Abstract
Prompt intravenous fluid therapy is a fundamental treatment for patients with septic shock. However, the optimal approach for administering intravenous fluid in septic shock resuscitation is unknown. Two competing strategies are emerging—a liberal fluids approach consisting of a larger volume of initial fluid [50 – 75 ml/kg (4–6 liters in an 80 kg adult) over the first 6 hours] and later use of vasopressors, versus a restrictive fluids approach consisting of a smaller volume of initial fluid [≤30 ml/kg (≤2–3 liters)] with earlier reliance on vasopressor infusions to maintain blood pressure and perfusion. Early fluid therapy may enhance or maintain tissue perfusion by increasing venous return and cardiac output. However, fluid administration may also have deleterious effects by causing edema within vital organs, leading to organ dysfunction and impairment of oxygen delivery. Conversely, a restrictive fluids approach primarily relies on vasopressors to reverse hypotension and maintain perfusion while limiting the administration of fluid. Both strategies have some evidence to support their use, but lack robust data to confirm the benefit of one strategy over the other, creating clinical and scientific equipoise. As part of the National Heart, Lung and Blood Institute (NHLBI) Prevention and Early Treatment of Acute Lung Injury (PETAL) Network, we designed a randomized clinical trial to compare the liberal and restrictive fluids strategies—the Crystalloid Liberal Or Vasopressor Early Resuscitation in Sepsis (CLOVERS) trial. The purpose of this manuscript is to review the current literature on approaches to early fluid resuscitation in adults with septic shock and outline the rationale for the upcoming trial.
INTRODUCTION
For the past two decades, clinicians in the emergency department (ED) and intensive care unit (ICU) have routinely administered large volumes of intravenous fluid (IVF) to patients with septic shock, often totaling greater than 5 liters (L) in the first several hours of resuscitation.1–5 However, an improved mechanistic understanding of potential harm from excessive fluid administration6–8 and emerging observational data associating positive fluid balance with higher mortality9–15 have recently challenged the paradigm of large-volume fluid resuscitation.
With inadequate evidence to support a specific IVF strategy for the management of early septic shock, two alternative approaches have emerged: (1) a liberal fluids approach that relies on a larger volume of initial IVF administration [often 50 – 75 ml/kg (4–6 liters in an 80 kg adult)]; and (2) a restrictive fluids approach consisting of a smaller volume of initial IVF [often ≤30 ml/kg (≤2–3 liters)] and earlier use of vasopressors. Because of the equipoise surrounding these competing treatment strategies, we designed a randomized clinical trial to compare a liberal versus restrictive approach to IVF resuscitation—the Crystalloid Liberal Or Vasopressor Early Resuscitation in Sepsis (CLOVERS) trial. The goal of this manuscript is to describe the current state of the literature regarding IVF resuscitation in early septic shock and the rationale for the upcoming CLOVERS trial.
LIBERAL FLUIDS APPROACH
A “liberal” fluids approach to septic shock management is characterized by the administration of several liters (typically 50 – 75 ml/kg) of IVF during the first several hours of treatment.1,16,17 Vasopressor infusions are added immediately if the patient is profoundly hypotensive (e.g., systolic blood pressure <70 mm Hg), or remains hypotensive despite large volume fluid resuscitation. This liberal fluids strategy dominates current ED care in the United States (US), based in part on the initial Surviving Sepsis Campaign recommendations and Early Goal Directed Therapy (EGDT).1,2,5 A liberal fluids approach is also encouraged by the SEP-1 Core Measure from the Centers for Medicare and Medicaid Services (CMS) and Joint Commission, which recommends an infusion of at least 30 ml/kg of crystalloid fluid within 3 hours of septic shock recognition.18–19
Septic shock patients manifest decreased vasomotor tone and intravascular volume depletion from loss of fluid into the extravascular space via capillary endothelial dysfunction, both which contribute to hypotension.5 IVF administration replenishes intravascular fluid lost to the extravascular space and increases volume within dilated vessels, potentially increasing cardiac pre-load, stroke volume, and cardiac output, leading to increased tissue perfusion and oxygen delivery. Fluid boluses may also improve microvascular perfusion by increasing the driving pressure across capillary beds. These potential advantages to the microcirculation may be present even when the patient does not exhibit traditional signs of “fluid responsiveness,” such as an increase in stroke volume or cardiac output following a fluid challenge.20
Reversal of hypotension with fluid boluses may allow clinicians to avoid or limit vasopressors, which have the potential to cause patient harm, including cardiac dysrhythmias, increased myocardial oxygen demand, digital, renal and mesenteric ischemia, and soft tissue damage from extravasation.21 Using fluids instead of vasopressors to treat hypotension may also allow clinicians to avoid some ICU admissions in hospitals that require all patients on vasopressors to be admitted to an ICU, thus preserving ICU bed capacity.
