Abstract
Purpose
The purpose of the study was to investigate the effects of a family-based self-management support intervention for adults with type 2 diabetes (T2DM).
Methods
Using a 2-group, experimental repeated measures design, 157 dyads (participant with T2DM and family member) were randomly assigned to an intervention (education, social support, home visits, and telephone calls) or a wait list control group. Data were collected at baseline, postintervention (3 months), and 6 months postintervention. A series of 2 × 3 repeated measures ANOVAs were used to test the hypotheses with interaction contrasts to assess immediate and sustained intervention effects.
Results
Significant changes over time were reported in diet self-management, exercise self-management, total self-management, diabetes self-efficacy for general health and total diabetes self-efficacy, physician distress, regimen distress, interpersonal distress, and total distress. There were likewise sustained effects for diet self-management, total self-management, diabetes self-efficacy for general health, total self-efficacy, physician distress, regimen distress, and interpersonal distress.
Conclusions
Results support and extend prior research documenting the value of culturally relevant family-based interventions to improve diabetes self-management and substantiate the need for intensive, longer, tailored interventions to achieve glycemic control.
Diabetes, an escalating global health threat, has more than doubled among adults over the past 3 decades.1 Environmental/lifestyle factors are implicated for the increase in diabetes-related risk factors.2 Of the approximately 29 million (9.3%) adults in the US with diabetes, type 2 diabetes (T2DM) accounts for 90% to 95% of adult cases.3 Minorities, including 50 million Hispanics who represent 16% of the US population, are disproportionately affected by diabetes (12.8%) compared to non-Hispanic Caucasians (7.6%).3 Hispanics experience higher rates of obesity, sedentary lifestyles, poorer eating habits and family histories of diabetes,4 diabetes-related death rates (51%),5 and increased risk for diabetes-related complications such as neuropathy, nephropathy, diabetic retinopathy, and cardiovascular disease than non-Hispanics.6 Among persons of Mexican origin, the largest Hispanic subgroup, 18.3% have diabetes.7
The chronic and complex trajectory of T2DM requires daily engagement in self-management activities to achieve glycemic control and prevent future complications.8 Inadequate metabolic control is evidenced nationally by only 36% to 69% of persons with diabetes achieving glycemic control.9 Less than 40% (36.8%) of Hispanics with T2DM have controlled A1C.10
Diabetes self-management education and support (DSME/S) builds knowledge, skills, and abilities for successful T2DM self-management; decreases A1C and weight and lowers health care costs.8 DSME/S has demonstrated a reduction in A1C by 1% and a positive effect on other clinical indicators in persons with T2DM. DSME/S improves lifestyle.11 Persons not receiving DSME/S have a 4-fold increase for developing major diabetes-related complications compared to individuals receiving DSME/S.12 Despite DSME/S benefits, the majority of persons with diabetes who receive DSME/S is quite small.11,13 Barriers to accessing DSME/S are substantial: 56% of persons with diabetes mellitus nationally and 54.9% of Arizonans report never having attended a DSME/S class.14
Diabetes self-management commonly occurs in a family environment.15 Family values and a family-oriented world-view influence diabetes self-management, and in turn, diabetes control affects the health and well-being of the entire family.16 Family support and social relationships are critical in improving diabetes self-management in Hispanics with T2DM.17 While family social support in diabetes self-management has a positive impact on behavior changes,17,18 families have also been identified as a barrier to T2DM management and glycemic control.19,20 Therefore, engaging family members in DSME/S and promoting family support may be pivotal in facilitating lifestyle changes in Hispanics with T2DM. The focus of diabetes self-management in Hispanics needs to shift from traditional individual approaches to family-focused interventions.
Several studies have emphasized the significance of including both adults diagnosed with T2DM and their family members to improve diabetes outcomes.20–22 Lifestyle modification programs including family support and tailored to Hispanic culture demonstrated improvement in patients’ self-efficacy, perceived support, knowledge, and self-care.23–25 However, these interventions focused primarily on individuals and did not fully integrate family members26 and thus may not be sustainable in the family-centered Hispanic culture. Only 1 study conducted with Latinos reported family members’ participation and outcomes.21 Family members improved in diabetes knowledge and physical health–related quality of life. The paucity of family-based interventions with Hispanics with T2DM and heterogeneity across study designs and interventions contribute to a gap in our understanding of the effect of family participation on diabetes outcomes.
The effect of a family-based self-management support intervention on behavioral and biological outcomes was investigated. Adults with T2DM and a family member were involved in all aspects of the intervention. It was hypothesized that Mexican American adults with T2DM in the 12-week family-based diabetes intervention group would show greater improvements than the wait list control group immediately after the intervention period and 6 months after the intervention in: (1) behavioral outcomes of diabetes self-management, diabetes self-efficacy, diabetes-related distress, nutrition, and physical activity and (2) the biological outcome of glycemic control (A1C).
Research Design and Methods
In a 2-group, experimental repeated measures design, the effectiveness of a culturally tailored family-based T2DM self-management social support intervention previously refined by a Family Action Board (FAB)20 was tested. Participants were randomly assigned to either the intervention or wait list control group. A total of 157 dyads (participant with T2DM and their family member) participated (83 intervention dyads, 74 control dyads). Culturally responsive recruitment methods27 that were successful in our previous studies were used. Potential participants were recruited by bilingual/bicultural promotoras. In addition, the FAB helped to identify, reach out, and motivate potential participants to participate. Inclusion criteria were Mexican Americans diagnosed with T2DM for at least 1 year, between 35 and 74 years, of Mexican origin, spoke and read Spanish or English, A1C of 8.0% (64 mmol/mol) or greater, had not participated in a diabetes education program in the prior year, able to walk at least 1 mile (determined by self-report), access to and ability to talk on telephone, and had 1 adult family member willing to participate. Participants were excluded if they were pregnant, had a disability, or had an advanced or terminal condition. The participant with T2DM identified a family member who was 18 years or older, spoke and read Spanish or English, either lived in same house as participant with T2DM or saw them weekly to share meals or visit or shopping, and were willing to participate. Figure 1 outlines recruitment, enrollment, intervention, and follow-up. Both the adult with T2DM and family members received grocery certificates for $25, $30, and $40 after each data collection, respectively.
Figure 1.
