Abstract
To explore the role of hScrib in the pathogenesis of endometriosis.
This was a retrospective study of 240 women in our hospital between January 2014 and January 2017. The expression of hScrib in endometrium (EM), endometriosis (EMs), and endometrial adenocarcinoma (EC) was investigated, and compared the differences among them. Serum levels, protein expressions, localizations, and correlations of hScrib and E-cadherin were determined.
The levels of serum soluble hScrib and E-cadherin were significantly highest in EC, followed by EMs, and healthy women (P < .05). hScrib protein content was opposite result in 3 tissues (P < .05), and was negatively correlated with r-AFS stage in EMs. The location changed from membrane to cytoplasm. Co-localization of hScrib with E-cadherin was found at extensive cell–cell boundaries in EMs.
hScrib and E-cadherin may be as new diagnostic markers of endometriosis. Low expression of hScrib leads to the loss of cell polarity and stability. Also, hScrib may induce EMT through regulating E-cadherin, might play an important role in pathogenesis of endometriosis.
Keywords: E-cadherin, endometrial adenocarcinoma, endometrium, hScrib, ovarian endometriosis
1. Introduction
Endometriosis (EMs) refers to the presence of functional endometrium (glands and mesenchymal cells) outside the uterine cavity.[1] The ectopic endometrium invades distant parts of the body and is most commonly seen in ovaries, uterosacral ligaments, and peritoneum. Although the pathological morphology appears benign, but it has the ability to plant, erode, and metastasize like malignant tumor,[2] and hence called “benign gynecological disease similar to cancer.” With the changes in environment and speed up of the work rhythm, the incidence of endometriosis is rising and trending in younger women. Due to its malignant behavior, the vast number of women had great physical and mental damage. Therefore, the pathogenesis of endometriosis grabbed the attention of many scholars, becoming the research hotspot in the field of gynecological diseases recently.
hScrib is a human homolog of Drosophila. As a transmembrane protein, it is localized at the broad basolateral membrane and extensive cell–cell boundaries in the epithelium.[3] hScrib has recently been identified in the establishment of apical-basal polarity and acts as an integrity determinant in the epithelia, and plays a critical role in enhancing cell–cell adhesion and inhibition of cell proliferation and migration.[4] Numerous studies have shown that hScrib was unusually reduced in many types of human malignancies,[5] such as cervical cancer, endometrial cancer, breast cancer, colon cancer, etc., and as a tumor suppressor is involved in the pathogenesis of several different types of malignant tumors.[6–10] We believe that hScrib might be involved in the pathogenesis of endometriosis as the ectopic endometrial cells have malignant potential.
Hence, the hScrib involvement in the progression of endometriosis and its specific role need to be addressed. Our study investigated the localization and expression of hScrib and E-cadherin, an EMT (epithelial–mesenchymal transition) protein in endometriosis, analyzed the relationship between the protein expression of hScrib and the clinical parameters of endometriosis. Also the expression of hScrib was compared among the disease conditions, such as endometrium, ovarian endometriosis and endometrial adenocarcinoma.
2. Materials and methods
2.1. Reagents and materials
ELISA Kit was purchased from R&D Systems (Minneapolis, MN). Goat antihuman hScrib and mouse antihuman E-cadherin antibodies were purchased from R&D Systems (Minneapolis, MN). Donkey antigoat and rabbit antimouse secondary antibodies (Labelled Polymer-HRP, Dako). Allexa 488 and Alexa-568 conjugated antibodies (Invitrogen, Eugene, OR).
2.2. Clinical data
This was a retrospective study of 240 women in our hospital between January 2014 and January 2017.We collected serum and tissues samples from 80 ovarian endometriosis patients, 80 endometrial adenocarcinoma patients and 80 women donor venous blood specimens. The stages of endometriosis were I–II–III–IV as classified by r-AFS stage in all the 20 cases. According to the FIGO classification of endometrial carcinoma,[11] 80 cases were classified as clinical stages I and II, with 40 cases each. These patients received no medication for at least 6 months before surgery. Patient's age ranged between 23 and 55. All the specimens were confirmed by postoperative pathology and had complete clinical information. Women donors live in Beijing for more than 10 years. They were all in good health through complete physical examination in the outpatient department of our hospital. The examinations of blood bio-chemistry laboratory and tumor markers were normal. Virus serology examination was negative. The x-ray and imaging examinations were normal. They had no past history of internal medical, surgical, gynecological, tumor, infectious, and mental diseases (including no endometriosis).
