Abstract
Dengue virus (DENV) currently circulates in more than 100 countries and causes an estimated 390 million infections per year. While most cases manifest as a self-resolving fever, ~1.5% of infections develop into a more severe dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS), which causes ~20,000 deaths annually. The underlying pathological feature of DHF/DSS, also known as Severe Dengue, is an acute increase in vascular permeability leading to hypovolemia and shock. Angiogenic factors and cytokines, such as vascular endothelial growth factor (VEGF) and tumor necrosis factor (TNF), have been implicated in the increased vascular permeability, suggesting a potential therapeutic strategy for Severe Dengue. Here, we employed a mouse model of antibody-dependent enhancement of DENV infection, which recapitulates the fatal capillary leakage and shock of human Severe Dengue, to investigate the effects of approved VEGF- and TNF-targeting drugs. DENV infection caused a significant increase in serum VEGF levels within 2 days and resulted in ~80% mortality within 8 days of infection. Treatment of mice with sunitinib, a VEGF receptor tyrosine kinase inhibitor, once (day 2) or twice (days 1 and 2) post-infection reduced mortality by 50–80% compared with untreated mice. Notably, sunitinib treatment decreased serum TNF levels, white blood cell counts, and hematocrit levels relative to untreated mice, but had only marginal effects on tissue viral burden. Combination therapy with anti-TNF antibody and sunitinib significantly reduced vascular leakage and synergized to provide superior protection from lethal DENV infection compared with either agent alone. These data suggest that a two-pronged anti-angiogenic and anti-inflammatory approach may be useful for the rapid treatment of DHF/DSS.
Keywords: Severe dengue, mouse model, antibody-dependent enhancement of infection, vascular leakage, angiogenesis, TNF, therapeutics, dual treatment
1. Introduction
Dengue virus (DENV) is a member of the flavivirus family and is primarily transmitted to humans by female Aedes aegypti mosquitoes. Uncontrolled urbanization, globalization, and the spread of DENV-transmitting mosquitoes have resulted in co-circulation of the four DENV serotypes (DENV1–4), increasing the frequency of epidemics and severity of disease. The majority of the ~390 million new infections annually (Bhatt et al., 2013) result in self-limiting acute dengue fever (DF). However, ~1.5% of cases evolve into the life-threatening dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS), also known as Severe Dengue, which requires hospitalization in intensive care units and represents a significant economic burden for countries where DENV is endemic (Stanaway et al., 2016). Despite extensive experience and training of physicians in these countries, the complex physiological changes occurring in DHF/DSS patients can cause major complications, with a mortality rate of around 0.2% (Stanaway et al., 2016).
There are currently no effective treatments or vaccines for DENV. Moreover, therapeutic approaches that target the virus itself could have unforeseen deleterious consequences, not only by promoting the emergence of resistant strains but also by exacerbating the disease. Multiple laboratories, including our own, have demonstrated that DENV-specific antibodies can convert a mild illness into a lethal disease through antibody-dependent enhancement (ADE) of infection in both mice (Balsitis et al., 2010; Zellweger et al., 2010) and humans (Halstead, 2017; Katzelnick et al., 2017). Thus, vaccine-induced antibodies may paradoxically precipitate severe disease upon subsequent infection. Ideally, vaccine and antiviral strategies must target all four serotypes of DENV as well as multiple genotypes within each serotype.
Targeting of host pathways is an alternative therapeutic strategy that could avoid eliciting drug resistance and be effective against multiple DENV genotypes/serotypes. The major pathophysiologic feature of Severe Dengue is an acute increase in vascular permeability, leading to fluid leakage into tissues and severe hypovolemia. Although the precise role of endothelial cells in this event is poorly understood, several studies suggest a role for pro-angiogenic factors in DENV-induced endothelial cell dysfunction. For example, patients with DHF/DSS have high circulating levels of vascular endothelial growth factor (VEGF) (Furuta et al., 2012; Srikiatkhachorn et al., 2007; Thakur et al., 2016; Tseng et al., 2005), and DENV infection leads to upregulation of VEGF receptor-2 (VEGFR-2) in human umbilical vein endothelial cells (Srikiatkhachorn et al., 2007) and of VEGF in a human pulmonary endothelial cell line (Azizan et al., 2009). These results implicate VEGF in the regulation of vascular permeability during DENV infection, raising the possibility that approved anti-VEGF/VEGFR therapies could be useful for preventing or ameliorating DHF/DSS. Additional studies suggest that combination therapy may have superior efficacy compared to treatment using only one VEGF inhibitor (Kanakaraj et al., 2012; Kang and Chung, 2010; Ozdemir et al., 2013; Veritti et al., 2012). Indeed, combination therapy with multiple VEGF inhibitors or with concurrent VEGF- and tumor necrosis factor (TNF)-targeting agents have been used to treat corneal neovascularization and rheumatoid arthritis in animal models and humans (Kanakaraj et al., 2012; Kang and Chung, 2010; Ozdemir et al., 2013; Veritti et al., 2012). In addition, we and others have demonstrated that antibody-mediated blockade of TNF prevents Severe Dengue in DENV-infected mice (Ng et al., 2014; Phanthanawiboon et al., 2016; Shresta et al., 2006; Watanabe et al., 2015; Zellweger et al., 2010).