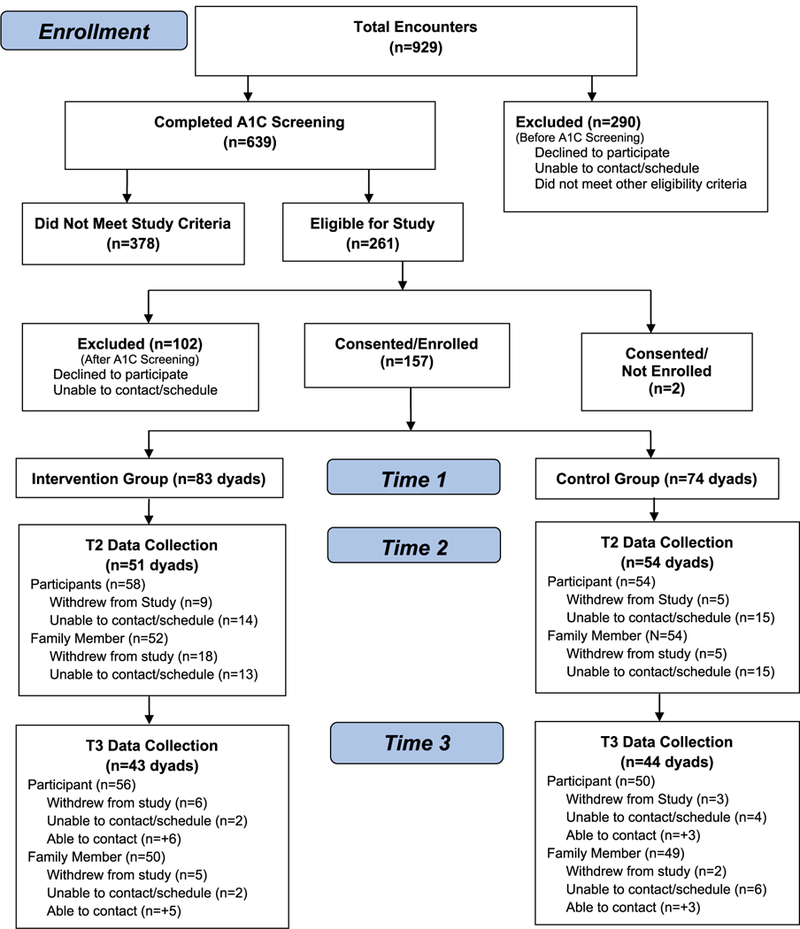
Study flow chart.
The study was approved by the Institutional Review Board of the participating institution. All participants provided signed informed consent for A1C screening (person with T2DM only) and study participation. All consent documents were available in English and Spanish.
Intervention Group
The family-based intervention was conducted in Hispanic urban neighborhoods in the Arizona border region. The 12-week intervention program included 3 successive components: (1) six 2-hour educational and social support group sessions conducted weekly for 6 weeks, (2) three 2-hour home visits scheduled weekly for 3 weeks, and (3) three 20-minute telephone calls scheduled weekly for 3 weeks. The intervention was consecutively delivered to 12 cohorts with 5 to 12 dyads (10–24 people) in each intervention cohort. The educational and support sessions included information about managing diabetes to improve glycemic control and prevent complications through food consumed, physical activity, and stress management. A nurse who is a certified diabetes educator (CDE) conducted the educational sessions, and a promotora conducted the social support sessions, home visits, and telephone calls. The home visits built on and tailor knowledge and skills acquired in the group sessions tailored to the family context. Goals established in the group sessions using the SMART (Specific, Measurable, Attainable, Relevant, and Time-bound) goal approach were evaluated and redefined if needed in each home visit. The promotora made telephone calls to follow up on the participants’ progress and/or barriers in meeting their SMART goals for healthy eating, physical activity, and managing diabetes-related distress. All intervention sessions were audiotaped.
Wait List Control Group
After the final data collection, a nurse educator conducted the wait list control group program. Two-hour educational sessions were provided weekly for 3 weeks. Session topics were the same as those delivered to the intervention group.
Measures
All instruments were available in English and Spanish. With exception of A1C, all data were collected from the participant with T2DM and the family member at baseline, 3 months (post intervention), and 9 months (6 months post intervention).
Descriptive Measures
The Acculturation Rating Scale for Mexican Americans (ARMSA-II), a 30-item Likert-type scale, was administered at baseline. The scale includes 2 subscales, Mexican Orientation (MOS, 17 items) and Anglo orientation (AOS, 13 items). Mean scores were computed for each subscale. The MOS mean score is subtracted from the AOS mean score to obtain a linear acceleration score, which results in acculturation level (5 levels).28 Cronbach’s alpha was .93 for the AOS and .87 for the MOS.
The Diabetes Knowledge Questionnaire (DKQ), a 24-item questionnaire, measured knowledge of diet, exercise, blood sugar, foot care, and complications. Response options (yes, no, I don’t know) were scored as correct or not correct29 and summed for a total score, with higher scores indicating greater knowledge. Cronbach’s alpha was .72.
Diabetes health literacy was measured with the Newest Vital Sign, a 6-item instrument.30 Questions ask about information on a nutrition label. Items are scored as correct or incorrect, with higher scores indicating greater health literacy. Cronbach’s alpha was .68.
Height and weight were measured with the Seca 215 height rod and a balanced scale after the participant removed his or her shoes and any hats or scarves. Recorded height and weight were used to calculate the BMI from tables available on the National Heart, Lung, & Blood Institute (NHLBI) website.31 Waist and hip measurements were obtained in a private location with the tape measure snugly around but not compressing the skin at the waist (midpoint between the inferior margin of the last rib and the crest of the ileum) and hip (the widest part of the buttocks) with participant relaxed and after exhalation. The measurement was repeated and recorded to the nearest 0.1 inch. The Waist-Hip Ratio was calculated as the waist measurement divided by the hip measurement.
Behavioral Outcomes
The 14-item Diabetes Self-Care Activities Questionnaire asked the frequency (past 7 days) participants engaged in diet, exercise, blood sugar testing, foot care, and taking prescribed diabetes medication. A mean score was calculated, with higher scores indicating greater self-management activities performed.24 Subscale Cronbach’s alpha coefficients ranged from .69 to .94 and was .80 for the total scale.
The Self-Efficacy for Diabetes Scale32 measures how confident participants feel in managing their diet, exercise, blood sugar, and illnesses specific to diabetes. Item responses range from 1 (not at all confident) to 5 (totally confident), with the descriptors anchoring the beginning (1) and end of the scale (5). The 8-item scale contains 2 subscales: Diabetes Self-efficacy for Health Behaviors (5 items) and Diabetes Self-efficacy for General Health (3 items). A mean score was computed for each subscale and the total scale, with higher scores representing greater self-efficacy. Subscale Cronbach’s alpha coefficients were .78 and .74, respectively, and was .81 for the total scale.