2.3. Collection of samples
The study protocol was approved by the institutional review board of Beijing Tongren Hospital of Capital Medical University. After obtaining the written informed consent, undergoing surgery, the cyst walls were obtained from ovarian endometriosis patients (n = 80), the malignant tumor tissue samples were got from uterine cavity of endometrial adenocarcinoma patients (n = 80), the venous blood samples were form all patients (n = 160) and women donors (n = 80). Because endometrium can only be obtained through surgery, and a highly trauma, it cannot be obtained from healthy female volunteers. So the endometria in our study were obtained by curettage from patients with ovarian endometriosis when surgery.
Tissue samples were collected under sterile conditions and transported to the laboratory by placing them on ice in PBS. The venous blood samples were first centrifuged for a speed of 4000 r/min at 4°C for 15 minutes to obtain serum. Then the supernatants were transferred into 1.5 mL microcentrifuge polypropylene tubes, stored at −80°C in a freezer prior to use. When analyzed, the serum samples were melted on ice, centrifuged at 12000 r/min at 4°C for 15 minutes to remove the impurities. Few tissue samples were fixed with 4% polyformaldehyde and made into paraffin tissue blocks for immunohistochemistry. The other tissue samples were embedded in OCT compound, snap frozen in liquid nitrogen, and stored at −80°C in a freezer until analysis.
2.4. ELISA analysis
The serum concentration of soluble hScrib and E-cadherin were measured using a specific ELISA kit (Genzyme/Techne, Minneapolis, MN) based on monoclonal antibodies. All samples were measured by an investigator who was blinded to the clinical details and coded data sheet. Each sample was measured twice. Serum was diluted according to the manufacturer's protocol. Preparation of microtiter plates including coating with diluted supernatant and standard solutions, washing and blotting were performed. Then the microtiter plates were incubated at 37°C for 60 minutes with 100 μL/well of hScrib and E-cadherin primary antibodies. The samples were washed, and then 100 μL HRP-labeled secondary antibodies were added to each well, incubated in a humidified box under 37°C for 30 minutes. After washing, 100 μL of substrate solution was added to each well and showed a dark color for 20 minutes. Then add 100 μL termination liquid to complete the experiment. The reaction resulted in color development with intensities proportional to the concentrations of hScrib and E-cadherin that were presented in the samples and standards. The color developed was measured with the micro-titre plate reader for measuring the absorbance at 450 nm. Accurate sample concentrations of hScrib and E-cadherin were determined by comparing the specific absorbances with those obtained from the standards plotted on a standard curve. Serum levels were expressed as mg/l. Values were standardized against the controls to generate relative fold change.
2.5. Immunohistochemistry
Paraffin-embedded tissue specimens were sliced at a 5 μm thickness. These slide sections were deparaffinized and rehydrated. Antigens were retrieved by buffer at 98°C. Endogenous peroxidase was blocked by incubation for 20 minutes with a solution of 1% hydrogen peroxidase. After blocking with nonspecific staining blocking reagent, the sections were incubated with goat antihuman hScrib antibody (Santa Cruz Biotechnology, Santa Cruz, CA) diluted at 1:1000 in PBS for 60 minutes at room temperature. After which, the samples were incubated with donkey antigoat secondary antibody (Labelled Polymer-HRP anti-Goat, Dako) for 30 minutes. Staining was performed using the vector novaRED substrate kit (funakoshi). All sections were counterstained with hematoxylin and evaluated under a light microscope. As a positive control, we stained MDCK cells.
Immuno-reactive expressions of hScrib were used to determine the percentage of positive cells and the intensity of staining. Score according to the intensity and range of the staining. Dyeing intensity: colorless (0 score), pale yellow (1 score), pale brown (2 score), brown (3 score). Dyeing range: < 5%(0 score), 5% ∼ 25%(1 score), 26%– 50% (2 score), >50% (3 score). The intensity and range of the staining accumulated 0–1 score are negative (–), 2–3 score are weak positive (+), 4–5 score are positive (++), and 6 score are strongly positive (+++).