Clinical Evidence Supporting a Liberal Fluids Approach
In the 1990s, in-hospital mortality rates for septic shock were 40%−50% for hospitals in developed countries.5 In 2001, Rivers et al22 published results of a trial noting lower in-hospital mortality with EGDT, a protocolized resuscitation strategy targeting CVP, mean arterial pressure (MAP), and central venous oxygen saturation (ScvO2). Patients in the EGDT group received larger fluid volumes during the first 6 hours of treatment than those in the standard therapy group (mean volume of IVF administration: 5.0 L vs 3.5 L), and experienced a lower in-hospital mortality (31% vs 47%).22
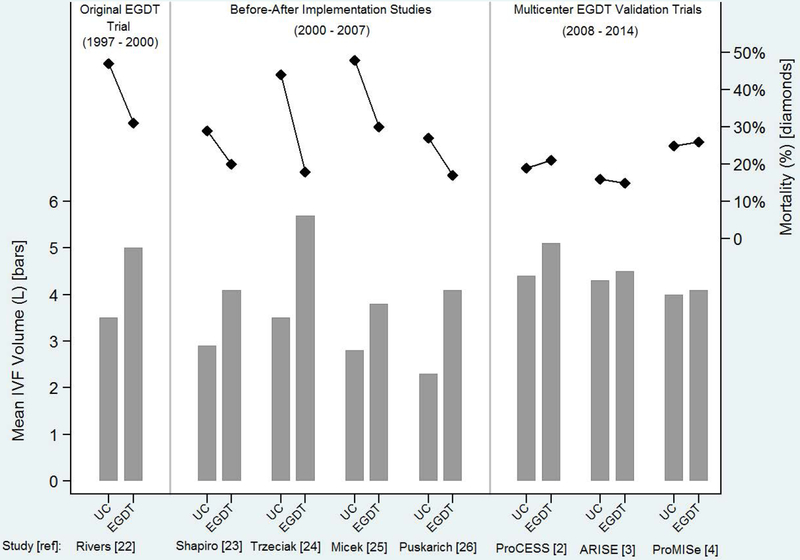
Following the Rivers et al trial22, early large volume fluid resuscitation was widely adopted in the US.1,2,5,16 Observational studies at many institutions during the next 10 years suggested that implementation of EGDT protocols, even with incomplete adherence, were associated with larger volumes of fluid administration and lower mortality (Figure 1–2).5,23–26 For example, Puskarich et al26 conducted a before-after analysis of EGDT therapy implementation at their institution and found a substantial increase in the volume of IVF administered during the first 6 hours of resuscitation (mean 2.3 L before EGDT vs 4.1 L with EGDT) and decline in in-hospital mortality (27% vs 17%). However, most of these early studies evaluating the impact of EGDT involved implementation of a multifaceted bundle of sepsis care, and the effects of different volumes of fluid resuscitation were not separated from the effects of other bundle components, such as early sepsis recognition, prompt antibiotics, and specialized sepsis response teams.27–28 A recent meta-analysis suggested that the mortality benefit associated with EGDT in observational studies was largely due to earlier and more appropriate antibiotics, not fluid volumes or achievement of hemodynamic goals.29
Figure 1.
Volumea of early intravenous fluid administration (bars; left axis) and mortalityb (diamonds; right axis) in severe sepsis and septic shock studies comparing usual care to Early Goal Directed Therapy. Bars show the volume of fluid administered in liters for the usual care and Early Goal Directed Therapy groups in each study. The connected dots demonstrate the percentage of patients who died in usual care and Early Goal Directed Therapy groups in each study. Patients in the usual care group of later studies tended to receive more fluid than those in the usual care group of earlier studies, and similar to patients in the Early Goal Directed Therapy groups. Mortality was higher in the usual care group of studies in which usual care patients received less fluid than Early Goal Directed Therapy patients, but similar in the later studies in which the usual care and Early Goal Directed Therapy groups received similar volumes of fluid. UC: usual care; EGDT: Early Goal Directed Therapy; L: liter
Footnotes:
a. Time window for reported mean fluid volumes: first 6 hours after ED presentation: Rivers22, Shapiro23, Puskarich26; total volume during ED stay: Trzeciak24, Micek25; pre-randomization period plus 6 hours post-randomization: ProCESS2, ARISE3, ProMISe4.
b. Time window for reported mortality: in-hospital: Rivers22, Trzeciak24, Puskarich26, ProMISe4; 28-day in-hospital: Shapiro23; 28-day: Micek25; 60-day in-hospital: ProCESS2, ARISE3.
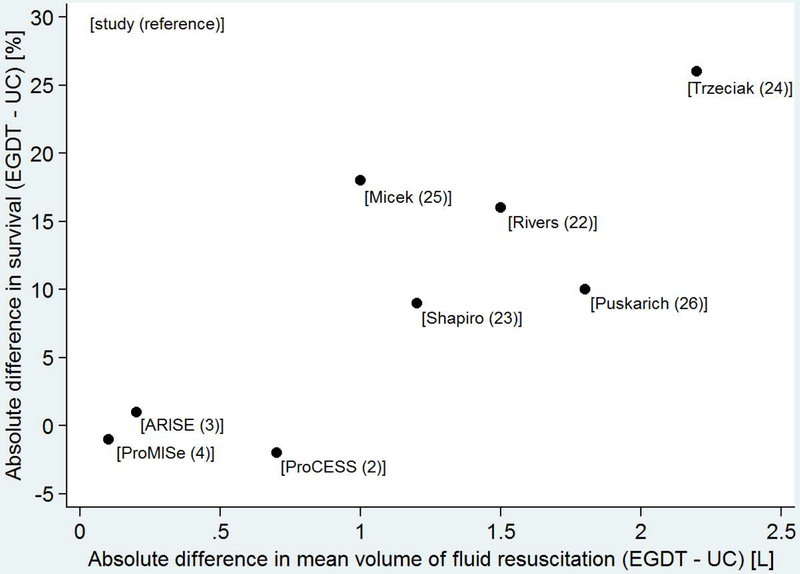
Figure 2.
Scatterplot demonstrating the relationship between the difference in mean volume of fluid resuscitationa in the Early Goal Directed Therapy group and usual care group (x-axis) verses difference in survivalb in the Early Goal Directed Therapy group and usual care group (y-axis) among 8 studies comparing Early Goal Directed Therapy and usual care for early sepsis treatment. Studies with a larger difference in fluid volumes between groups tended to have a larger difference in survival. The reference for each study is listed in brackets. UC: usual care; EGDT: Early Goal Directed Therapy; L: liter
Footnotes:
a. Time window for reported mean fluid volumes: first 6 hours after ED presentation: Rivers22, Shapiro23, Puskarich26; total volume during ED stay: Trzeciak24, Micek25; pre-randomization period plus 6 hours post-randomization: ProCESS2, ARISE3, ProMISe4.
b. Time window for reported survival: in-hospital: Rivers22, Trzeciak24, Puskarich26, ProMISe4; 28-day in-hospital: Shapiro23; 28-day: Micek25; 60-day in-hospital: ProCESS2, ARISE3.