The Diabetes Distress Scale, a 17-item questionnaire, contains 4 subscales assessing emotional burden, physician distress, regimen distress, and interpersonal distress. Response options are no problem, sometimes a problem, and serious problem. Scores were calculated for subscales and the total scale, with higher scores representing greater diabetes distress.33 Subscale Cronbach’s alpha coefficients ranged from .79 to .91 and .91 for total scale.
Healthy eating was measured with the Fat, Fruit, and Vegetable questionnaire (23 items) that assessed frequency of consuming specific foods. Sixteen items measure frequency of consumption of foods containing fats (once a month or less to 5 or more times per week). Seven items measure frequency of fruit and vegetable consumption (less that once per week to 2 or more times a day).34 Cronbach’s alpha was .80 for fat, .51 for fruit, and .73 for vegetable.
Physical activity was measured with the International Physical Activity Questionnaire (IPAQ). Seven items assess the number of days and hours/minutes per day participants engaged in vigorous physical activity, moderate physical activity, walking, and sedentary (sitting) activities in the prior 7 days. Vigorous physical activity, moderate physical activity, and walking were used to compute metabolic equivalent task (MET) minutes per week.35
Biologic Outcome
Participants’ A1C was obtained by finger stick and measured using the DCA machine (DCA 2000). The test is considered valid and reliable.36
Power Analysis
Based on descriptive statistics from preliminary studies, effect size estimates for the interaction effects in the 2 × 3 ANOVAs were made. Given these effect sizes (f), an alpha level of .05, and a sample size of 156 (78 per group), power would be .99 for mean diabetes self-care activities (f = .45), .89 for exercise self-care activities (f = .28), .66 for diet self-care activities (f = .21), .80 for diabetes knowledge (f = .25), .78 for interpersonal distress (f = .24), and .93 for total diabetes distress (f = .30). For A1C, power would be .86 to detect a 1.0 difference (f = .27).
Data Analysis Plan
SPSS version 23.0 was used. Descriptive statistics described the sample. Chi-square and t tests were performed to assess baseline group differences. A series of 2 × 3 repeated measures ANOVAs with interaction contrasts were used to test the hypotheses. The between-subjects factor was group with 2 levels (intervention and wait list control), and the within-subjects factor (repeated measure) was time with 3 levels (baseline, time 2 [T2], and time 3 [T3]). However, it was the interaction contrasts that assessed the immediate and sustained effects. The immediate effectiveness of the intervention was indicated by a significant interaction contrast assessing differential change between the intervention and wait list control groups from baseline to immediate postintervention (T2). The sustainability of the intervention was evaluated with the interaction contrast assessing differential change between the intervention and wait list control groups from T2 through T3.
Results
Participants with T2DM ranged in age from 35 to 75 (mean = 53.53, SD = 9.0). The majority were female (65%), married (71%), had less than a high school education (68%), and had an annual income of $20 000 or less (65%). They had T2DM for 11.52 years (SD = 7.8, range, 1–40 years). They had lived in the U.S for 28.18 years (SD = 16.2, range, 0.16–69). They tended to be overweight or obese (93.6%), with an average BMI of 33.31 (SD = 6.9, range, 18.6–56.3). Waist circumference ranged from 29 to 61 (mean = 42.00, SD = 6.3). The majority were taking medications for diabetes (93%), with 45% taking oral medications for diabetes. Almost two-thirds (65%) reported a very Mexican orientation, and health literacy scores indicated very limited health literacy. There were no differences between the groups on any of the demographic characteristics.
Family members ranged in age from 18 to 88 years (mean = 47.27, SD = 16.1). The majority were female (72.6%), married (64.3%), had less than a high school education (53%), and had an annual income of $20 000 or less (59%). They had lived in the US for 28.01 years (SD = 16.5, range, 3–87). They tended to be overweight or obese (80.3%), with an average BMI of 33.40 (SD = 7.4, range, 21.0–66.6). Waist circumference ranged from 29 to 64 (mean = 40.92, SD = 6.3). They had limited health literacy, and half (50%) reported a very Mexican orientation. Demographic characteristics for participants and family members are presented in Table 1.
Table 1.
Demographic Characteristics: Participant and Family Member
| Characteristic | Participant With Type 2 Diabetes |
Family Member |
||||||
|---|---|---|---|---|---|---|---|---|
| Total n = 157 |
Control n = 74 |
Intervention n = 83 |
P | Total n = 157 |
Control n = 74 |
Intervention n = 83 |
P | |
| Gender | .069 | .440 | ||||||
| Female, n (%) | 102 (65.0) | 53 (71.6) | 49 (59.0) | 114 (72.6) | 55 (74.3) | 59 (71.1) | ||
| Male, n (%) | 55 (35.0) | 21 (28.4) | 34 (41.0) | 42 (26.8) | 19 (25.7) | 23 (27.7) | ||
| Age | .875 | .792 | ||||||
| Mean (SD) | 53.53 (9.0) | 53.41 (8.4) | 53.64 (9.6) | 47.27 (16.1) | 47.65 (17.4) | 46.95 (14.9) | ||
| Range | 35−75 | 35−75 | 35−73 | 18−88 | 18−84 | 18−88 | ||
| Marital status | .493 | .041 | ||||||
| Single, n (%) | 21 (13.4) | 13 (17.6) | 8 (9.6) | 35 (22.3) | 17 (23.0) | 18 (21.7) | ||
| Married, n (%) | 111 (70.7) | 51 (68.9) | 60 (72.3) | 101 (64.3) | 46 (62.2) | 55 (66.3) | ||