2.6. Immunofluorescence
Endometriotic tissue samples were washed in PBS, embedded in OCT compound (Sakura, Tokyo, Japan), and snap frozen in liquid nitrogen. Cryo-sections were cut at 8 μm thickness and mounted on poly-L-lysine-treated slides. Sections were then fixed in acetone for 30 minutes on ice and washed in PBS for 5 minutes twice. After blocking with nonspecific staining blocking reagent, the sections were incubated with goat anti-hScrib antibody and mouse anti- E-cadherin diluted at 1:1000 in PBS for 60 minutes at room temperature and incubated with donkey antigoat and rabbit anti-mouse Allexa 488 and 568 conjugated antibodies (Invitrogen, Eugene, OR) for 30 minutes and avoided direct light. Expression of protein was investigated under confocal fluorescence microscopy.
2.7. Statistical analysis
Data were evaluated using SAS 9.4 using analysis of variance and Spearman correlation. P < .05 was accepted as statistically significant.
3. Results
3.1. Serum expressions of soluble hScrib and E-cadherin in the women donors, ovarian endometriotic patients and endometrial adenocarcinoma patients
As shown in Figure 1, the serum concentration of soluble hScrib among women donors ranged from 0.423 to 1.778 mg/L, with a mean value of 1.169 ± 0.356 mg/L. In endometriosis, the concentration was 2.924–5.561 mg/L, with an average of 4.562 ± 0.315 mg/L. The serum hScrib concentration of endometrial adenocarcinoma was 4.423 to 8.678 mg/L, with an average of 6.173 ± 0.325 mg/L. Variance among the 3 groups was statistically significant (P < .05).
Figure 1.
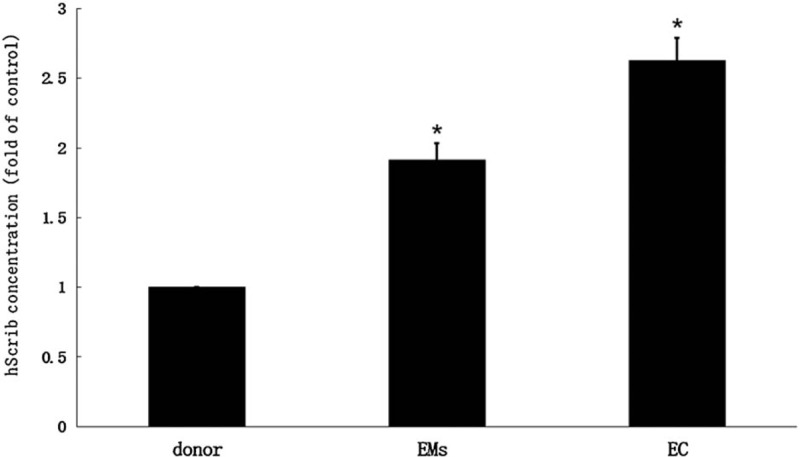
The serum concentration of soluble hScrib among women donors ranged from 0.423 to 1.778 mg/L, with a mean value of 1.169 ± 0.356 mg/L.
Figure 2 showed that the serum expressions of soluble E-cadherin in women donors ranged from 3.586 to 6.178 mg/L, with a mean value of 4.295 ± 0.531 mg/L, and in endometriosis was 6.429–9.316 mg/L, with an average of 7.534 ± 0.529 mg/L. The serum E-cadherin concentration in endometrial adenocarcinoma was 8.734–11.712 mg/L, with an average of 9.253 ± 0.568 mg/L. The variance among the 3 groups was statistically significant (P < .05). In addition, the mean value of serum concentration of soluble hScrib in stage IV endometriosis was 5.126 ± 0.371 mg/L, and in stage I endometrial carcinoma was 5.229 ± 0.393 mg/L., the results were similar.
Figure 2.