In 2014–2015, results of 3 large multicenter trials evaluating EGDT were published. Each of these trials—ProCESS2 in the US, ARISE3 mostly in Australia and New Zealand, and ProMISe4 in England—demonstrated no incremental mortality benefit between patients initially resuscitated according to EGDT versus usual care. While the timing of fluid administration varied between arms, overall IVF volume between ED presentation and 6 hours post-enrollment was approximately 4 – 5 liters in all groups of all trials. This suggests that early large volume fluid resuscitation was part of usual care (Figure 1–2). Therefore, the ProCESS, ARISE, and ProMISe trials cannot provide insight on the comparative effects of a liberal versus restrictive fluid strategy. However, these trials, plus other observational studies30, demonstrated a substantial decline in the short-term mortality risk for patients with septic shock (currently 15% – 25%) since the 1990s (approximately 40% - 50%), when early large volume fluid resuscitation was less common.5,31 Of note, several factors other than fluid resuscitation likely contributed to a decline in reported sepsis mortality over time, including implementation of early sepsis screening, diagnosing less severely ill patients as having sepsis, and changes to administrative coding for sepsis.29,32,33 Nonetheless, a concurrent decline in sepsis mortality during the same time period in which usual care shifted toward larger volume fluid resuscitation suggests adoption of a liberal fluid strategy may have contributed to a decrease in sepsis mortality during the past two decades.
RESTRICTIVE FLUIDS APPROACH
A “restrictive” fluids approach to septic shock management is characterized by the administration of smaller fluid volumes (often ≤30 ml/kg) and earlier use of vasopressors to reduce vasodilation and improve tissue perfusion.17 With a restrictive fluids approach, the primary method of maintaining blood pressure and systemic perfusion is through vasopressor titration, with fluid boluses added when there is evidence of extreme hypovolemia or when tissue hypoperfusion is suspected despite high vasopressor infusion rates. Historically, the common practice of requiring central venous access for vasopressor infusion hampered early use of vasopressors.34,35 However, current data suggest that norepinephrine administration through large peripheral intravenous catheters for short intervals (hours to days) with appropriate monitoring is safe,36–37 facilitating early vasopressor use for sepsis resuscitation.
The physiologic rationale for a restrictive fluids strategy includes data suggesting that IVF boluses only transiently increase intravascular volume, but subsequently lead to pathologic extravascular fluid leakage (edema), which interferes with cellular function in several organs, including the kidneys, liver, heart and lungs.6–9 Several days of diuresis after shock resolution are often necessary to remove this excess fluid generated by an initial liberal fluids strategy.14 By decreasing venous capacitance (thereby converting unstressed volume to stressed volume without a change in overall volume), vasopressors can increase venous return and cardiac output in a fashion similar to an IVF bolus without burdening tissues with excess extravascular fluid.38
Increasing CVP with IVF boluses may decrease tissue perfusion by narrowing the gradient between arterial pressure and venous pressure, which drives tissue perfusion.39 Some hypothesize that the peripheral vasoconstrictive response to shock is beneficial by selectively providing perfusion to essential organs at the expense of non-vital tissues; rapid reversal of this adaptive physiologic response with IVF boluses may be harmful.40
Physiology studies suggest that between one-third and one-half of septic shock patients never experience an increase in cardiac output with fluid boluses, and when cardiac output does increase, it typically only does so for 30–60 minutes.6,7,41–44 Thus, many septic patients treated with IVF potentially experience limited benefit in terms of increased cardiac output, but are exposed to the negative consequences of tissue edema.
Recommendations for resuscitation of hemorrhagic shock after trauma have evolved over the past two decades and now emphasize the avoidance of large volume crystalloid administration, in favor of blood product transfusion and selective use of permissive hypotension.45–47 A shift in sepsis resuscitation from a liberal to restrictive fluids strategy would parallel this recent change in hemorrhagic shock resuscitation.
Observational Clinical Studies Evaluating Early Fluid Administration and Mortality
Seymour et al48 analyzed the New York State Department of Health administrative databases to evaluate associations between the timing of several individual components of early sepsis treatment and in-patient mortality. They found that earlier antibiotics, earlier blood cultures, and earlier lactate measurement were all associated with lower mortality. However, earlier administration of a 30 ml/kg IVF bolus was not associated with improved mortality; a lapse of each subsequent hour until bolus completion had no association with mortality (odds ratio: 1.01 per hour, 95% CI: 0.99 to 1.02). Although confounding is likely in this observational study, these data suggest that early fluid boluses may not be a key component for optimizing sepsis survival.
Furthermore, a growing body of observational literature suggests larger volumes of IVF and larger positive net fluid balances are associated with increased mortality in sepsis.9–15,49–54 For example, in a recent severity-adjusted multivariable analysis of 23,513 septic adults, each additional liter of IVF up to 5 L on the first day of treatment was associated with a small decrease in mortality (−0.7% absolute change per liter, 95% CI: −1.0% to −0.4%); however, each additional liter beyond 5 L was associated with an increase in mortality (+2.3% absolute change per liter of IVF, 95% CI: +2.0 to +2.5%).54
Table 1 summarizes data from 7 recent studies evaluating the association between early net fluid balance and mortality. Cumulatively, these studies included over 3,500 septic patients from 5 continents managed according to local usual care. Patients with higher net positive fluid balances consistently experienced higher mortality.9–15 While these results provide rationale for questioning the safety of large volume fluid boluses and pursuing interventional trials, the high risk of confounding in these observational studies precludes a causality assessment or defining an optimal clinical approach.55,56 Severity of illness is a strong potential confounder in the association between volume of fluid administration (and net fluid balance) and mortality, because more severely ill septic patients tend to receive more IVF during routine clinical care.54 Although each of these studies used multivariable modeling to adjust for illness severity, potential residual confounding and reverse causality remain concerns.55,56
Table.