| Divorced, n (%) | 18 (11.5) | 7 (9.5) | 11 (13.3) | 13 (8.3) | 4 (5.4) | 9 (10.8) | ||
| Widowed, n (%) | 6 (3.8) | 3 (4.1) | 3 (3.6) | 6 (3.8) | 6 (8.1) | |||
| Education | .931 | .297 | ||||||
| Never attended school, n (%) | 4 (2.5) | 1 (1.4) | 3 (3.6) | 4 (2.5) | 2 (2.7) | 2 (2.4) | ||
| Grade school, n (%) | 78 (49.7) | 38 (51.4) | 40 (48.2) | 53 (33.8) | 32 (43.2) | 21 (25.3) | ||
| Some high school, n (%) | 24 (15.3) | 11 (14.9) | 13 (15.7) | 26 (16.6) | 11 (14.9) | 15 (18.1) | ||
| High school graduate, n (%) | 18 (11.5) | 9 (12.2) | 9 (10.8) | 27 (17.2) | 12 (16.2) | 15 (18.1) | ||
| Some college, n (%) | 24 (15.3) | 11 (14.9) | 13 (15.7) | 27 (17.2) | 12 (16.2) | 15 (18.1) | ||
| College graduate, n (%) | 8 (5.1) | 4 (5.4) | 4 (4.8) | 16 (10.2) | 4 (5.4) | 12 (14.5) | ||
| Graduate school, n (%) | 1 (0.6) | 1 (1.2) | 2 (1.3) | 1 (1.4) | 1 (1.2) | |||
| Years lived in US | .143 | .361 | ||||||
| Mean (SD) | 28.18 (16.2) | 26.13 (15.0) | 29.96 (17.1) | 28.01 (16.5) | 26.70 (16.5) | 29.16 (16.5) | ||
| Range | 0.16−69 | 0.16−69 | 1−65 | 3−87 | 5−68 | 3−87 | ||
| Language speak at home | .391 | .153 | ||||||
| English, n (%) | 28 (17.8) | 10 (13.5) | 18 (21.7) | 35 (22.3) | 12 (16.2) | 23 (27.7) | ||
| Spanish, n (%) | 112 (71.3) | 56 (75.7) | 56 (67.5) | 92 (58.6) | 49 (66.2) | 43 (51.8) | ||
| Both, n (%) | 16 (10.2) | 8 (10.8) | 8 (9.6) | 29 (18.5) | 13 (17.6) | 16 (19.3) | ||
| Have paying job | .671 | .112 | ||||||
| No, n (%) | 78 (49.7) | 37 (50.0) | 41 (49.4) | 73 (46.5) | 36 (48.6) | 37 (44.6) | ||
| Yes, n (%) | 62 (39.5) | 31 (41.9) | 31 (37.3) | 67 (42.7) | 27 (36.5) | 40 (48.2) | ||
| Retired, n (%) | 16 (10.2) | 6 (8.1) | 10 (12.0) | 16 (10.2) | 11 (14.9) | 5 (6.0) | ||
| Annual family income | .067 | .025 | ||||||
| More than $25 000, n (%) | 24 (15.3) | 6 (8.1) | 18 (21.7) | 33 (21.0) | 10 (13.5) | 23 (27.7) | ||
| $20 000−$25 000, n (%) | 19 (12.1) | 8 (10.8) | 11 (13.3) | 21 (13.4) | 8 (10.8) | 13 (15.7) | ||
| $15 000−$20 000, n (%) | 28 (17.8) | 16 (21.6) | 12 (14.5) | 22 (14.0) | 13 (17.6) | 9 (10.8) | ||
| $10 000−$15 000, n (%) | 29 (18.5) | 18 (24.3) | 11 (13.3) | 27 (17.2) | 10 (13.5) | 17 (20.5) | ||
| <$10 000, n (%) | 45 (28.7) | 20 (27.0) | 25 (30.1) | 41 (26.1) | 26 (35.1) | 15 (18.1) | ||
| Don’t know, n (%) | 4 (2.5) | 1 (1.4) | 3 (3.6) | 2 (1.3) | 2 (2.4) | |||
| Relationship to family member in study | .227 | .411 | ||||||
| Spouse/partner, n (%) | 75 (47.8) | 30 (40.5) | 45 (54.2) | 72 (45.9) | 31 (41.9) | 41 (49.4) | ||
| Adult daughter, n (%) | 34 (21.7) | 20 (27.0) | 14 (16.9) | 26 (16.6) | 12 (16.2) | 14 (16.9) | ||
| Adult son, n (%) | 6 (3.8) | 4 (5.4) | 2 (2.4) | 1 (0.6) | 1 (1.2) | |||
| Other family member, n (%) | 37 (23.6) | 18 (24.3) | 19 (22.9) | 56 (35.7) | 31 (41.9) | 25 (30.1) | ||
| Years with diabetes | .492 | |||||||
| Mean (SD) | 11.52 (7.8) | 11.05 (7.3) | 11.92 (8.3) | |||||
| Range | 1−40 | 1−40 | 1.5−40 | |||||
| Medication take for diabetes | .121 | |||||||
| Oral, n (%) | 70 (44.6) | 36 (48.6) | 34 (41.0) | |||||
| Insulin, n (%) | 14 (8.9) | 3 (4.1) | 11 (13.3) | |||||
| Oral plus insulin, n (%) | 45 (28.7) | 21 (28.4) | 24 (28.9) | |||||
| Acculturation level | .316 | .330 | ||||||
| Very Mexican oriented, n (%) | 102 (65.0) | 49 (66.2) | 53 (63.9) | 79 (50.3) | 39 (52.7) | 40 (48.2) | ||
| Mexican oriented to bicultural, n (%) | 28 (17.8) | 16 (21.6) | 12 (14.5) | 42 (26.8) | 23 (31.1) | 19 (22.9) | ||
| Slightly Anglo oriented bicultural, n (%) | 19 (12.1) | 7 (9.5) | 12 (14.5) | 23 (14.6) | 7 (9.5) | 16 (19.3) | ||
| Strongly Anglo oriented, n (%) | 8 (5.1) | 2 (2.7) | 6 (7.2) | 11 (7.0) | 5 (6.8) | 6 (7.2) | ||
| Very assimilated; Anglicized, n (%) | 1 (0.6) | 1 (1.2) | ||||||
| BMI calculated | .841 | .225 | ||||||
| Mean (SD) | 33.31 (6.9) | 33.20 (6.7) | 33.42 (7.2) | 33.40 (7.4) | 32.63 (8.1) | 34.15 (6.6) | ||
| Range | 18.6−56.3 | 20.2−56.3 | 18.6−54.8 | 21.0−66.6 | 21.0−66.6 | 22.2−56.1 | ||
| BMI categories | .034 | .186 | ||||||
| Normal weight (18.5−24.9), n (%) | 10 (6.4) | 8 (10.8) | 2 (2.4) | 13 (8.3) | 8 (10.8) | 5 (6.0) | ||
| Overweight (25−29.9), n (%) | 47 (29.9) | 17 (23.0) | 30 (36.1) | 37 (23.6) | 22 (29.7) | 15 (18.1) | ||
| Obesity (BMI of 30 or greater), n (%) | 100 (63.7) | 49 (66.2) | 51 (61.4) | 89 (56.7) | 39 (52.7) | 50 (60.2) | ||
| Diabetes knowledge (% correct) | .634 | .064 | ||||||
| Mean (SD) | 68.60 (14.8) | 69.20 (14.0) | 68.07 (15.5) | 61.57 (21.0) | 58.28 (21.5) | 64.51 (20.3) | ||
| Range | 8.33−95.83 | 25.00−95.83 | 8.33−95.83 | 0−100 | 0−100 | 0−95.83 | ||
| Newest vital sign | .533 | .029 | ||||||
| Mean (SD) | 2.51 (1.7) | 2.42 (1.8) | 2.59 (1.6) | 3.04 (1.9) | 2.70 (1.9) | 3.35 (1.7) | ||
| Range | 0−6 | 0−6 | 0−6 | 0−6 | 0−6 | 0−6 | ||
Hypothesis 1
Participants with T2DM had significant changes over time (group by time interaction) in diet self-management, F(2, 188) = 7.37, P = .001; exercise self-management, F(2, 188) = 3.77, P = .025; total self-management, F(2, = 6.88, P = .001; diabetes self-efficacy for health behaviors, F(1.8, 168.6) = 4.50, P = .015; diabetes self-efficacy for general health, F(2, 190 = 3.55), P = .031; total diabetes self-efficacy, F(1.8, 173.7) = 4.98, P = .010; physician distress, F(2, 190) = 3.42, P = .035; regimen distress, F(2, 190) = 9.75, P < .001; interpersonal distress, F(1.9, 177.0) = 4.12, P = .020; and total diabetes distress, F(1.8, 172.7) = 9.07, P < .001. Participant changes over time are presented in Table 2.