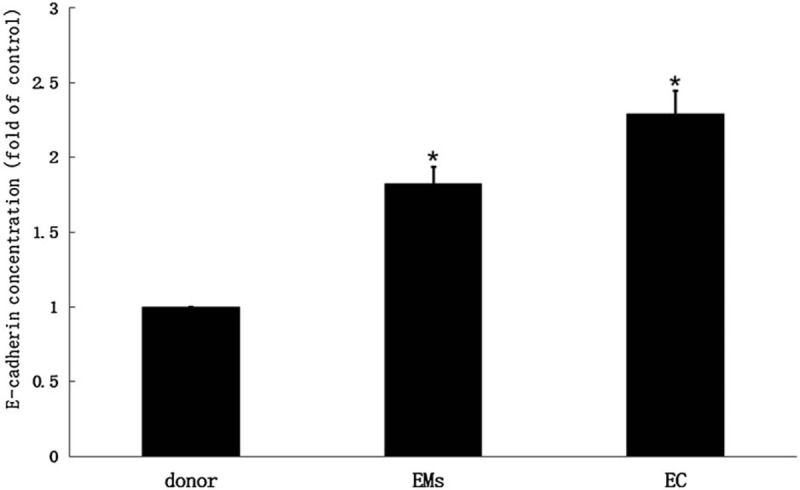
The serum expressions of soluble E-cadherin in women donors ranged from 3.586 to 6.178 mg/L, with a mean value of 4.295 ± 0.531 mg/L, and in endometriosis was 6.429–9.316 mg/L, with an average of 7.534 ± 0.529 mg/L.
These results indicated that both hScrib and E-cadherin showed an increasing trend in the serum concentration expressions in the healthy female volunteers, endometriosis, and endometrial adenocarcinoma. In addition, the serum concentrations of soluble hScrib and E-cadherin in endometriosis were positively correlated with Spearman analysis (r = 0.583).
3.2. Immuno-reactive expressions of hScrib in the endometrial tissues, emdometriotic tissues, and endometrial adenocarcinoma tissues
As shown in Table 1, hScrib positive rate was highest in the endometrial tissues, followed by ovarian endometriotic tissues, and with the lowest being in the endometrial adenocarcinoma tissues. Comparison of the differences among the 3 groups were statistically significant.
Table 1.
Expression of hScrib in tissues.

Figure 3 showed that the positive cells stained for hScrib were present at the cell–cell boundaries and at broad basolateral membrane of the glandular epithelium of the endometrium, and were strongly positive. Positive and strongly positive expressions of hScrib were frequently presented in the cases of early stages (I and II) endometriosis. Priority to the broad basolateral membrane position was given. Figure 4 showed that both in endometriosis of advanced stages (III and IV) and endometrial adenocarcinoma of early stages (I and II), the immune-reactive hScrib localization was changed from membrane to cytoplasm. hScrib expressions were often weakly positive, and the 2 expression intensities were similar. In addition, the expression of hScrib in endometriosis was negatively correlated with the stage of endometriosis as classified by r-AFS system using Spearman correlation analysis (r = −0.412).
Figure 3.
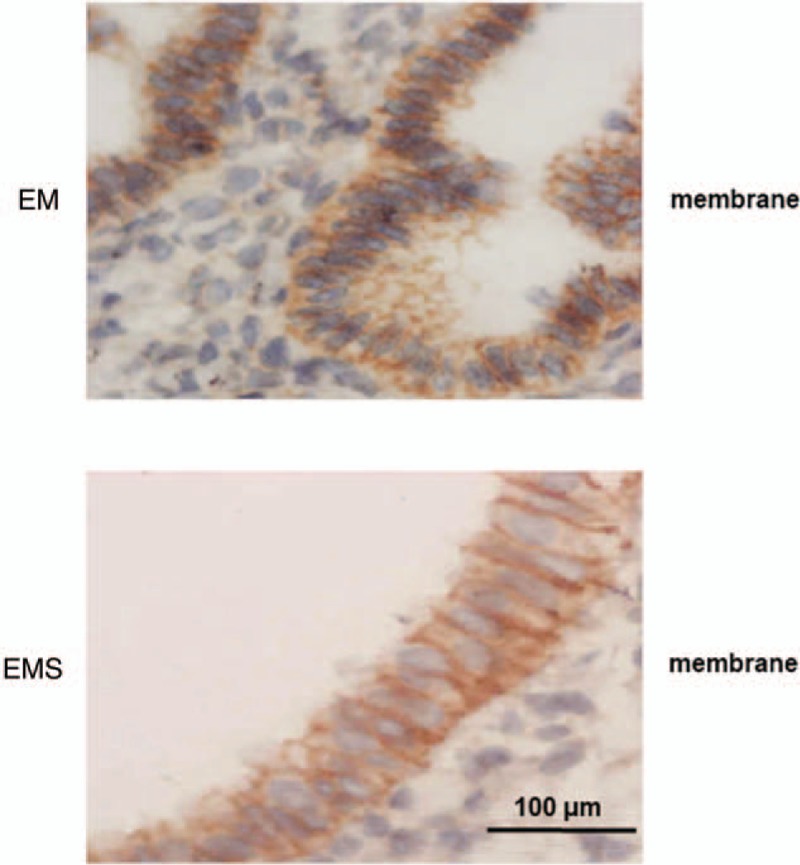
The positive cells stained for hScrib were present at the cell–cell boundaries and at broad basolateral membrane of the glandular epithelium of the endometrium, and were strongly positive.