Representative observational clinical studies published between 2010 and 2017 evaluating the association between early net fluid balance and mortality in adults with sepsis. RCT: randomized controlled trial; ICU: intensive care unit; CI: confidence interval; HR: hazard ratio; OR: odds ratio; US: United States
| Publication | Study Design [centers] | Population [sample size] | Exposure (Predictor) Variable(s) | Primary Outcome | Main findings |
|---|---|---|---|---|---|
| Boyd et al. Crit Care Med 20119 | Secondary analysis of a multicenter RCT [27 centers in Canada, Australia, USA] | Adults in ICU with septic shock on norepinephrine ≥5 mcg/min [n = 778] | Net fluid balance at 12 hours after initiation of resuscitation; patients classified according to quartile of net fluid balance | 28-day mortality | Compared to patients in the highest quartile of fluid balance (median 8.2 L) those in the lower quartiles of fluid balance (quartile 1: 0.7 L; quartile 2: 2.9 L) had lower risk of mortality in adjusted proportional hazard models [quartile 1 vs quartile 4: aHR 0.57 (95% CI: 0.41, 0.80); quartile 2 vs quartile 4: aHR 0.58 (0.41, 0.82). A fluid balance of +3 L at 12 hours correlated with optimal survival. |
| Micek et al. Crit Care 201310 | Retrospective cohort study [1 center in US] | Adults in ICU with septic shock (vasopressor use >12 hours) [n = 163] | Net fluid balance at 24 hours after shock recognition; patients classified according to quartile of net fluid balance | In-hospital mortality | In an adjusted proportional hazards model, patients in the highest quartile of positive fluid balance at 24 hours had increased in-hospital mortality compared to those in the first quartile (p=0.001) and second quartile (p=0.034). |
| Sadaka et al. J Intensive Care Med 201411 | Retrospective cohort study [1 center in US] | Adults in ICU with septic shock [n = 350] | Net fluid balance at 24 hours after ICU admit; patients classified into 4 categories according to net fluid balance: <6L, 6–12 L, 12–18L, 18–24L. | In-hospital mortality | In an adjusted proportional hazards model, compared to patients with <6 L fluid balance, those with 6–12L, 12–18L, and 18–24 L positive fluid balance had higher mortality risk [aHR: 1.52 (1.35, 1.69), 1.74 (1.47, 2.01), 1.62 (1.20, 2.04), respectively]. |
| Acheampong & Vincent. Crit Care 201512 | Prospective cohort study [1 center in Belgium] | Adults in ICU >48 hours with sepsis (infection & ≥1 organ failure) [n = 173] | Net daily fluid balance for first 7 days of ICU stay; daily fluid balance analyzed on a continuous scale | ICU mortality | On a continuous scale, more positive daily fluid balance was associated with increased ICU mortality in an adjusted proportional hazards model [aHR 1.014 per ml/kg increase (95% CI: 1.007, 1.022)]. |
| de Oliveira et al. J Crit Care 201513 | Retrospective cohort study [1 center in Brazil] | Adults in ICU with sepsis (infection & ≥1 organ failure) [n = 116] | Net fluid balance between 24 and 48 hours after first recognition of organ dysfunction | In-hospital mortality | A net positive fluid balance >3 L was associated with increased hospital mortality in an adjusted logistic regression model [aOR 3.19 (1.19, 8.54)]. |
| Kelm et al. Shock 201514 | Retrospective cohort study [1 center in US] | Adults in ICU with sepsis (infection & ≥1 organ failure) [n = 405] | Signs of fluid overload on day 1 (new pitting edema, crackles, anasarca on exam or new vascular congestion, pulmonary edema or pleural effusion on CXR) | In-hospital mortality | Patients with at least one sign of fluid overload on ICU day #1 had higher risk of in-hospital mortality in an adjusted logistic regression model [aOR: 2.27 (95% CI: 1.31, 4.09)]. |
| Sakr et al. Crit Care Med 201715 | Prospective cohort study [multicenter, multinational audit over 10 days] | Adults in ICU with sepsis (infection & ≥1 organ failure) [n = 1,808] | Net fluid balance at 24 hours and 72 hours after ICU admission; patients classified according to quartile of net fluid balance | 28-day in-hospital mortality | Fluid balance at 24 hours was not associated with mortality; however, higher fluid balance at 72 hrs was associated with increased mortality. Compared with patients in the lowest quartile of fluid balance at 72 hrs, adjusted hazard ratios for quartiles 2, 3, and 4 were 1.36 (1.03, 1.80), 1.47 (1.12, 1.92), and 1.63 (1.25, 2.12), respectively. |
Clinical Trials Supporting a Restrictive Fluids Approach
Prior trials evaluating a liberal versus restrictive approach largely focused on the post-resuscitation period after the resolution of shock.52,57,58 In the largest of these trials, the Fluid and Catheter Treatment Trial (FACTT),57 the ARDS Network Investigators randomized 1,000 patients to a liberal versus conservative (restrictive) fluids strategy for up to 7 days following the diagnosis of ARDS; 85% of these patients had sepsis, pneumonia or aspiration as the primary etiology of ARDS, and the mean time from ICU admission to initiation of the fluid management protocol (governed largely by shock resolution) was approximately 40 hours. Compared with patients in the liberal fluids group, those in the restrictive group had lower net fluid balances (mean cumulative fluid balance after 7 days: −136 ml vs 6992 ml, p <0.01), similar 60-day mortality (25.5% vs 28.4%, p=0.30), and more days alive and free from mechanical ventilation (14.6 vs. 12.1, p<0.01). A post-hoc analysis of the subgroup with an initial CVP ≤8 mm Hg demonstrated substantially greater volumes of fluid administration and higher mortality in patients randomized to the liberal arm compared to the restrictive arm; in the subgroup with initial CVP >8, volume of fluid administered and mortality did not substantially differ between the randomized arms, suggesting lower fluid volumes administered in the restrictive arm may have been a primary contributor to improved outcomes.59 This and other similar trials52,58 established the safety of restrictive fluid management in the post-resuscitative phase of critical illness and have led investigators to question the practice of large volume fluid resuscitation during the initial, acute phase of sepsis treatment as well.