Table 2.
Participant Changes in Behavioral and Biological Outcomes Across Time
| Time 1 |
Time 2 |
Time 3 |
F (df) P Group × Time Contrast T1 vs. T2 Contrast T2 vs. T3 |
||||
|---|---|---|---|---|---|---|---|
| Control Mean (SD) |
Intervention Mean (SD) |
Control Mean (SD) |
Intervention Mean (SD) |
Control Mean (SD) |
Intervention Mean (SD) |
||
| Diet self-management activities | 3.58 (1.6) | 3.04 (1.5) | 4.09 (1.3) | 4.67 (1.2) | 4.19 (1.3) | 4.32 (1.2) | 7.37 (2,188) .001 |
| 14.08 (1,94) .000 | |||||||
| 2.68 (1,94) .105 | |||||||
| Exercise self-management activities | 2.72 (2.1) | 2.41 (2.3) | 2.95 (1.8) | 3.77 (1.9) | 3.27 (1.9) | 3.18 (2.0) | 3.77 (2,188) .025 |
| 6.06 (1,94) .016 | |||||||
| 5.25 (1,94) .024 | |||||||
| Blood sugar self-management activities | 3.41 (2.9) | 3.95 (2.8) | 3.42 (2.6) | 4.29 (2.5) | 3.67 (2.8) | 3.90 (2.7) | 0.59 (2,188) .553 |
| 0.32 (1,94) .574 | |||||||
| 1.19 (1,94) .278 | |||||||
| Foot care self-management activities | 4.96 (2.0) | 4.95 (2.2) | 5.30 (2.0) | 5.96 (1.7) | 5.20 (2.0) | 5.91 (1.5) | 3.04 (2,188) .050 |
| 3.84 (1,94) .053 | |||||||
| 0.02 (1,94) .892 | |||||||
| Medication self-management activities | 6.32 (1.6) | 6.74 (0.7) | 6.48 (1.5) | 6.77 (1.1) | 6.45 (1.3) | 6.72 (1.1) | 0.16 (2,178) .855 |
| 0.22 (1,89) .642 | |||||||
| 0.004 (1,89) .951 | |||||||
| Total self-management activities | 3.94 (1.4) | 3.78 (1.4) | 3.95 (1.2) | 4.58 (1.0) | 4.36 (1.3) | 4.63 (0.9) | 6.88 (2,188) .001 |
| 12.63 (1,94) .001 | |||||||
| 3.74 (1,94) .056 | |||||||
| Diabetes self-efficacy for health behaviors | 3.69 (1.1) | 3.71 (1.1) | 3.63 (1.1) | 4.25 (0.7) | 3.84 (1.0) | 4.01 (0.8) | 4.50 (1.8,168.6) .015 |
| 7.58 (1,94) .007 | |||||||
| 6.95 (1,94) .010 | |||||||
| Diabetes self-efficacy for general health | 3.82 (1.4) | 3.58 (1.1) | 3.94 (1.2) | 4.30 (1.0) | 4.18 (1.1) | 4.45 (0.7) | 3.55 (2,190) .031 |
| 5.26 (1,95) .024 | |||||||
| 0.22 (1,95) .639 | |||||||
| Total diabetes self-efficacy | 3.76 (1.1) | 3.66 (0.9) | 3.76 (1.1) | 4.27 (0.7) | 3.98 (0.9) | 4.18 (0.7) | 4.98 (1.8,173.7) .010 |
| 8.92 (1,95) .004 | |||||||
| 3.88 (1,95) .052 | |||||||
| Emotional Burden subscale | 4.34 (2.8) | 5.04 (3.0) | 3.74 (2.7) | 3.28 (2.6) | 3.47 (2.2) | 3.32 (2.6) | 2.67 (2,190) .072 |
| 4.56 (1,95) .035 | |||||||
| 0.47 (1,95) .494 | |||||||
| Physician Distress subscale | 1.64 (2.1) | 2.24 (2.3) | 1.77 (2.4) | 1.22 (1.9) | 1.36 (2.0) | 1.44 (2.0) | 3.42 (2,190) .035 |
| 6.34 (1,95) .013 | |||||||
| 2.64 (1,95) .108 | |||||||
| Regimen Distress subscale | 4.23 (3.0) | 5.50 (2.8) | 4.30 (3.2) | 3.20 (2.3) | 3.83 (3.0) | 3.66 (2.4) | 9.75 (2,190) .000 |
| 19.81 (1,95) .000 | |||||||
| 3.34 (1,95) .071 | |||||||
| Interpersonal Distress subscale | 2.06 (1.9) | 2.72 (2.0) | 1.81 (1.8) | 1.40 (1.7) | 1.51 (1.7) | 1.52 (1.9) | 4.12 (1.9,177.0) .020 |
| 7.33 (1,95) .008 | |||||||
| 1.71 (1,95) .194 | |||||||
| Total diabetes distress | 12.28 (7.3) | 15.50 (8.5) | 11.62 (8.5) | 9.10 (6.6) | 10.17 (6.6) | 9.94 (7.0) | 9.07 (1.8,172.7) .000 |
| 15.99 (1,95) .000 | |||||||
| 4.14 (1,95) .045 | |||||||
| Fats subscale | 1.43 (0.6) | 1.47 (0.6) | 1.16 (0.6) | 1.12 (0.5) | 1.11 (0.6) | 1.15 (0.5) | 0.50 (2,190) .606 |
| 0.72 (1,95) .399 | |||||||
| 0.81 (1,95) .371 | |||||||
| Fruits subscale | 1.91 (1.4) | 1.86 (1.1) | 1.92 (1.2) | 1.68 (1.0) | 1.86 (1.3) | 1.65 (0.9) | 0.33 (2,188) .716 |
| 0.53 (1,94) .470 | |||||||
| 0.03 (1,94) .875 | |||||||
| Vegetables subscale | 2.16 (1.0) | 2.11 (0.9) | 2.12 (1.0) | 2.41 (0.9) | 2.04 (1.0) | 2.13 (0.9) | 1.49 (2,190) .229 |
| 3.49 (1,95) .065 | |||||||
| 1.21 (1,95) .273 | |||||||
| IPAQ vigorous activity (MET min/wk) |
1706.05 (3222.0) | 1939.53 (4444.2) | 1887.44 (4090.0) | 2003.72 (3381.4) | 2081.86 (5343.2) | 2138.16 (4425.8) | 0.01 (1.9,155.7) .984 |
| 0.01 (1,84) .906 | |||||||
| 0.002 (1,84) .963 | |||||||
| IPAQ moderate activity (MET min/wk) |
1600.00 (3195.0) | 639.50 (1336.0) | 1135.79 (2217.0) | 1165.50 (1873.2) | 1271.58 (2077.0) | 1057.50 (2306.8) | 1.26 (1.8,136.1) .285 |
| 2.86 (1,76) .095 | |||||||
| 0.17 (1,76) .686 | |||||||
| IPAQ walking (MET min/wk) |
1105.93 (1860.6) | 762.67 (1400.2) | 1790.25 (2820.5) | 1669.71 (3108.4) | 1829.33 (3022.0) | 1390.13 (2200.0) | 0.09 (2,144) .915 |
| 0.08 (1,72) .778 | |||||||
| 0.15 (1,72) .697 | |||||||
| IPAQ total activity (MET min/wk) | 3877.17 (5284.2) | 4278.16 (7291.3) | 4406.01 (7090.4) | 4874.10 (6803.7) | 5027.09 (8791.5) | 4322.76 (7294.5) | 0.25 (2,190) .783 |