Figure 4.
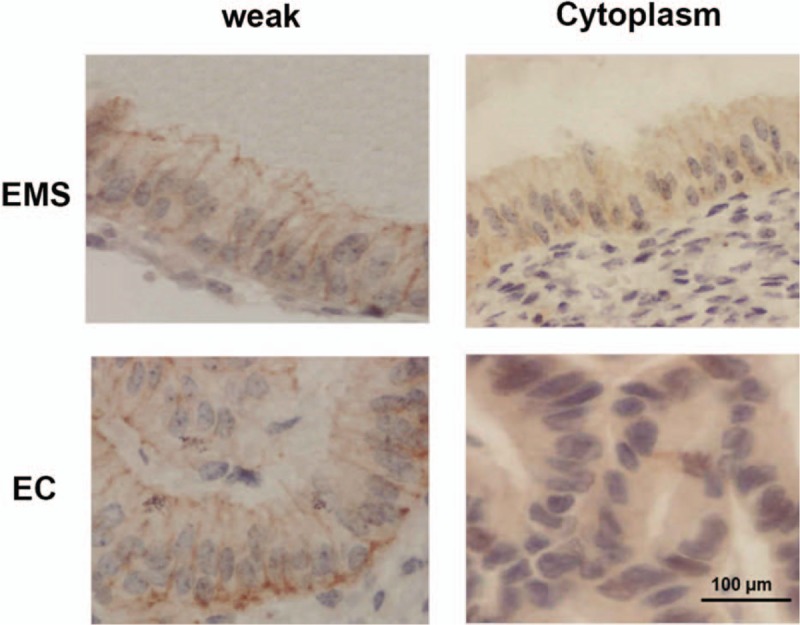
Both in endometriosis of advanced stages (III and IV) and endometrial adenocarcinoma of early stages (I and II), the immune-reactive hScrib localization was changed from membrane to cytoplasm.
3.3. Fluorescent staining of hScrib and E-cadherin in endometriosis
As shown in Figure 5, immunofluorescence confocal microscopy was provided further evidence for the co-localization of hScrib with E-cadherin. hScrib was labeled with green fluorescent dye and E-cadherin was labeled with red fluorescent dye. Fusion of the colors produced yellow color, which was used to quantify co-localization of hScrib and E-cadherin. Confocal microscopy revealed co-localization of hScrib with E-cadherin at the extensive cell–cell boundaries and broad basolateral membrane in the glandular epithelium of the ovarian endometriosis with stage I.
Figure 5.
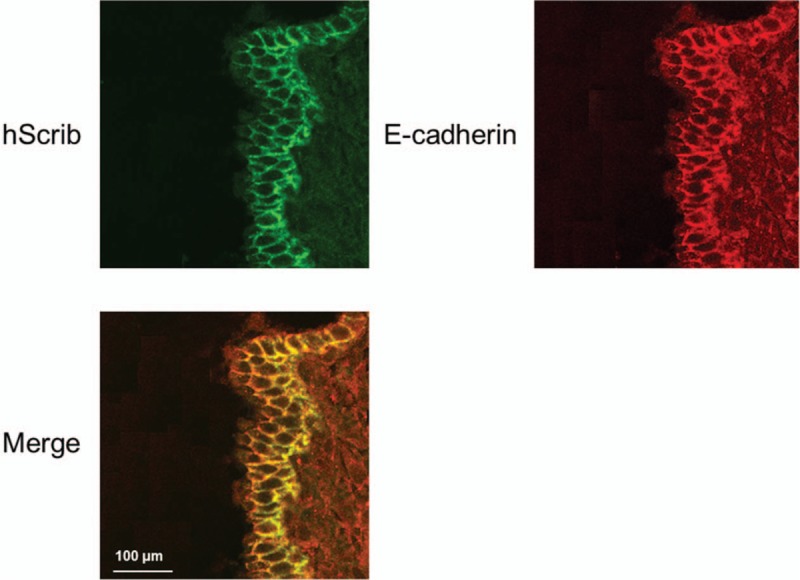
Immunofluorescence confocal microscopy was provided further evidence for the co-localization of hScrib with E-cadherin. hScrib was labeled with green fluorescent dye and E-cadherin was labeled with red fluorescent dye.