No large clinical trials powered for mortality and conducted in developed countries with advanced critical care capabilities have compared the liberal and restrictive fluid approaches for adults with septic shock during the acute resuscitative phase of management. However, two trials in Africa (FEAST40 and the Simplified Severe Sepsis Protocol Trial60) and a recent small pilot trial in Northern Europe (CLASSIC17) suggested potential benefit from an early restrictive approach. Each of these 3 trials is described below.
FEAST Trial
FEAST (Fluid Expansion As Supportive Therapy) was an unblinded randomized trial evaluating early IVF boluses versus usual care without fluid boluses in 3,141 septic children in sub-Saharan East African hospitals.40 During the first 8 hours of treatment, children in the bolus group received a median fluid volume of 40 ml/kg, while those in the usual care group received a median of 10 ml/kg. Children in the bolus therapy group had higher mortality at 48 hours compared to those in the usual care control group (10.5% vs 7.3%; relative risk: 1.45, 95% CI: 1.13 to 1.86). Higher mortality for the bolus therapy group was observed across a broad range of sub-populations, including those with respiratory illnesses, neurologic illness, severe anemia, and acidosis.8,40 The pathway toward death was more commonly cardiovascular collapse rather than syndromes characterized by overt fluid overload, such as pulmonary or cerebral edema.8 Several characteristics of the FEAST trial limit its generalizability to adults with septic shock in developed countries, including a study population of children, malaria as the most common infection, and the absence of advanced critical care capabilities (patients were managed on pediatric wards without the availability of mechanical ventilation). Nonetheless, these data suggest that early large volume-fluid boluses are not universally beneficial in early sepsis management.
Simplified Severe Sepsis Protocol Trial
Andrews et al60 conducted a randomized trial among 212 adults with septic shock in Zambia to evaluate the effectiveness of the Simplified Severe Sepsis Protocol, which is a quantitative resuscitation protocol similar to EGDT modified for hospitals in developing countries. The study excluded patients with signs of respiratory failure (arterial oxygen saturation <90% and respiratory rate >40 breaths per minute) based on prior work in the same setting suggesting the sepsis protocol was harmful for patients with respiratory failure.61 Patients were randomized to fluid management according to the sepsis protocol versus usual care. The sepsis protocol consisted of an initial 2 L IVF bolus within 1 hour of sepsis recognition, then an additional 2 L over the subsequent 4 hours. Fluids were stopped if the patient experienced any of the following: decrease in oxygen saturation by 3%, increase in respiratory rate by 5 breaths per minute, or increase in jugular venous pressure to 3 cm above the sternal angle. Usual care in this setting did not include routine large volume fluid boluses. Patients in the sepsis protocol group received more IVF than those in the usual care group (median 3.5 L vs 2.0 L, p <0.01). In-hospital death was more common in the sepsis protocol group than the usual care group (48% vs 33%, p=0.03). Mechanical ventilation and ICU care were generally not available in this study; therefore, results are not directly generalizable to sepsis management in hospitals with advanced critical care capabilities. However, these results suggest larger initial fluid boluses may be detrimental in resource-limited settings.
CLASSIC Trial
Hjortrup et al17 recently published CLASSIC (Conservative versus Liberal Approach to fluid therapy of Septic Shock in Intensive Care). This was an unblinded pilot trial of 151 adults in 9 Northern European ICUs with septic shock to test whether separation in fluid volumes could be achieved between an intervention group (restrictive fluids approach) and usual care group (liberal fluids approach). After ICU admission and initial fluid administration of at least 30 ml/kg, patients were randomized to: (1) restrictive fluids, in which additional fluid could only be administered for overt signs of severe hypoperfusion, such as MAP <50 mm Hg despite norepinephrine infusion, plasma lactate >4 mml/L, skin mottling proximal to the knee, or urine output <0.1 ml/kg/hr; versus (2) usual care, in which additional fluid was allowable as long as fluid challenges were thought by the treating clinicians to improve hemodynamics. Patients randomized to the restrictive fluids group received less resuscitation fluid over 5 days than those in the usual care group (absolute difference: −1.2 L, 95% CI: −2.0, −0.4). Although this trial was not powered to detect differences in clinical outcomes, patients in the restrictive fluid group were less likely to have worsening kidney injury (OR: 0.46, 95% CI: 0.23, 0.93) and had a non-significant point estimate favoring lower 90-day mortality (OR: 0.71, 95% CI: 0.36, 1.40).
CLOVERS: AN UPCOMING TRIAL
Recognizing the equipoise around IVF management during early sepsis resuscitation and the critical importance of high quality data in this area to promote continued improvement in sepsis outcomes, the National Heart, Lung and Blood Institute (NHLBI) Prevention and Early Treatment of Acute Lung Injury (PETAL) Clinical Trials Network (www.petalnet.org) developed the CLOVERS trial. PETAL consists of emergency medicine and critical care researchers at more than 40 enrolling centers dedicated to conducting randomized controlled trials for improving the care of critically-ill ED and ICU patients with or at risk for acute respiratory distress syndrome (ARDS).