These observations prompted us to hypothesize that concurrent targeting of angiogenesis and inflammation with VEGF and TNF inhibitors may improve endothelial barrier function and provide superior protection against DENV-induced DHF/DSS compared with single-agent therapy. In this study, we used a mouse model of DENV with ADE developed in our laboratory (Zellweger et al., 2010) to examine the effects of sunitinib, a small molecule inhibitor of multiple receptor tyrosine kinases, including VEGFR-1–3 (O’Farrell et al., 2003), in combination with an anti-TNF monoclonal antibody (mAb). Both agents are FDA-approved for multiple indications, including treatment of gastrointestinal stromal tumors, advanced renal cell carcinoma and advanced pancreatic neuroendocrine tumors for sunitinib, and chronic immune-mediated inflammatory diseases such as rheumatoid arthritis, Crohn’s disease, and psoriasis for anti-TNF mAb.
Injection of AG129 mice (deficient in type I and II interferon receptors in the 129/Sv genetic background) with an anti-DENV mAb followed by inoculation with DENV recapitulates most aspects of lethal DHF/DSS. This model is more clinically relevant than high-dose viral inoculation in the absence of enhancing Abs, since Severe Dengue is mostly observed when DENV-specific Abs are already present (i.e., individuals with secondary DENV infection and infants born to DENV-immune mothers).
Here, we show that VEGF levels are elevated in the AG129 mouse model of ADE-induced lethal dengue disease, and treatment with sunitinib significantly reduces mortality without affecting viral load. Moreover, combination therapy with sunitinib and anti-TNF mAb reduces vascular leakage in the spleen, liver, and small and large intestines and synergistically improves survival, indicating that simultaneous targeting of host angiogenic and inflammatory pathways is a promising option for ameliorating Severe Dengue.
2. Materials and methods
Key reagents, antibodies, primers, and probes used in this study are described in Supplementary Table S1.
2.1. Virus and cells
The mouse-adapted DENV2 D2S10 was derived from the clinical isolate PL046 as previously described (Shresta et al., 2006) by passaging PL046 ten times between mice and C6/36 mosquito cells. S221 is a triple plaque-purified clone derived from D2S10 (Yauch et al., 2009; Zellweger et al., 2014; Zellweger et al., 2013). S221 virus stock titer was determined by qRT-PCR analysis of isolated RNA (QIAmp Viral RNA Mini Kit, Qiagen) as described (Prestwood et al., 2008). Viral doses are expressed as genome equivalents (GE).
2.2. Mice and lethal infection model
AG129 mice (129/Sv mice deficient in type I and II interferon receptors) were bred at La Jolla Institute for Allergy and Immunology under standard pathogen-free conditions. All experiments were approved by the Institutional Animal Care and Use Committee under protocol number AP028-SS1-0615. All experiments included age- and sex-matched mice (males and females, 5–6 weeks of age).
To infect via ADE, mice were injected via intraperitoneal (i.p.) route with 5 μg anti-DENV1–4 prM mAb (clone 2H2, IgG2a) diluted in PBS. One hour later, the mice were inoculated via intravenous (i.v.) route with 1.0 × 109 GE of DENV2 S221.
2.3. VEGF and TNF ELISA
Blood was collected into serum collection tubes (Sarstedt, #41.1500.005) by cardiac puncture and centrifuged at 13,000 rpm for 15 min at 4°C to isolate serum. VEGF and TNF levels were quantified using a mouse VEGF Quantikine ELISA kit and TNF Quantikine ELISA kit (R&D Systems), respectively, according to the manufacturer’s instructions. All samples were analyzed in duplicate.
2.4. Hematologic Analysis
Blood was collected into blood collection tubes (Sarstedt, # 41.1504.105) by cardiac puncture. Hematocrit and white blood cell (WBC) and platelet counts were measured using a Hemavet instrument (Hemavet 950; Drew Scientific Group) according to the manufacturer’s instructions.
2.5. Viral RNA quantification
RNA was isolated from mouse sera (collected as described above) using a QIAmp Viral RNA Mini Kit (Qiagen). Tissues were collected into RNAlater (Invitrogen) and homogenized, RNA was then isolated using an RNeasy Mini Kit (Qiagen). DENV RNA levels in sera and tissues were quantified by qRT-PCR and normalized to 18S (Prestwood et al., 2008). Specific primers and probes are listed in Table S1.
2.6. Vascular permeability assay
Vascular permeability was measured by detecting leakage of Evans blue dye (Fisher Scientific) into spleen and liver as described previously (Barone et al., 1989; Zellweger et al., 2010). Mice were infected with DENV as described above and injected with Evans blue (0.2 mL, 0.5% [w/v] in PBS) via i.v. route at day 3.5 post-infection (p.i.). After 20 min, the mice were euthanized and extensively perfused with PBS. Spleens and livers were collected into formamide (Sigma) and incubated with agitation at 37°C for 24 h. The dye was quantified by comparing sample absorbance at 610 nm with a standard curve. Data are presented as ng Evans blue per mg tissue. For visual inspection of hemorrhage in the intestinal tract, mice were perfused with PBS.
2.7. Drug treatments
Sunitinib (LC Laboratories) was prepared in 60% dimethyl sulfoxide, 36% water, 2% ethanol, and 2% Kolliphor (v/v) according to the manufacturer’s instructions and was stored at −80°C until use. Rat anti-mouse TNF mAb and rat IgG1 isotype control were from BioXcell (#BE0058 and #BE0088, respectively). Mice were injected via i.p. route with 600 or 1200 μg sunitinib (50 μL/mouse) and/or 5, 10, or 20 μg anti-TNF mAb (100 μL/mouse) once at 24 h or twice at 24 and 48 h p.i., as indicated in the legends.