4. Discussion
In the present study, we first demonstrated that the expression of hScrib was almost present at the broad basolateral membrane of the glandular epithelium and at the cell–cell boundaries in normal endometrium and stages I–II ovarian endometriosis, but the expression was rarely observed in the plasma. With the progression of clinical stage, the expression of hScrib becomes weaker and weaker, and the hScrib localization was changed from membrane to cytoplasm in the advanced stages of III-IV ovarian endometriosis and early stages of I–II endometrial adenocarcinoma. Our data showed the evidence of involvement of hScrib in the maintenance of tissue architecture, integrity, and polarity of cell in endometriosis for the first time. By reducing the cell–cell adhesion and connection, producing ectopic endometrial cells were pulled away from the original lesions. This resulted in the spread and distant metastasis, promoting the development of endometriosis. In addition, we also found that the concentrations of hScrib in serum were similar, and the immuno-reactive hScrib localization and intensity were near to stage IV endometriosis and stage I endometrial adenocarcinoma. This also proved that the ectopic endometrial cells of r-AFS stage IV in morphology and biology were close to the endometrial glandular cancer cells of FIGO stage I, and the ectopic endometrial cells indeed has the potential for malignant transformation.
E-cadherin is a transmembrane protein, which plays a very important role in cell–cell adhesion and connection, and is essential for apical/basal polarization, morphological and organizational structure maintenance of the epithelial cells. E-cadherin was widely considered to be an important tumor suppressor, could be disrupted during the process of human malignancies.[12,13] Several reports suggested that the serum expression of soluble E-cadherin in many human epithelial malignant tumors was significantly higher than the healthy donors. The appearance of E-cadherin fragments in blood might be the cause of protein degradation in tumor cells. Hence, many scholars have suggested that serum soluble E-cadherin can act as one of the important markers in diagnosing epithelial malignancy.[14,15] Jedryka found that the serum concentration of soluble E-cadherin in patients with endometriosis was also significantly higher than that in healthy women,[16,17] and our study showed similar results. The reason may be that the ectopic endometrial cells also have the ability to promote proteolysis due to the molecular biological characteristics of malignant tumor cells. We then showed that the serum expressions of soluble hScrib and E-cadherin in endometriosis patients were both elevated, and their expressions were positively correlated. Therefore, we hypothesized that hScrib and E-cadherin might be applied in future clinical practice as 2 new markers for serological diagnosis of endometriosis. Our results provided a preliminary experimental basis and research direction. However, the cut-off value of the 2 diagnostic indexes and association with clinical stage in endometriosis still needs to be further elucidated.
The retrograde menstruation theory of Sampson is the most widely accepted theory to explain the pathogenesis of endometriosis. The pathogenesis of endometriosis involves adhesion, invasion, and angiogenesis processes.[18] However, since retrograde menstruation occurs in most of the reproductive aged women, it is clear that there must be other factors that contribute to the implantation of endometrial cells and their subsequent development into endometriotic disease. In the recent years, based on the systemic research data of endometriosis, Professor Lang Jinghe proposed a new theory for endometriosis, the “Eutopic endometrium determination theory.” The eutopic endometrium of women with endometriosis has some fundamental molecular differences compared to the eutopic endometrium of women without endometriosis. These differences determine the susceptibility to develop into endometriosis. There is substantial evidence to support that the alterations including number and activation of adhesion molecules contribute to the pathogenesis of endometriosis. E-cadherin, a typical adhesion molecule, was associated with abnormal expression in endometriosis.[19,20] E-cadherin may play a possible role in the migration and dissemination of ectopic endometrial cells.[19]
It is generally conceptualized that E-cadherin is a key factor in the EMT process. The changes of E-cadherin, N-cadherin, and Snail remain important characteristics of EMT process. EMT is defined as epithelial cells, having polarity, compact connection, and strong adhesive force transform into nonpolar, and liquid mesenchymal cells. EMT is characterized by lost polarity, cytoskeleton remodeling, weakened adhesion, and enhanced migration. The process of EMT was involved in many human malignant tumors.[21–25] It was also widely reported that EMT is a promoting factor that promotes the formation and metastasis of ectopic lesions in the pathogenesis of endometriosis.[26,27] The expressions of hScrib and E-cadherin in ectopic tissues were significantly reduced. E-cadherin downregulation indicated the occurrence of EMT, which also confirms the metastasis and invasion of endometriosis related to EMT. Our study demonstrated the co-localization of hScrib with E-cadherin at broad basolateral membrane of the glandular epithelium and at the extensive cell–cell boundaries in endometriosis. Our data showed that hScrib may participate in the process of endometriosis contributed to EMT by regulating the expression of E-cadherin.