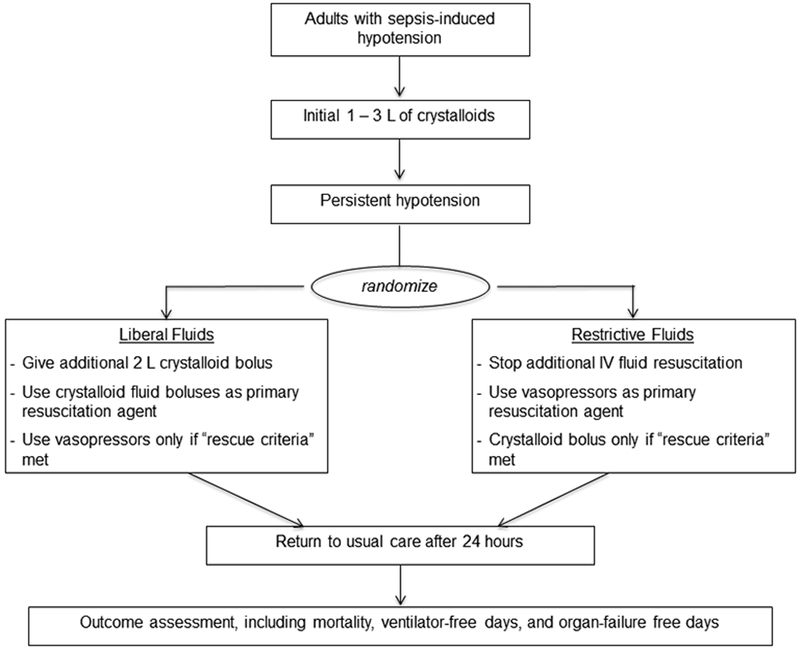
CLOVERS will be a multicenter, unblinded clinical trial comparing liberal and restrictive fluid resuscitation strategies for the first 24 hours of septic shock management among adults in the US (Figure 3). The liberal strategy will consist of IVF management similar to the usual care groups in ProCESS2, ARISE3, and ProMISe4, in which fluid administration is encouraged as first line treatment for signs of hypoperfusion without overt fluid overload. The restrictive strategy will consist of early vasopressor initiation after an initial modest fluid bolus (≤3 L), with additional fluids administered only for signs of extreme intravascular volume depletion. This will enable direct comparison between liberal and restrictive fluid strategies for early sepsis resuscitation. Unlike the CLASSIC trial17, in which enrolled patients received a median of 4 – 5 L of IVF prior to randomization, enrollment for CLOVERS will be in the ED, with patients randomized as soon as possible (but no more than 4 hours) after receiving 1 L of fluid. Patients randomized to the restrictive strategy will be started on a vasopressor infusion to support mean arterial pressure, while patients randomized to the liberal strategy will receive an additional 2 L of IV fluid before considering vasopressors. The primary outcome will be in-hospital mortality to day 90, with key secondary outcomes including ventilator-free days and organ-failure-free days to day 28.
Figure 3.
Trial design summary for the Crystalloid Liberal Or Vasopressor Early Resuscitation in Sepsis (CLOVERS) trial.
In conclusion, despite significant progress during the past two decades, morbidity and mortality from septic shock remain unacceptably high and additional improvement is needed. IVF resuscitation is considered an important initial step in sepsis management, but the optimal dosing for IVF and timing for vasopressors are unknown. Although large-volume fluid boluses of 4–5 L within the first 6 hours of treatment are common, this practice is based on low quality evidence. A growing body of literature has highlighted potential adverse effects from rapid, large-volume fluid boluses. Shifting toward earlier vasopressors and less IVF during initial resuscitation for septic shock is a potential avenue to improve outcomes; however, current evidence for this approach on patient-centered outcomes is lacking. The upcoming CLOVERS trial will directly compare a liberal and restrictive fluids strategy for early septic shock management in EDs and ICUs in the US with the goal of providing patient outcome data needed to inform and guide clinical practice.
Acknowledgments
Funding:
This work was supported by National Heart, Lung and Blood Institute U01 grants: HL123009, HL123010, HL123004, HL123022, HL122989, HL123008, HL123027, HL123020, HL123018, HL123031, HL123033, HL122998, and HL123023. Dr. Self was supported in part by K23GM110469 from the National Institute of General Medical Sciences.
Potential Conflicts of Interest:
All authors are supported by the National Heart, Lung and Blood Institute (NHLBI) within the National Institutes of Health (NIH) for participation in the Prevention and Early Treatment of Acute Lung Injury (PETAL) Clinical Trials Network. No investigators report potential conflicts of interest for the submitted manuscript other than research support from NIH. Disclosures outside the submitted manuscript are outlined below. WHS grants from Cheetah Medical; consultant fees from Abbott Point of Care, Cempra Pharmaceuticals, Ferring Pharmaceuticals, and BioTest AG; travel funds from Gilead Sciences and Pfizer. SMB reports fees from Faron Pharmaceuticals for serving on a steering committee for a clinical trial in ARDS. AAG reports consulting fees from Coalition for Sepsis Survival (a not-for-profit foundation) to develop sepsis-related algorithms. KDL reports stock ownership with Amgen; consultant fees from Achaogen, Durect, Z S Pharma, Theravance, Quark, Potrero Medical; travel funds from the American Society of Nephrology and National Policy Forum on Critical Care and Acute Renal Failure; and compensation for an editor position from the National Kidney Foundation. CDM reports grants from Abbott, Ferring, Siemens; provision of software from Siemens. TWR reports consultant fees from Cumberland Pharmaceuticals, Inc, personal fees from Avisa Pharma, LLC. ISD reports a relationship with Cheetah Medical in which he is principal investigator for an industry-sponsored sepsis trial; payments for his PI role and study-site enrollment are to his employer, an academic hospital. NIS reports Research funding from the NIH, Siemens, Lajolla pharmaceuticals, and ThermoFisher; and, advisory board income from Baxter.
Footnotes
Meetings:
None
Footnote:
The opinions expressed in this article are those of the authors and do not necessarily represent the US Department of Health and Human Services, the National Institutes of Health, or the National Heart, Lung, and Blood Institute.