2.8. Statistical analyses
All data were analyzed with Prism software version 7.0 (GraphPad Software, Inc.) and are expressed as the mean ± SEM. Survival was analyzed by the Kaplan–Meier method with the log-rank test. All other group differences were compared using Student’s t-test. Values * P ≤ 0.5, ** P < 0.01, *** P < 0.001 were considered statistically significant.
3. Results
3.1. Serum VEGF levels are elevated in DENV-infected mice
Since VEGF is increased in patients with DHF/DSS (Furuta et al., 2012; Srikiatkhachorn et al., 2007; Thakur et al., 2016; Tseng et al., 2005), we examined serum VEGF levels in our mouse model of DENV with ADE. For this, AG129 mice were injected with 2H2 anti-DENV mAb (5 μg via i.p. route) and then infected with DENV2 strain S221 (1.0 × 109 GE via i.v. route) 1 h later. In this protocol, the mice develop symptoms within 2-3 days and generally succumb to severe disease within 4 to 5 days (Zellweger et al., 2010). Similar to the observations in humans, we found that serum VEGF levels began to rise within 1 day and were significantly increased by day 2 p.i. compared to naïve AG129 mice (Figure 1). These results reveal that serum VEGF levels are elevated in this model and validate its use to investigate the role of VEGF in DHF/DSS.
Figure 1. Serum VEGF levels are increased in DENV-infected mice.
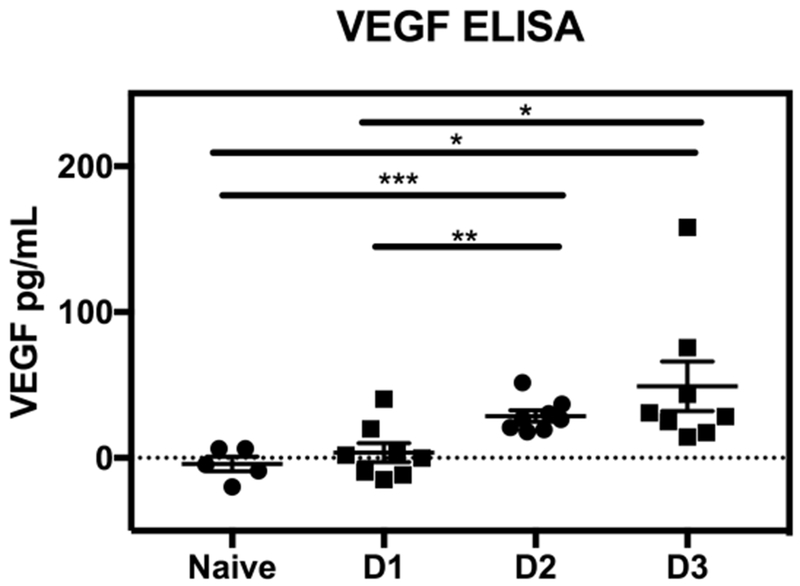
AG129 mice were injected with 5 μg anti-DENV1–4 prM mAb 2H2 via i.p. route and then inoculated with 1 × 109 GE DENV2 strain S221 via i.v. route. Sera were collected from uninfected (naïve, n = 5) or infected mice (n = 8) at 24 h (D1), 48 h (D2), or 72 h (D3) p.i. VEGF protein levels were quantified by ELISA. Data are presented as the mean ± SEM. * P ≤ 0.5, ** P < 0.01, *** P < 0.001 by Student’s t-test.
3.2. Sunitinib treatment improves the survival of mice with lethal DENV infection
To assess the effect of treatment with a potential VEGF pathway blocker in this DENV model, mice were administered 1200 μg of sunitinib once on day 2 or twice on days 1 and 2 after infection. This dose was selected because it is comparable to the approved human dose by allometric scaling (Nair and Jacob, 2016), it is below the maximum tolerated dose in mice, and is non-toxic in this mouse model (Bekerman et al., 2017; Mendel et al., 2003; Nair and Jacob, 2016). While only 20% of the vehicle-treated mice were still alive 8 days after infection, the survival rate for the groups receiving one or two doses of 1200 μg sunitinib was 50% and 80%, respectively (Figure 2A), indicating that treatment with sunitinib as late as 2 days p.i. conferred significant protection.
Figure 2. Sunitinib treatment improves survival of DENV-infected mice.
