT. The prevalence of asymptomatic carotid artery disease in patients with peripheral vascular disease. Clin Radiol 2000;55:56–61. [DOI] [PubMed] [Google Scholar]
- [24].House AK, Bell R, House J, et al. Asymptomatic carotid artery stenosis associated with peripheral vascular disease: a prospective study. Cardiovasc Surg 1999;7:44–9. [DOI] [PubMed] [Google Scholar]
- [25].Ascher E, DePippo P, Salles-Cunha S, et al. Carotid screening with duplex ultrasound in elderly asymptomatic patients referred to a vascular surgeon: is it worthwhile? Ann Vasc Surg 1999;13:164–8. [DOI] [PubMed] [Google Scholar]
- [26].Ahmed B, Al-Khaffaf H. Prevalence of significant asymptomatic carotid artery disease in patients with peripheral vascular disease: a meta-analysis. Eur J Vasc Endovasc Surg 2009;37:262–71. [DOI] [PubMed] [Google Scholar]
- [27].Sen S, Lynch DR, Kaltsas E, et al. Association of asymptomatic peripheral arterial disease with vascular events in patients with stroke or transient ischemic attack. Stroke 2009;40:3472–7. [DOI] [PubMed] [Google Scholar]
- [28].Cina CS, Safar HA, Maggisano R, et al. Prevalence and progression of internal carotid artery stenosis in patients with peripheral arterial occlusive disease. J Vasc Surg 2002;35:75–82. [DOI] [PubMed] [Google Scholar]
- [29].Johansson EP, Wester P. Carotid bruits as predictor for carotid stenosis detected by ultrasonography: an observational study. BMC Neurol 2008;8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]