REFERENCES
- 1.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017; 45: 486–552. [DOI] [PubMed] [Google Scholar]
- 2.Investigators ProCESS, Yealy DM Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014; 370: 1683–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ARISE Investigators, ANZICS Clinical Trials Group, Peake SL, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014; 371: 1496–506. [DOI] [PubMed] [Google Scholar]
- 4.Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015; 372: 1301–11. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen HG, Jaehne AK, Jayaprakash N, et al. Early goal directed therapy in severe sepsis and septic shock: Insights and comparisons to ProCESS, ProMISe and ARISE. Crit Care 2016; 20: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marik PE. Iatrogenic salt water drowning and the hazards of a high central venous pressure. Ann Intensive Care. 2014; 4: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glassford NJ, Eastwood GM, Bellomo R. Physiologic changes after fluid bolus therapy in sepsis: A systematic review of contemporary data. Crit Care. 2014; 18: 696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maitland K, George ED, Evans JA, Kiguli S, Olupot-Olupot P, Akech SO, et al. Exploring mechanisms of excess mortality with early fluid resuscitation: Insights from the FEAST trial. BMC Medicine. 2013; 11: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: A positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 2011; 39: 259–65. [DOI] [PubMed] [Google Scholar]
- 10.Micek ST, McEvoy C, McKenzie M, Hampton N, Doherty JA, Kollef MH. Fluid balance and cardiac function in septic shock as predictors of hospital mortality. Crit Care 2013; 17: R246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadaka F, Juarez M, Naydenov S, O’Brien J. Fluid resuscitation in septic shock: The effect of increasing fluid balance on mortality. J Intensive Care Med 2014; 29: 213–7. [DOI] [PubMed] [Google Scholar]
- 12.Acheampong A, Vincent JL. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit Care. 2015; 19: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Oliveira FS, Freitas FG, Ferreira EM, de Castro I, Bafi AT, de Azevedo LC, Machado FR. Positive fluid balance as a prognostic factor for mortality and acute kidney injury in severe sepsis and septic shock. J Crit Care. 2015; 30: 97–101. [DOI] [PubMed] [Google Scholar]
- 14.Kelm DJ, Perrin JT, Cartin-Ceba R, et al. Fluid overload in patients with severe sepsis and septic shock treated with early goal directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock. 2015; 43: 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakr Y, Rubatto Birri PN, Kotfis K, Nanchal R, Shah B, Kluge S, et al. Higher fluid balance increases the risk of death from sepsis: Results from a large international audit. Crit Care Med 2017; 45: 386–394. [DOI] [PubMed] [Google Scholar]
- 16.Dellinger RP Levy MM, Rhodes A, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013; 4: 580–637. [DOI] [PubMed] [Google Scholar]
- 17.Hjortrup PB, Haase N, Bundgaard H et al. Restricting volumes of resuscitation fluid in septic shock after the initial management: The CLASSIC randomised, parallel-group, multicenter feasibility trial. Intensive Care Med. 2016; 42: 1695–1705. [DOI] [PubMed] [Google Scholar]
- 18.Motzkus CA, Lilly CM. Accountability for sepsis treatment: The SEP-1 Core Measure. Chest. 2017; 151:955–957. [DOI] [PubMed] [Google Scholar]
- 19.National Quality Forum. NQF-endorsed voluntary consensus standards for hospital care, Measure set: Sepsis. Available at: https://www.nhfca.org/psf/resources/Updates1/SEP-1%20Measure%20Information%20Form%20(MIF).pdf. Accessed 08 January 2018.
- 20.Ospina-Tascon G, Neves AP, Occhipinti G, Donadello K, Buchele G, Simion D, et al. Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med 2010; 36: 949–955. [DOI] [PubMed] [Google Scholar]
- 21.Anantasit N, Boyd JH, Wlley KR, Russell JA. Serious adverse events associated with vasopressin and norepinephrine infusion for septic shock. Crit Care Med 2014; 42: 1812–20. [DOI] [PubMed] [Google Scholar]
- 22.Rivers E, Nguyen B, Havstad S, et al. Early goal directed therapy in severe sepsis and septic shock. N Engl J Med. 2001; 345: 1368–77. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro NI, Howell MD, Talmor D, Lahey D, Ngo L, Buras J, et al. Implementation and outcomes of the Multiple Urgent Sepsis Therapies (MUST) protocol. Crit Care Med 2006; 34: 1025–32. [DOI] [PubMed] [Google Scholar]
- 24.Trzeciak S, Dellinger RP, Abate NL, et al. Translating research to clinical practice: A 1-year experience with implementing early goal-directed therapy for septic shock in the emergency department. Chest 2006; 129: 225–232. [DOI] [PubMed] [Google Scholar]
- 25.Micek ST, Roubinian N, Heuring T, et al. Before-after study of a standardized hospital order set for the management of septic shock. Crit Care Med 2006; 34: 2707–2713. [DOI] [PubMed] [Google Scholar]
- 26.Puskarich MA, Marchick MR, Kline JA, Steuerwald MT, Jones AE. One year mortality of patients treated with an emergency department based early goal directed therapy protocol for severe sepsis and septic shock: A before and after study. Crit Care. 2009; 13: R167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis RJ. Disassembling goal-directed therapy for sepsis: A first step. JAMA. 2010; 303: 777–9. [DOI] [PubMed] [Google Scholar]
- 28.Jones AE. Unbundling early sepsis resuscitation. Ann Emerg Med. 2014; 63: 654–5. [DOI] [PubMed] [Google Scholar]
- 29.Kalil AC, Johnson DW, Lisco SJ, Sun J. Early-goal directed therapy for sepsis: A novel solution for discordant survival outcomes in clinical trials. Crit Care Med. 2017; 45: 607–614. [DOI] [PubMed] [Google Scholar]
- 30.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA 2014; 311:1308–16. [DOI] [PubMed] [Google Scholar]
- 31.Lilly CM. The ProCESS trial: A new era of sepsis management. N Engl J Med. 2014; 370:1750–1. [DOI] [PubMed] [Google Scholar]
- 32.Lindenauer PK, Lagu T, Shieh MS, Pekow PS, Rothberg MB. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003–2009. JAMA 2012; 307: 1405–13. [DOI] [PubMed] [Google Scholar]