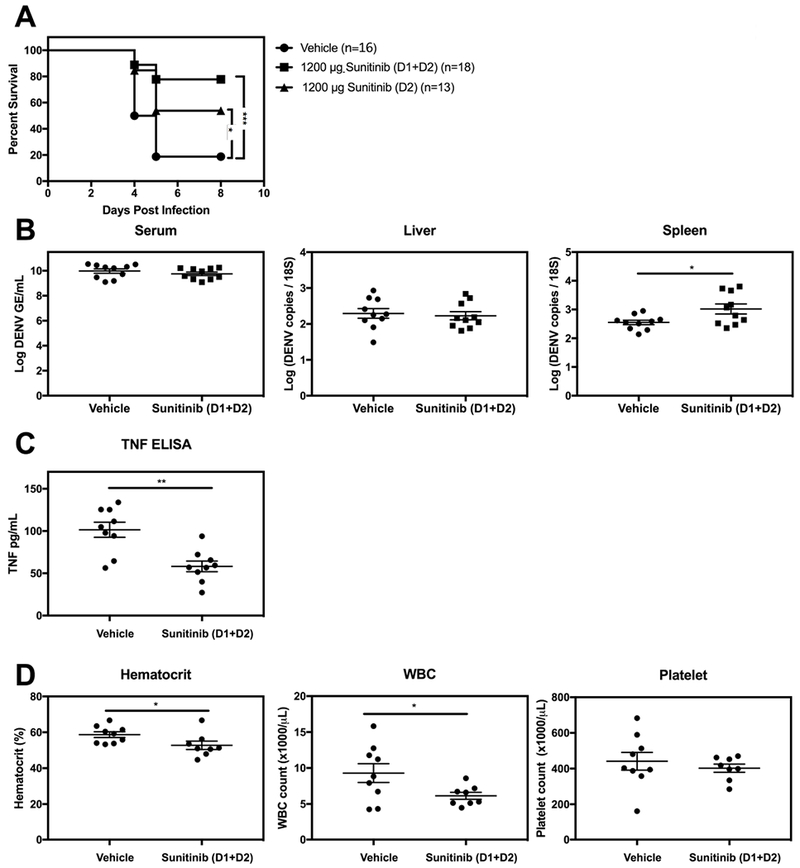
AG129 mice were injected with 5 μg 2H2 mAb via i.p. route and then challenged with 1 × 109 GE DENV2 strain S221 via i.v. route. At 24 h (D1) and/or 48 h (D2) after DENV challenge, mice were injected via i.p. route with vehicle or 1200 μg sunitinib. (A) Kaplan–Meier survival curves of treated mice over 8 days post-infection. (B) qRT-PCR analysis of viral RNA levels in the serum, liver, and spleen at day 3 p.i. (n = 10 mice/group). (C) TNF protein levels in serum at day 3 p.i. were quantified by ELISA. (D) Hematocrit, WBC count , and platelet count were measured at day 3.5 p.i. using a Hemavet analyzer. Data are presented as the mean ± SEM. * P ≤ 0.5, *** P < 0.001, by log-rank test (A) or Student’s t-test (B-D).
To understand how sunitinib treatment improved survival of DENV-infected mice, we first asked whether sunitinib treatment impacted viral replication by measuring viral RNA levels in tissues at day 3 p.i. by qRT-PCR. Mice with sunitinib treatment had similar levels of DENV2 RNA in the serum and liver but higher levels in the spleen (0.5-fold, * P < 0.05) relative to mice with vehicle-treatment (Figure 2B), indicating that sunitinib treatment had no effect on viral burden in the serum or liver and modestly enhanced DENV burden in the spleen. In humans, sunitinib treatment has been reported to reduce hematocrit and both WBC and platelet cell counts (Korkmaz et al., 2014; Kucharz et al., 2016; Zhu et al., 2011). Moreover, elevated TNF levels, hematocrit, and WBC count and thrombocytopenia are associated with Severe Dengue (Chuansumrit and Chaiyaratana, 2014; WHO, 2009). We thus compared these parameters in mice with sunitinib versus vehicle treatment. Mice with sunitinib treatment had reduced serum TNF levels (1.8-fold, ** P < 0.01), hematocrit (1.1-fold, * P < 0.05), and WBC count (1.5-fold, * P < 0.05) relative to mice with vehicle treatment, whereas similar platelet counts were observed in the two mouse groups (Figure 2C and 2 D). Collectively, these results demonstrate that treatment with sunitinib improves the survival of DENV-infected mice without decreasing tissue viral burden, and suggest that sunitinib treatment likely prevents Severe Dengue by reducing factors associated with DENV-induced vascular leakage such as TNF levels and hematocrit and WBC counts.
3.3. Sunitinib and anti-TNF mAb act synergistically to enhance survival of DENV-infected mice
Previous studies have established a beneficial role of anti-TNF mAb treatment in reducing the mortality of mice infected with DENV without ADE (Phanthanawiboon et al., 2016; Shresta et al., 2006) or with ADE (Ng et al., 2014; Watanabe et al., 2015; Zellweger et al., 2010). Based on our finding that sunitinib treatment decreased TNF levels in the serum of DENV-infected mice relative to vehicle treatment (Figure 2C), we hypothesized that treatment with anti-TNF mAb confers an additional survival advantage compared with sunitinib treatment alone. To test this hypothesis, we first established sub-optimal doses of anti-TNF mAb and sunitinib. In agreement with published studies, we found that 100%, 50%, and 20% of mice receiving 20 μg, 10 μg, and 5 μg of anti-TNF mAb, respectively, survived to day 8 p.i., whereas all of the vehicle-treated mice had succumbed by day 5 (Figure 3A, left panel). We selected a dose of 600 μg sunitinib to test the combination therapy because it resulted in a survival rate of 35%, mid-way between that of the vehicle (0%) and 1200 μg sunitinib (50%) dose groups (Figure 3A, right panel). Remarkably, 80% of the mice treated with both sunitinib 600 μg and anti-TNF 5 μg were alive on day 8 p.i., compared with only 35%, 20%, and 5% for mice receiving sunitinib alone, anti-TNF alone, and vehicle, respectively (Figure 3B). Consistent with our findings in sunitinib-treated mice, we observed no clinically meaningful differences in viral load between the serum, liver, spleen, small and large intestines of vehicle-treated and anti-TNF plus sunitinib-treated mice (Figure 4A). Taken together, these results demonstrate that anti-angiogenic and anti-inflammatory therapy act synergistically to reduce mortality from severe dengue without significantly impacting tissue viral burden.
Figure 3. Combination treatment with anti-TNF mAb and sunitinib improves survival of DENV-infected mice.