| 0.001 (1,95) .970 | |||||||
| 0.38 (1,95) .538 | |||||||
| A1C | 9.87 (1.6) 84 mmol/mol | 9.99 (1.6) 86 mmol/mol | 9.48 (1.9) 80 mmol/mol | 8.93 (1.8) 74 mmol/mol | 9.20 (2.0) 77 mmol/mol | 9.19 (2.1) 77 mmol/mol | 1.89 (2,186) .154 |
| 3.11 (1,93) .081 | |||||||
| 2.81 (1,93) 0.97 | |||||||
Abbreviations: IPAQ, International Physical Activity Questionnaire; MET, metabolic equivalent task.
For diet self-management, a significant difference was noted between the 2 groups at baseline, t(155) = 2.04, P = .043, with diet self-management activities greater for the control group. From baseline to T2, diet self-management activities increased for both groups, with the increase greater for the intervention group. From T2 to T3, a slight decrease was reported for the intervention group while there was little change for the control group. The intervention effect was sustained for 6 months.
An increase in exercise self-management activities was reported from baseline to T2 for both groups, with the increase greater for the intervention group. From T2 to T3, the intervention group decreased in exercise self-management activities while the control group continued to increase such that at T3, the control group scored higher than the intervention group. The intervention effect was not sustained for 6 months.
For total diabetes self-management, the intervention group increased from baseline to T2 while there was little change for the control group. From T2 to T3, there was an increase for both groups, with a slight increase for the intervention group. The intervention effect was sustained for 6 months.
Diabetes Self-efficacy for health behaviors increased for the intervention group and decreased for the control group between baseline and T2. The control group increased in diabetes self-efficacy for health behaviors from T2 to T3 while the intervention group decreased. The intervention effect was not sustained for 6 months post intervention.
There was an increase in diabetes self-efficacy for general health for both groups from baseline to T2 with the increase greater for the intervention group. Both groups increased from T2 to T3. The intervention effect was sustained for 6 months post intervention.
Total diabetes self-efficacy increased for both groups with the increase greater for the intervention group from baseline to T2. From T2 to T3, there was a slight decrease in total diabetes self-efficacy for the intervention group and an increase for the control group. The intervention effect was sustained for 6 months post intervention.
From baseline to T2, physician distress decreased for the intervention group while there was a slight increase for the control group. From T2 to T3, there was a decrease in physician distress for the control group and a slight increase for the intervention group. The intervention effect was sustained for 6 months.
For regimen distress, there was a significant difference between the two groups at baseline t(155) = 2.17, P = .032, with regimen distress greater for the intervention group. From baseline to T2, there was a decrease in regimen distress for the intervention group whereas the control group increased slightly. From T2 to T3, the intervention group increased slightly while the control group decreased slightly. The intervention effect was sustained for 6 months.
Interpersonal distress decreased for both groups with the decrease greater for the intervention group from baseline to T2. From T2 to T3, interpersonal distress decreased for the control group but slightly increased for the intervention group. The intervention effect was sustained for 6 months.
A significant difference in total diabetes distress was observed between the 2 groups at baseline, t(155) = 2.07, P = .040, with total diabetes distress greater for the intervention group. There was a decrease in total diabetes distress for both groups from baseline to T2 with the decrease greater for the intervention group. The control group continued to decrease in total distress from T2 to T3 while the intervention group increased in total distress. The intervention effect was not sustained for 6 months.
Hypothesis 2
Participants’ A1C did not significantly change over time (group by time interaction). For both groups, A1C decreased slightly from baseline to T2, with the decrease greater for the intervention group. The control group continued to decrease from T2 to T3 while the intervention group increased slightly.