The limitation of the present study is that the endometriotic tissues were obtained from ovarian endometriomas. It has been argued that ovarian endometrioma is a different entity from peritoneal endometriosis. Therefore, it remains possible that non-ovarian endometriotic cells may show different responses from the present results.
In summary, the present study demonstrated that the decreased expression and mis-localization of hScrib was associated with clinical stage of endometriosis. As a direct mediator for the maintenance of integrity, polarity of cell and cell–cell adhesion and contribute to EMT by regulating E-cadherin, we suggested that hScrib may be an important player in the development of endometriosis. In addition, we found that the serum concentrations of soluble hScrib and E-cadherin were significantly increased in endometriotic patients. Further studies are warranted to study regarding the 2 new markers in serological diagnosis of endometriosis. In future, we increase the sample size to further investigate the detailed gene and molecular biological effects of hScrib in the pathophysiology of endometriosis. Further studies using in vivo animal models would be warranted to elucidate a definitive role of hScrib in endometriosis.
Author contributions
OZ conceived and supervised the study; OZ designed experiments; SJ performed experiments; TX provided new tools and reagents; CM analysed data; OZ wrote the manuscript; ZJ made manuscript revisions. All authors reviewed the results and approved the final version of the manuscript.
Conceptualization: Zhuo Ouyang.
Data curation: Minxiu Chen, Jinping Sun.
Investigation: Jinping Sun.
Methodology: Zhuo Ouyang, Minxiu Chen, Jinping Sun.
Project administration: Zhuo Ouyang.
Resources: Jianjun Zhai.
Software: Minxiu Chen.
Supervision: Zhuo Ouyang.
Visualization: Jianjun Zhai.
Writing – original draft: Zhuo Ouyang, Jianjun Zhai.
Writing – review & editing: Zhuo Ouyang.
Footnotes
Abbreviations: EC = endometrial adenocarcinoma, EM = endometrium, Ems = endometriosis, EMT = epithelial–mesenchymal transition.
Consent for publication: Not applicable.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.(Ethical approval number:TRECKY-231).
Funding: This study was supported by National Natural Science Fund for the year 2011 (No. 8100409).
The authors have no conflicts of interest to disclose.
References
- [1].Matalliotakis M, Zervou MI, Matalliotaki C, et al. The role of gene polymorphisms in endometriosis. Mol Med Rep 2017;16:5881–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Taniguchi F. New knowledge and insights about the malignant transformation of endometriosis. J Obstet Gynaecol Res 2017;43:1093–100. [DOI] [PubMed] [Google Scholar]
- [3].Khursheed M, Bashyam MD. Apico-basal polarity complex and cancer. J Biosci 2014;39:145–55. [DOI] [PubMed] [Google Scholar]
- [4].Martin-Belmonte F, Perez-Moreno M. Epithelial cell polarity, stem cells and cancer. Nat Rev Cancer 2011;12:23–38. [DOI] [PubMed] [Google Scholar]
- [5].Enomoto M, Igaki T. Deciphering tumor-suppressor signaling in flies: genetic link between Scribble/Dlg/Lgl and the Hippo pathways. J Genet Genomics 2011;38:461–70. [DOI] [PubMed] [Google Scholar]
- [6].Nakagawa S, Yano T, Nakagawa K, et al. Analysis of the expression and localisation of a LAP protein, human scribble, in the normal and neoplastic epithelium of uterine cervix. Br J Cancer 2004;90:194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Smith A, Teknos TN, Pan Q. Epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Oral Oncol 2013;49:287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Navarro C, Nola S, Audebert S, et al. Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene 2005;24:4330–9. [DOI] [PubMed] [Google Scholar]