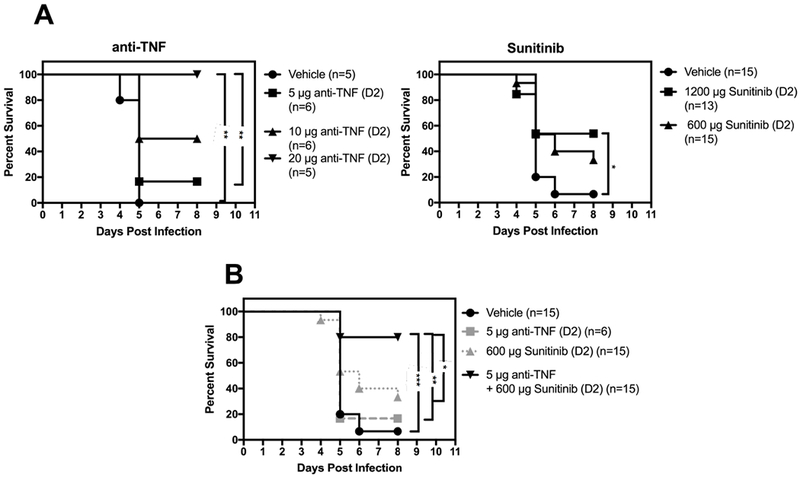
Kaplan–Meier survival curves of AG129 mice injected with 5 μg 2H2 mAb via i.p. route, followed by challenge with 1 × 109 GE DENV2 S221 via i.v. route. At 48 h (D2) p.i., mice were injected with vehicle or (A, left panel) 5 μg, 10 μg, or 20 μg anti-TNF mAb, (A, right panel) 600 μg or 1200 μg sunitinib, or (B) 5 μg anti-TNF mAb and/or 600 μg sunitinib. * P ≤ 0.5, ** P < 0.01, *** P < 0.001 by log-rank test.
Figure 4. Combination treatment with anti-TNF mAb and sunitinib reduces vascular permeability in DENV-infected mice.
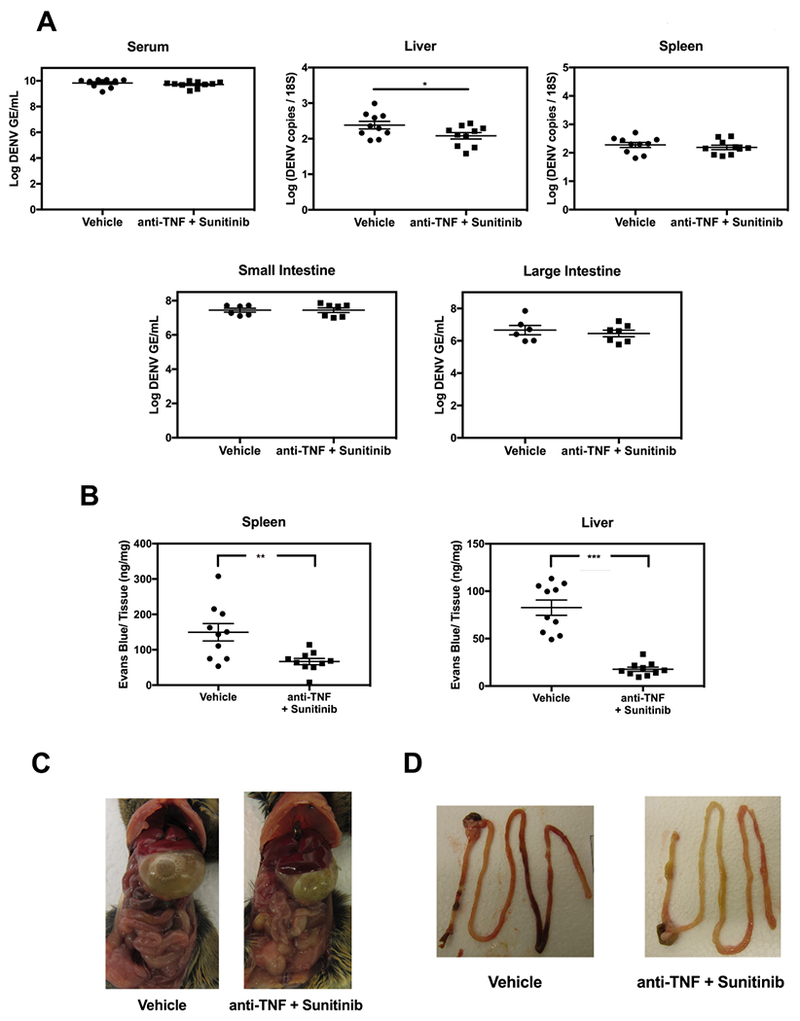
AG129 mice (n = 6-10/group) were injected with 5 μg 2H2 mAb via i.p. route and then inoculated with 1 × 109 GE DENV2 S221via i.v. route. At 48 h after DENV challenge, mice were injected with vehicle or 5 μg anti-TNF mAb and 600 μg sunitinib. (A) qRT-PCR analysis of viral RNA levels in the serum, liver, spleen, small and large intestines at day 3 p.i. (B) Quantification of Evans blue dye in the spleen and liver at day 3.5 p.i. (C) Fluid accumulation in visceral organs at day 3.5 p.i. (D) Small and large intestinal bleeding at day 3.5 p.i. Tissues from one representative animal per group are shown in (C) and (D). * P ≤ 0.5, ** P < 0.01, *** P < 0.001 by Student’s t-test.