Discussion
Study findings indicated that the diabetes self-management support intervention increased diabetes self-management for heathy eating and physical activity and decreased physician distress, regimen distress, interpersonal distress and total diabetes distress. stress. Results are consistent with prior research that has documented that DSME/Ss are effective in improving self-care activities to manage one’s diabetes regimen.8,12 While diabetes self-management for medications was not significant, participants scored high on this subscale, supporting prior research and reinforcing that medication adherence is easier than changing lifestyle behaviors. Prior research has also reported that improvements in diabetes self-management behaviors results in improved clinical outcomes, including a lower A1C.8 Although the differential change in A1C was not significant, a decrease from 9.99% (86 mmol/mol) to 8.93% (74 mmol/mol) was found immediately following the intervention, which is a clinically significant decrease associated with decreased mortality, myocardial infarction, and microvascular complications.37
A recent Cochrane review of 33 culturally appropriate health education interventions for T2DM in ethnic minorities that averaged about 8 months in length found improvements in glycemic control at 3, 6, and 12 months following the intervention.38 The greatest improvements were in the short term and with interventions lasting longer than 3 months.
Although diet and physical activity self-management significantly increased, no significant improvements in healthy eating or physical activity were reported. This finding has also been reported in low-income adults with T2DM.39 Participants were low income, had limited education, and had low dietary intake of fruits and vegetables as in our study. Dietary changes have consistently been reported to be the most difficult, especially in low-income persons with diabetes, as factors such as culture, lifelong habits, and family and socioeconomic resources influence dietary intake. A meta-analysis describing biobehavioral determinants of glycemic control40 found that dietary adherence was a significant predictor of glycemic control, with self-efficacy being the most consistent predictor of dietary adherence. Participants in the study reported numerous barriers to regular physical activity, including family responsibilities, irregular working hours, lack of areas in neighborhoods to walk, as well as lack of exercise facilities. These barriers have been reported in other research with T2DM.41 Diabetes distress, the emotional burden experienced by adults with T2DM, has been shown to influence glycemic control (A1C).42 Physician, regimen, and interpersonal distress significantly decreased following the intervention and were sustained for 6 months. Interesting, although both physician and interpersonal distress decreased, baseline levels were below 2.5. However, regimen distress was high at baseline, indicating that participants worried about managing their illness regimen and preventing complications. Interventions have consistently been shown to be effective in reducing diabetes distress.43 In the REDEEM Trial,44 the intervention was effective in reducing distress and increasing diabetes self-management skills, but the A1C was not reduced, indicating additional intervention strategies are necessary.
Participants’ diabetes self-efficacy for supporting healthy behaviors, general health, and total diabetes self-efficacy increased following the intervention. Participants increased confidence in general diabetes health behaviors, and this was sustained over time. Scale means indicated that levels of diabetes self-efficacy were moderate at baseline (3.58–3.71 on a 5-point scale), with the highest level of confidence for supporting diabetes management health behaviors. This finding raises the question of the level of confidence needed to influence diabetes self-management and adherence as significant changes in dietary and physical activity behaviors were not found, although our participants reported moderate to high level of confidence to manage their diabetes. A recent meta-analysis40 found that self-efficacy was the most consistent predictor of adherence behaviors. This finding is consistent with prior reviews of self-efficacy. While inclusion of this concept is essential in future research, further exploration of levels that predict successful management and adherence as well as additional factors that predict adherence are needed. Inclusion of this concept is critical in future research as interventions need to be designed that empower family members to have the confidence needed to support their family member with diabetes. In addition, since self-efficacy is a consistent predictor of dietary self-management, interventions that target both individual and family efficacy should be tested.
In conclusion, findings from our randomized intervention trial support and extend research using culturally appropriate diabetes self-management and support interventions for Mexican Americans. Longer interventions with intensive sessions tailored to specific needs and booster sessions are necessary to achieve and maintain glycemic control. Although such interventions are complex, time-intensive, and costly, they are necessary to improve diabetes self-management and decrease complications. Also, our low-income sample had limited education and acculturation, poor health literacy, few economic and community resources, and experienced ongoing family and financial crises. New strategies are needed to address these multiple challenges in diabetes self-management, including greater community participation.
Implications
Application of these findings to DSME/S for adults of Mexican origin has implications for the health care provider, other members of the health care team including the CDE, and the health care organization.13 To affect long-term positive outcomes, the health care team and CDE must actively and consistently collaborate with the family member(s) during clinical encounters. The health care organizational structure must demonstrate a commitment to quality, culturally tailored, family-based DSME/S as an integral component of diabetes care. Integration of community stakeholders such as promotoras who represent the local community and are knowledgeable of the cultural norms that influence diabetes self-management and T2DM self-management in the family context are vital to an effective program.
Acknowledgments:
The project described was supported by Grant R01MD005837 from the National Institute for Minority Health and Health Disparities. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute for Minority Health and Health Disparities or the National Institute of Health. The authors acknowledge Gwen Gallegos, FNP, RN, CDE, and Josefina Meranza, Promotora, for their expertise in culturally tailoring and delivering the family-based intervention.
Financial Support: Grant Support: R01MD005837, National Institute for Minority Health and Health Disparities.
References
- 1.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011;378(9785):31–40. [DOI] [PubMed] [Google Scholar]
- 2.Hu F Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 2011;34(6):1249–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States Atlanta, GA: United States Department of Health and Human Services, Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 4.National Alliance for Hispanic Health. The State of Diabetes Among Hispanics Washington, DC: National Alliance for Hispanic Health; 2010. [Google Scholar]
- 5.Dominguez K, Penman-Aguilar A, Chang MH, et al. Vital signs: leading causes of death, prevalence of diseases and risk factors, and use of health services among Hispanics in the United States—2009–2013. MMWR Morb Mortal Wkly Rep 2015;64(17): 469–478. [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Living With Diabetes: Complications Arlington, VA: American Diabetes Association; 2016. [Google Scholar]
- 7.American Diabetes Association. Diabetes Among Hispanics: All Are Not Equal Arlington, VA: American Diabetes Association; 2014. [Google Scholar]
- 8.Standards of medical care in diabetes—2016. Diabetes Care 2016;39(suppl 1):S11–S112. [DOI] [PubMed] [Google Scholar]
- 9.Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care 2013;36(8):2271–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agency for Healthcare Research and Quality. 2010 National Healthcare Disparities Report Rockville, MD: US Department of Health and Human Services; 2011. [Google Scholar]
- 11.Powers MA, Bardsley J, Cypress M, et al. Diabetes self-manage-ment education and support in type 2 diabetes: a joint position statement of the American Diabetes Association, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics. Diabetes Educ 2015;41(4):417–430. [DOI] [PubMed] [Google Scholar]
- 12.Boren SA, Gunlock TL, Schaefer J, Albright A. Reducing risks in diabetes self-management: a systematic review of the literature. Diabetes Educ 2007;33(6):1053–1077. [DOI] [PubMed] [Google Scholar]
- 13.Haas LB. National standards for diabetes self-management education and support. Diabetes Care 2013;36(1):S100–S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arizona Department of Health Services. Arizona diabetes burden report: 2011. http://azdhs.gov/documents/prevention/tobacco-chronic-disease/diabetes/reports-data/AZ-Diabetes-Burden-Report-2011.pdf. Accessed April 7, 2017.