- [9].Wallesch M, Pachow D, Blucher C, et al. Altered expression of E-cadherin-related transcription factors indicates partial epithelial-mesenchymal transition in aggressive meningiomas. J Neurol Sci 2017;380:112–21. [DOI] [PubMed] [Google Scholar]
- [10].Gardiol D, Zacchi A, Petrera F, et al. Human discs large and scrib are localized at the same regions in colon mucosa and changes in their expression patterns are correlated with loss of tissue architecture during malignant progression. Int J Cancer 2006;119:1285–90. [DOI] [PubMed] [Google Scholar]
- [11].Denschlag D, Ulrich U, Emons G. The diagnosis and treatment of endometrial cancer: progress and controversies. Dtsch Arztebl Int 2010;108:571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Coopman P, Djiane A. Adherens Junction and E-Cadherin complex regulation by epithelial polarity. Cell Mol Life Sci 2016;73:3535–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hu QP, Kuang JY, Yang QK, et al. Beyond a tumor suppressor: soluble E-cadherin promotes the progression of cancer. Int J Cancer 2016;138:2804–12. [DOI] [PubMed] [Google Scholar]
- [14].Tsalikidis C, Papachristou F, Pitiakoudis M, et al. Soluble E-cadherin as a diagnostic and prognostic marker in gastric carcinoma. Folia Med (Plovdiv) 2013;55:26–32. [DOI] [PubMed] [Google Scholar]
- [15].Okugawa Y, Toiyama Y, Inoue Y, et al. Clinical significance of serum soluble E-cadherin in colorectal carcinoma. J Surg Res 2012;175:e67–73. [DOI] [PubMed] [Google Scholar]
- [16].Jedryka M, Goluda M, Kuliczkowski K, et al. E-cadherin in the serum and the peritoneal fluid of women with endometriosis. Ginekol Pol 2001;72:418–21. [PubMed] [Google Scholar]
- [17].Fu C, Lang J. Serum soluble E-cadherin level in patients with endometriosis. Chin Med Sci J 2002;17:121–3. [PubMed] [Google Scholar]
- [18].Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol 1927;3:93. [PMC free article] [PubMed] [Google Scholar]
- [19].Shaco-Levy R, Sharabi S, Benharroch D, et al. Matrix metalloproteinases 2 and 9, E-cadherin, and beta-catenin expression in endometriosis, low-grade endometrial carcinoma and non-neoplastic eutopic endometrium. Eur J Obstet Gynecol Reprod Biol 2008;139:226–32. [DOI] [PubMed] [Google Scholar]
- [20].Scotti S, Regidor PA, Schindler AE, et al. Reduced proliferation and cell adhesion in endometriosis. Mol Hum Reprod 2000;6:610–7. [DOI] [PubMed] [Google Scholar]
- [21].Prieto-Garcia E, Diaz-Garcia CV, Garcia-Ruiz I, et al. Epithelial-to-mesenchymal transition in tumor progression. Med Oncol 2017;34:122. [DOI] [PubMed] [Google Scholar]
- [22].Zhou XM, Zhang H, Han X. Role of epithelial to mesenchymal transition proteins in gynecological cancers: pathological and therapeutic perspectives. Tumour Biol 2014;35:9523–30. [DOI] [PubMed] [Google Scholar]
- [23].Liu N, Peng SM, Zhan GX, et al. Human chorionic gonadotropin beta regulates epithelial–mesenchymal transition and metastasis in human ovarian cancer. Oncol Rep 2017;38:1464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mirantes C, Espinosa I, Ferrer I, et al. Epithelial-to-mesenchymal transition and stem cells in endometrial cancer. Hum Pathol 2013;44:1973–81. [DOI] [PubMed] [Google Scholar]
- [25].Jiang C, Xu R, Li XX, et al. p53R2 overexpression in cervical cancer promotes AKT signaling and EMT, and is correlated with tumor progression, metastasis and poor prognosis. Cell Cycle 2017;16:1673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yang YM, Yang WX. Epithelial-to-mesenchymal transition in the development of endometriosis. Oncotarget 2017;8:41679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bilyk O, Coatham M, Jewer M, et al. Epithelial-to-mesenchymal transition in the female reproductive tract: from normal functioning to disease pathology. Front Oncol 2017;7:145. [DOI] [PMC free article] [PubMed] [Google Scholar]