3.4. Combination sunitinib and anti-TNF mAb treatment reduces vascular permeability in DENV-infected mice
Vascular leakage is the major pathogenic feature of Severe Dengue in infected humans (Ngono and Shresta, 2018) and mice infected via the ADE pathway (Balsitis et al., 2010; Zellweger et al., 2010). Since TNF and VEGF both have well-characterized roles in regulating vascular permeability (Bates, 2010; Hofmann et al., 2002; Inyoo et al., 2017), we examined the effects of anti-TNF mAb plus sunitibin treatment on vascular leakage in the spleen, liver, and small and large intestines of infected mice (Figure 4B-D and S1). Mice were treated with vehicle or anti-TNF mAb plus sunitinib on day 2 p.i., injected with Evans blue i.v. on day 3.5, and sacrificed 20 min later for collection of the spleen and liver. This dye binds avidly to albumin and is therefore a quantitative marker of fluid leakage from the vasculature into organs. Lower levels of Evans blue were observed in the spleen (2-fold, ** P < 0.01) and liver (5-fold, *** P < 0.001) of mice with the combination treatment relative to those with vehicle treatment (Figure 4B), demonstrating that the combination treatment reduced DENV-induced vascular leakage. To confirm these results, mice without Evans blue injection were visually inspected for fluid accumulation in visceral organs (characterized by swollen intestine and stomach) and intestine hemorrhage (characterized by the presence of blood). The extent of gastrointestinal tract swelling and intestinal bleeding was reduced in mice with the combination treatment relative to mice with vehicle treatment (Figure 4C, 4D and S1). Thus, the dual therapy with anti-TNF mAb and sunitinib promotes survival of DENV-infected mice in part by reducing vascular leakage.
4. Discussion
This study was designed to answer an important question in DENV research; namely, whether existing FDA-approved therapies could be repurposed to treat patients with severe dengue. The therapy should optimally be safe and carry a low risk of eliciting resistance. We hypothesized that drugs blocking host pathways implicated in the pathogenesis of DHF/DSS might be the best candidates, and we tested the efficacy of the VEGFR inhibitor sunitinib with an anti-TNF mAb in order to target both angiogenic and inflammatory pathways.
In agreement with studies showing that plasma VEGF levels are significantly higher in patients with DHF/DSS than in those with mild disease (Furuta et al., 2012; Srikiatkhachorn et al., 2007; Thakur et al., 2016; Tseng et al., 2005), we observed that VEGF levels are elevated in the serum of DENV-infected mice compared to naïve mice. This result, together with a study showing high levels of VEGF in mice with maternal anti-DENV Ab-mediated dengue disease (Ng et al., 2014), implicate VEGF in the pathogenesis of DHF/DSS. In another study, treatment with recombinant angiopoietin-1, which signals through tunica interna endothelial cell kinase 2 (Tie2) to counteract VEGF-induced vascular permeability (Gavard et al., 2008; Hansen et al., 2010; Thurston et al., 1999), improved the survival of mice infected with DENV (Phanthanawiboon et al., 2016). Collectively, these findings implicate a role for angiogenic pathways in DHF/DSS pathogenesis.
Sunitinib treatment reduced mortality in our mouse model. Similar results were obtained in a study in which mice were infected with DENV alone without ADE (Bekerman et al., 2017). Also in agreement with our results, no significant effects of sunitinib on viral burden was observed in the published study. Our results thus confirm and extend the published finding by demonstrating that sunitinib and anti-TNF acted synergistically to improve survival without affecting tissue viral burden. This is consistent with studies showing that sunitinib or anti-TNF treatment alone had no effects on viremia (Bekerman et al., 2017; Phanthanawiboon et al., 2016). In addition to the known effects of sunitinib on angiogenesis and vascular permeability (Hofmann et al., 2002; Inyoo et al., 2017; Marzola et al., 2005), TNF blockade alone has been shown to decrease vascular leakage in DENV-infected mice (Ng et al., 2014; Watanabe et al., 2015). Our observation that dual treatment reduces vascular permeability following DENV infection is consistent with these findings and suggests that maintenance or repair of vascular integrity is important for survival.
In summary, we found that a single injection of sunitinib and anti-TNF mAb at 2 days p.i. dramatically improved survival. In our model, delaying the treatment for 2 days not only avoids impairment of the early innate immune response but also more closely mimics the clinical situation, where patients are seen when the disease has already manifested itself. However, a therapeutic regimen involving expensive biologics such as anti-TNF might not be immediately realized in many resource poor countries that are impacted by DENV. Once reliable early markers of Severe Dengue are discovered, or the cost of developing TNF blockers decreases over time due to expiration of patents, treatments targeting TNF might represent a viable option worldwide. Regardless, the dual approach to targeting host pathways is a particularly attractive strategy, since it is less likely to elicit resistant strains, minimizes toxicity due to the reduction in dose, and is independent of the viral serotype or genotype. Further studies should investigate whether dual sunitinib and anti-TNF treatment, or other anti-angiogenic/inflammatory agents, could be useful for the treatment of hemorrhagic fevers induce by other viruses such as Ebola, Lassa, and Yellow fever.
Supplementary Material
Figure S1: Combination treatment with anti-TNF mAb and sunitinib reduces vascular permeability in the gastrointestinal tract of DENV-infected mice
AG129 mice (n = 6-8/group) were injected with 5 μg 2H2 mAb via i.p. route and then inoculated with 1 × 109 GE DENV2 S221via i.v. route. At 48 h after DENV challenge, mice were injected with vehicle or 5 μg anti-TNF mAb and 600 μg sunitinib. Vascular leakage-associated fluid accumulation in visceral organs and intestinal bleeding at day 3.5 p.i. in (A) mice with vehicle treatment and (B) mice with anti-TNF plus sunitinib treatment are shown.