- 15.Garcia-Solano B, Gallegos-Cabriales EC, Gomez-Meza MV, Garcia-Madrid G, Flores-Merlo M, Garcia-Solano M. Hierarchical clusters in families with type 2 diabetes. SAGE Open Med 2015;3:2050312115622957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosland AM, Heisler M, Choi HJ, Silveira MJ, Piette JD. Family influences on self-management among functionally independent adults with diabetes or heart failure: dDo family members hinder as much as they help? Chronic Illn 2010;6(1):22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fortmann AL, Gallo LC, Philis-Tsimikas A. Glycemic control among Latinos with type 2 diabetes: the role of social-environmental support resources. Health Psychol 2011;30(3):251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaccaro JA, Exebio JC, Zarini GG, Huffman FG. The role of family/friend social support in diabetes self-management for minorities with type 2 diabetes. World Journal of Nutrition and Health 2014;2(1):1–9. [Google Scholar]
- 19.Hu J, Amirehsani K, Wallace DC, Letvak S. Perceptions of barriers in managing diabetes: perspectives of Hispanic immigrant patients and family members. Diabetes Educ 2013;39(4):494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McEwen MM, Murdaugh C. Partnering with families to refine and expand a diabetes intervention for Mexican Americans. Diabetes Educ 2014;40(4):488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu J, Amirehsani KA, Wallace DC, McCoy TP, Silva Z. A family-based, culturally tailored diabetes intervention for Hispanics and their family members. Diabetes Educ 2016;42(3):299–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burns KK, Nicolucci A, Holt R, et al. Educational and Psychological Issues Diabetes Attitudes, Wishes and Needs Second Study (DAWN2TM): cross-national benchmarking indicators for family members living with people with diabetes. Diabet Med 2013;30(7):778–788. [DOI] [PubMed] [Google Scholar]
- 23.Pereira MG, Pedras S, Machado JC. Family variables as modera-tors between beliefs towards medicines and adherence to self-care behaviors and medication in type 2 diabetes. Fam Syst Health 2014;32(2):198–206. [DOI] [PubMed] [Google Scholar]
- 24.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care 2000;23(7):943–950. [DOI] [PubMed] [Google Scholar]
- 25.Trief PM, Fisher L, Sandberg J, et al. Health and psychosocial outcomes of a telephonic couples behavior change intervention in patients with poorly controlled type 2 diabetes: a randomized clinical trial. Diabetes Care 2016;39(12):2165–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baig AA, Benitez A, Quinn MT, Burnet DL. Family interventions to improve diabetes outcomes for adults. Ann N Y Acad Sci 2015;1353:89–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincent D, McEwen MM, Hepworth JT, Stump CS. Challenges and success of recruiting and retention for a culturally tailored diabetes prevention program for adults of Mexican descent. Diabetes Educ 2013;39(2):222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuellar I, Arnold B, Maldonado R. Acculturation rating scale for Mexican Americans-II: a revision of the original ARSMA scale. Hispanic Journal of Behavioral Sciences 1995;17(3):275–304. [Google Scholar]
- 29.Garcia AA, Villagomez ET, Brown SA, Kouzekanani K, Hanis CL. The Starr County Diabetes Education Study: development of the Spanish-language diabetes knowledge questionnaire. Diabetes Care 2001;24(1):16–21. [DOI] [PubMed] [Google Scholar]
- 30.Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med 2005;3(6):514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Heart, Lung and Blood Institute. Calculate your body mass index https://www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmicalc.htm. Accessed April 7, 2017.
- 32.Stanford Patient Education Research Center. Diabetes Self-Efficacy Scale http://patienteducation.stanford.edu/research/sediabetes.html. Accessed April 7, 2017.
- 33.Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care 2005;28(3):626–631. [DOI] [PubMed] [Google Scholar]
- 34.Block G, Gillespie C, Rosenbaum EH, Jenson C. A rapid food screener to assess fat and fruit and vegetable intake. Am J Prev Med 2000;18(4):284–288. [DOI] [PubMed] [Google Scholar]
- 35.Hagstromer M, Oja P, Sjostrom M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr 2006;9(6):755–762. [DOI] [PubMed] [Google Scholar]
- 36.Carter JS, Houston CA, Gilliland SS, et al. Rapid HbA1c testing in a community setting. Diabetes Care 1996;19(7):764–767. [DOI] [PubMed] [Google Scholar]
- 37.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Creamer J, Attridge M, Ramsden M, Cannings-John R, Hawthorne K. Culturally appropriate health education for type 2 diabetes in ethnic minority groups: an updated Cochrane review of randomized controlled trials. Diabet Med 2016;33(2):169–183. [DOI] [PubMed] [Google Scholar]
- 39.Compean Ortiz LG, Del Angel Perez B, Resendiz Gonzalez E, et al. Self-care behaviors and glycemic control in low-income adults in Mexico with type 2 diabetes mellitus may have implications for patients of Mexican heritage living in the United States. Clin Nurs Res 2016;25(2):120–138. [DOI] [PubMed] [Google Scholar]
- 40.Brown SA, Garcia AA, Brown A, et al. Biobehavioral determinants of glycemic control in type 2 diabetes: a systematic review and meta-analysis. Patient Educ Couns 2016;99(10):1558–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown SA, Hanis CL. Lessons learned from 20 years of diabetes self-management research with Mexican Americans in Starr County, Texas. Diabetes Educ 2014;40(4):476–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berry E, Lockhart S, Davies M, Lindsay JR, Dempster M. Diabetes distress: understanding the hidden struggles of living with diabetes and exploring intervention strategies. Postgrad Med J 2015;91(1075):278–283. [DOI] [PubMed] [Google Scholar]
- 43.Sturt J, Dennick K, Hessler D, Hunter BM, Oliver J, Fisher L. Effective interventions for reducing diabetes distress: systematic review and meta-analysis. International Diabetes Nursing 2015;12(2):40–55. [Google Scholar]
- 44.Fisher L, Hessler D, Glasgow RE, et al. REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care 2013;36(9):2551–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]