- 33.Rhee C, Dantes R, Epstein L, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. JAMA 2017; 318: 1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis R, Merchan C, Altshuler D, Papadopoulos J. Safety of peripheral administration of vasopressor agents. J Intensive Care Med 2017. January 1. doi 10.1177/0885066616686035. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Jones AE, Kline JA. Shock. Pages 41–56 In Marx JA, Hockberger RS, Walls RM, et al. (Eds). Rosen’s Emergency Medicine: Concepts and Clinical Practice, 6th Edition 2006. Philadelphia: Mosby Elsevier. [Google Scholar]
- 36.Cardenas-Garcia J, Schaub KF, Belchikov YG, et al. Safety of peripheral intravenous administration of vasoactive medication. J Hosp Med. 2015; 10: 581–5. [DOI] [PubMed] [Google Scholar]
- 37.Loubani OM, Green RS. A systematic review of extravasation and local tissue injury from administration of vasopressors through peripheral intravenous catheters and central venous catheters. J Crit Care. 2015; 30: 653.e9–e17. [DOI] [PubMed] [Google Scholar]
- 38.Hamzaoui O, Georger JF, Monnet X, et al. Early administration of norepinephrine increases cardiac preload and cardiac output in septic patients with life-threatening hypotension. Crit Care. 2010; 14: R142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Legrand M, Dupuis C, Simon C, et al. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: A retrospective observational study. Crit Care. 2013; 17: R278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maitland K, Kiguli S, Opoka RO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med 2011; 364: 2483–95. [DOI] [PubMed] [Google Scholar]
- 41.Monnet X, Marik P, Teboul JL. Passive leg raising for predicting fluid responsiveness: A systematic review and meta-analysis. Intensive Care Med. 2016; 42: 1935–1947. [DOI] [PubMed] [Google Scholar]
- 42.Dong ZZ, Fang Q, Zheng X, Shi H. Passive leg raising as an indicator of fluid responsiveness in patients with severe sepsis. World J Emerg Med. 2012; 3: 191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lanspa MJ, Brown SM, Hirshberg EL, et al. Central venous pressure and shock index predict lack of hemodynamic response to volume expansion in septic shock: a prospective observational study. J Crit Care 2012; 27: 609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nunes TS, Ladeira TR, Bafi AT, de Azevedo LC, Machado FR, Freitas FG. Duration of hemodynamic effects of crystalloids in patients with circulatory shock after initial resuscitation. Ann Intensive Care 2014; 4: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cannon JW, Khan MA, Raja AS, Cohen MJ, Como JJ, et al. Damage control resuscitation in patients with severe traumatic hemorrhage: A practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg 2017; 82: 605–617. [DOI] [PubMed] [Google Scholar]
- 46.American College of Surgeons. Advanced Trauma Life Support (ATLS): Student course manual, 9th edition Available at: https://www.44c.in.ua/files/book11.pdf. Accessed 17 March 2018.
- 47.Harris T, Thomas GO, Brohi K. Early fluid resuscitation in severe trauma. BMJ 2012; 345: e5752. [DOI] [PubMed] [Google Scholar]
- 48.Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med 2017; 376: 2235–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vincent J-L, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall J-R, Payen D, Sepsis Occurrence in Acutely Ill Patients Investigators. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344–353. [DOI] [PubMed] [Google Scholar]
- 50.Murphy CV, Schramm GE, Doherty JA, Reichley RM, Gajic O, Afessa B, Micek ST, Kollef MH. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136(1):102–109. doi: 10.1378/chest.08-2706. [DOI] [PubMed] [Google Scholar]
- 51.Sirvent JM, Ferri C, Baro A, Murcia C, Lorencio C (2015) Fluid balance in sepsis and septic shock as a determining factor of mortality. Am J Emerg Med 33:186–189. [DOI] [PubMed] [Google Scholar]
- 52.Silversides JA, Major E, Ferguson AJ, Mann EE, McAuley DF, Marshall JC, Blackwood B, Fan E. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med 2017; 43: 155–170. [DOI] [PubMed] [Google Scholar]
- 53.Smith SH, Perner A. Higher vs lower fluid volume for septic shock: clinical characteristics and outcome in unselected patients in a prospective, multicenter cohort. Crit Care. 2012; 16: R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marik PE, Linde-Zwirble WT, Bittner EA, Sahatjian J, Hansell D. Fluid administration in severe sepsis and septic shock, patterns and outcomes: Analysis of a large national database. Intensive Care Med 2017; 43: 625–632. [DOI] [PubMed] [Google Scholar]
- 55.Sjoding MW, Luo K, Miller MA, Iwashyna TJ. When do confounding by indication and inadequate risk adjustment bias critical care studies? A simulation study. Crit Care 2015; 19: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bosco JLF, Simmiman RA, Thwin SS, Geiger AM, Buist DSM, Prout MN, et al. A most stubborn bias: No adjustment method fully resolves confounding by indication in observational studies. J Clin Epidemiol 2010; 63: 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiedemann HP, Wheeler AP, National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006;354:2564–2575. [DOI] [PubMed] [Google Scholar]
- 58.Malbrain MLNG, Marik PE, Witters I et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014; 46:361–380. [DOI] [PubMed] [Google Scholar]
- 59.Semler MW, Wheeler AP, Thompson BT, et al. Impact of initial central venous pressure on outcomes of conservative versus liberal fluid management in acute respiratory distress syndrome. Crit Care Med 2016; 44: 782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andrews B, Semler MW, Muchemwa L, et al. Effect of an early resuscitation protocol on in-hospital mortality among adults with sepsis and hypotension: A randomized clinical trial. JAMA. 2017; 318:1233–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andrews B, Muchemwa L, Kelly P, Lakhi S, Heimburger DC, Bernard GR. Simplified severe sepsis protocol: a randomized controlled trial of modified early goal-directed therapy in Zambia . Crit Care Med. 2014; 42: 2315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]