Acknowledgements
This research was supported by NIH grants (R01 AI116813, R21 NS100477, and R21AI127988) to SS.
References
- Azizan A, Fitzpatrick K, Signorovitz A, Tanner R, Hernandez H, Stark L, Sweat M, 2009. Profile of time-dependent VEGF upregulation in human pulmonary endothelial cells, HPMEC-ST1.6R infected with DENV-1, −2, −3, and −4 viruses. Virol J 6, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsitis SJ, Williams KL, Lachica R, Flores D, Kyle JL, Mehlhop E, Johnson S, Diamond MS, Beatty PR, Harris E, 2010. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathog 6, e1000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone GW, Farley PC, Conerly JM, Flanagan TL, Kron IL, 1989. Morphological and functional techniques for assessing endothelial integrity: the use of Evans blue dye, silver stains, and endothelial derived relaxing factor. J Card Surg 4, 140–148. [DOI] [PubMed] [Google Scholar]
- Bates DO, 2010. Vascular endothelial growth factors and vascular permeability. Cardiovasc Res 87, 262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekerman E, Neveu G, Shulla A, Brannan J, Pu SY, Wang S, Xiao F, Barouch-Bentov R, Bakken RR, Mateo R, Govero J, Nagamine CM, Diamond MS, De Jonghe S, Herdewijn P, Dye JM, Randall G, Einav S, 2017. Anticancer kinase inhibitors impair intracellular viral trafficking and exert broad-spectrum antiviral effects. J Clin Invest 127, 1338–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI, 2013. The global distribution and burden of dengue. Nature 496, 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuansumrit A, Chaiyaratana W, 2014. Hemostatic derangement in dengue hemorrhagic fever. Thromb Res 133, 10–16. [DOI] [PubMed] [Google Scholar]
- Furuta T, Murao LA, Lan NT, Huy NT, Huong VT, Thuy TT, Tham VD, Nga CT, Ha TT, Ohmoto Y, Kikuchi M, Morita K, Yasunami M, Hirayama K, Watanabe N, 2012. Association of mast cell-derived VEGF and proteases in Dengue shock syndrome. PLoS Negl Trop Dis 6, e1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavard J, Patel V, Gutkind JS, 2008. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell 14, 25–36. [DOI] [PubMed] [Google Scholar]
- Halstead SB, 2017. Dengvaxia sensitizes seronegatives to vaccine enhanced disease regardless of age. Vaccine 35, 6355–6358. [DOI] [PubMed] [Google Scholar]
- Hansen TM, Singh H, Tahir TA, Brindle NP, 2010. Effects of angiopoietins-1 and −2 on the receptor tyrosine kinase Tie2 are differentially regulated at the endothelial cell surface. Cell Signal 22, 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann S, Grasberger H, Jung P, Bidlingmaier M, Vlotides J, Janssen OE, Landgraf R, 2002. The tumour necrosis factor-alpha induced vascular permeability is associated with a reduction of VE-cadherin expression. Eur J Med Res 7, 171–176. [PubMed] [Google Scholar]
- Inyoo S, Suttitheptumrong A, Pattanakitsakul SN, 2017. Synergistic Effect of TNF-alpha and Dengue Virus Infection on Adhesion Molecule Reorganization in Human Endothelial Cells. Jpn J Infect Dis 70, 186–191. [DOI] [PubMed] [Google Scholar]
- Kanakaraj P, Puffer BA, Yao XT, Kankanala S, Boyd E, Shah RR, Wang G, Patel D, Krishnamurthy R, Kaithamana S, Smith RG, LaFleur DW, Barbas CF 3rd, Hilbert DM, Kiener PA, Roschke VV, 2012. Simultaneous targeting of TNF and Ang2 with a novel bispecific antibody enhances efficacy in an in vivo model of arthritis. MAbs 4, 600–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Chung SK, 2010. The effect of subconjuctival combined treatment of bevacizumab and triamcinolone acetonide on corneal neovascularization in rabbits. Cornea 29, 192–196. [DOI] [PubMed] [Google Scholar]
- Katzelnick LC, Gresh L, Halloran ME, Mercado JC, Kuan G, Gordon A, Balmaseda A, Harris E, 2017. Antibody-dependent enhancement of severe dengue disease in humans. Science 358, 929–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkmaz S, Kılıçkap S, Terzi H, Sencan M, 2014. Sunitinib-Induced Microangiopathic Hemolytic Anemia: A Case Report. Erciyes Medical Journal, 3. [Google Scholar]
- Kucharz J, Giza A, Dumnicka P, Kuzniewski M, Kusnierz-Cabala B, Bryniarski P, Herman R, Zygulska AL, Krzemieniecki K, 2016. Macrocytosis during sunitinib treatment predicts progression-free survival in patients with metastatic renal cell carcinoma. Med Oncol 33, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzola P, Degrassi A, Calderan L, Farace P, Nicolato E, Crescimanno C, Sandri M, Giusti A, Pesenti E, Terron A, Sbarbati A, Osculati F, 2005. Early antiangiogenic activity of SU11248 evaluated in vivo by dynamic contrast-enhanced magnetic resonance imaging in an experimental model of colon carcinoma. Clin Cancer Res 11, 5827–5832. [DOI] [PubMed] [Google Scholar]
- Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, Murray LJ, Carver J, Chan E, Moss KG, Haznedar JO, Sukbuntherng J, Blake RA, Sun L, Tang C, Miller T, Shirazian S, McMahon G, Cherrington JM, 2003. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 9, 327–337. [PubMed] [Google Scholar]
- Nair AB, Jacob S, 2016. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7, 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng JK, Zhang SL, Tan HC, Yan B, Martinez JM, Tan WY, Lam JH, Tan GK, Ooi EE, Alonso S, 2014. First experimental in vivo model of enhanced dengue disease severity through maternally acquired heterotypic dengue antibodies. PLoS Pathog 10, e1004031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngono AE, Shresta S, 2018. Immune Response to Dengue and Zika. Annu Rev Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, Wong LM, Hong W, Lee LB, Town A, Smolich BD, Manning WC, Murray LJ, Heinrich MC, Cherrington JM, 2003. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood 101, 3597–3605. [DOI] [PubMed] [Google Scholar]
- Ozdemir O, Altintas O, Altintas L, Yildiz DK, Sener E, Caglar Y, 2013. Effects of subconjunctivally injected bevacizumab, etanercept, and the combination of both drugs on experimental corneal neovascularization. Can J Ophthalmol 48, 115–120. [DOI] [PubMed] [Google Scholar]
- Phanthanawiboon S, Limkittikul K, Sakai Y, Takakura N, Saijo M, Kurosu T, 2016. Acute Systemic Infection with Dengue Virus Leads to Vascular Leakage and Death through Tumor Necrosis Factor-alpha and Tie2/Angiopoietin Signaling in Mice Lacking Type I and II Interferon Receptors. PLoS One 11, e0148564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwood TR, Prigozhin DM, Sharar KL, Zellweger RM, Shresta S, 2008. A mouse-passaged dengue virus strain with reduced affinity for heparan sulfate causes severe disease in mice by establishing increased systemic viral loads. J Virol 82, 8411–8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shresta S, Sharar KL, Prigozhin DM, Beatty PR, Harris E, 2006. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J Virol 80, 10208–10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikiatkhachorn A, Ajariyakhajorn C, Endy TP, Kalayanarooj S, Libraty DH, Green S, Ennis FA, Rothman AL, 2007. Virus-induced decline in soluble vascular endothelial growth receptor 2 is associated with plasma leakage in dengue hemorrhagic Fever. J Virol 81, 1592–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanaway JD, Shepard DS, Undurraga EA, Halasa YA, Coffeng LE, Brady OJ, Hay SI, Bedi N, Bensenor IM, Castaneda-Orjuela CA, Chuang TW, Gibney KB, Memish ZA, Rafay A, Ukwaja KN, Yonemoto N, Murray CJL, 2016. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis 16, 712–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur P, Chakravarti A, Aggarwal S, Uppal B, Bhalla P, 2016. Elevated levels of vascular endothelial growth factor in adults with severe dengue infection. Virusdisease 27, 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM, 1999. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 286, 2511–2514. [DOI] [PubMed] [Google Scholar]
- Tseng CS, Lo HW, Teng HC, Lo WC, Ker CG, 2005. Elevated levels of plasma VEGF in patients with dengue hemorrhagic fever. FEMS Immunol Med Microbiol 43, 99–102. [DOI] [PubMed] [Google Scholar]
- Veritti D, Vergallo S, Lanzetta P, 2012. Triple therapy for corneal neovascularization: a case report. Eur J Ophthalmol 22 Suppl 7, S126–128. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Chan KW, Wang J, Rivino L, Lok SM, Vasudevan SG, 2015. Dengue Virus Infection with Highly Neutralizing Levels of Cross-Reactive Antibodies Causes Acute Lethal Small Intestinal Pathology without a High Level of Viremia in Mice. J Virol 89, 5847–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2009. Clinical Management and Delivery of Clinical Services, Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition, Geneva. [PubMed] [Google Scholar]
- Yauch LE, Zellweger RM, Kotturi MF, Qutubuddin A, Sidney J, Peters B, Prestwood TR, Sette A, Shresta S, 2009. A protective role for dengue virus-specific CD8+ T cells. J Immunol 182, 4865–4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger RM, Eddy WE, Tang WW, Miller R, Shresta S, 2014. CD8+ T cells prevent antigen-induced antibody-dependent enhancement of dengue disease in mice. J Immunol 193, 4117–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger RM, Miller R, Eddy WE, White LJ, Johnston RE, Shresta S, 2013. Role of humoral versus cellular responses induced by a protective dengue vaccine candidate. PLoS Pathog 9, e1003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger RM, Prestwood TR, Shresta S, 2010. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe 7, 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AX, Duda DG, Ancukiewicz M, di Tomaso E, Clark JW, Miksad R, Fuchs CS, Ryan DP, Jain RK, 2011. Exploratory analysis of early toxicity of sunitinib in advanced hepatocellular carcinoma patients: kinetics and potential biomarker value. Clin Cancer Res 17, 918–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Combination treatment with anti-TNF mAb and sunitinib reduces vascular permeability in the gastrointestinal tract of DENV-infected mice
AG129 mice (n = 6-8/group) were injected with 5 μg 2H2 mAb via i.p. route and then inoculated with 1 × 109 GE DENV2 S221via i.v. route. At 48 h after DENV challenge, mice were injected with vehicle or 5 μg anti-TNF mAb and 600 μg sunitinib. Vascular leakage-associated fluid accumulation in visceral organs and intestinal bleeding at day 3.5 p.i. in (A) mice with vehicle treatment and (B) mice with anti-TNF plus sunitinib treatment are shown.


