Supplemental Digital Content is available in the text
Keywords: cardiovascular events, hypercholesterolemia, low-density lipoprotein, meta-analysis, PCSK9 inhibitors
Abstract
Background:
The comparative efficacy and safety of PCSK9 inhibitors, statins, and ezetimibe to lower lipid levels in patients with hypercholesterolemia remain unknown. We aimed to investigate the benefits and harms of the lipid-lowering agents in these patients.
Methods:
PubMed, Embase, and the Cochrane Library were searched from January 1, 2000 to June 1, 2018 for relevant randomized controlled trials (RCTs). Frequentist network meta-analysis was used to pool all estimates. Ranking probabilities were used to rank the comparative effects of all drugs against placebo.
Results:
Eighty-four RCTs enrolled 246,706 patients were included. Most of the included were assessed as low risk of bias. The probabilities of PCSK9 inhibitors that ranked first in improving lipid outcomes were all 100%. The probability of statins that ranked first in reducing the risk of cardiovascular (CV) events was 60.6%, and the probability of PCSK9 inhibitor was 37.1%, while no significant difference of efficacy in reducing CV events was observed between the 2 agents (odds ratios [OR] 0.98, 95% CI 0.87–1.11). Statin ranked first in reducing all-cause and CV death. Compared with placebo, statins were associated with reduced risks of all-cause (OR 0.90, 95% CI 0.85–0.96) and CV death (OR 0.83, 95% CI 0.75–0.91) while PCSK9 inhibitors and ezetimibe were not. No agents caused adverse events (including neurocognitive events), except that statins therapy significantly increases the levels of alanine aminotransferase (ALT) (OR 1.89, 95% CI 1.42–2.51) and creatine kinase (CK) (OR 1.45, 95% CI 1.09–1.93) and the incidence of diabetes (OR 1.13, 95% CI 1.02–1.26).
Conclusions:
PCSK9 inhibitors were the most effective lipid-lowering agents in improving lipid levels. Furthermore, PCSK9 inhibitors achieved similar CV benefits like statins, while PCSK9 inhibitors were not associated with any increased risk of statin-related side-effects. Thus, PCSK9 inhibitors may also be recommended as promisingly first-line lipid-lowering treatment for patients with hypercholesterolemia, especially for these with statins intolerance or resistance.
1. Introduction
During the past 2 decades, statins have been treated as the principal pharmacological agents for reducing low-density lipoprotein cholesterol (LDL-C) and improving cardiovascular (CV) outcomes both in primary and secondary prevention of adults with hypercholesterolemia.[1,2] Nevertheless, a part of patients with hypercholesterolemia still fail to achieve the LDL-C target recommended by current guidelines, and some discontinue therapy due to statin-related side effects.[3,4] The problems are persistent even when treated with combination of statins and other lipid-modifying agents,[5] which highlights the requirement of a more effective and safe lipid-lowering therapy.
Several lipid-lowering agents, such as torcetrapib,[6] fenofibrate,[7] and niacin,[8] had been developed to fulfill lipid management need, but none demonstrated a benefit in terms of CV outcomes, despite they showing salutary effects in improving lipid levels. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, which are human monoclonal antibodies that bind human PCSK9 with high affinity to reduce LDL-C concentrations by decreasing the degradation of the LDL receptor available to recycle to the hepatocyte cell surface,[9] are novel agents for LDL-C reduction, more importantly with demonstrated effects of improving CV outcomes.[10]
Two types of PCSK9 inhibitors, alirocumab, and evolocumab, had been approved by the Food and Drug Administration for marketing, and both substantially reduced LDL-C level by approximately 50% to 60%,[11–14] with an apparently more effective lipid-lowering profile than statins and ezetimibe. Consequently, the pharmacology of lipid-lowering agents is becoming complex with the introduction of PCSK9 inhibitors, but whether the more intensive lipid-lowering therapies with PCSK9 inhibitors produce greater CV benefit than that of statins, and like statins, increase the levels of creatine kinase (CK) and alanine aminotransferase (ALT) and the incidence of new-onset diabetes is largely unknown, mainly due to a lack of head-to-head trials in current clinical setting. Understanding these questions is expected to be of great value for guiding the clinical management of dyslipidaemia. Although relevant meta-analyses[15–17] have been published or is being processed (CRD42017067529), these studies either omitted to focus on the efficacy and safety of PCSK9 inhibitors relative to placebo or missed some important large-scale randomized controlled trials (RCTs),[2,10,18–20] thus were unable or lack the power to reveal the comparative benefits and harms between PCSK9 inhibitors and statins. We, therefore, conducted an update network meta-analysis by summarizing up both direct and indirect evidence to evaluate and rank the comparative efficacy and safety among PCSK9 inhibitors, statins, and ezetimibe in patients with hypercholesterolemia.
2. Methods
We conducted and reported this systematic review with network meta-analysis in accordance with the extension of the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) statement. (Table S1)[21]
1.1. Data sources and searches
PubMed, Embase, and the Cochrane Library Central Register of Controlled Trials were searched for relevant RCTs between January 1, 2000 and June 1, 2018. Background medication used in the trials published before 2000 was different from that in the trials published after 2000. The different background medication is an important confounding factor that may cause potential bias for network comparisons. Therefore, we excluded the trials published before 2000 and searched evidence from 2000. Manual search was performed by searching the bibliographies of relevant meta-analyses, reviews, and the major CV conferences (European Society of Cardiology Congress and American College of Cardiology Congress) held in the past two years (the detail sources were listed in supplementary materials). The following key words were used: “hydroxymethylglutaryl-CoA reductase inhibitors,” “statins,” “ezetimibe,” “pcsk9,” “AMG 145,” “alirocumab,” “hypercholesterolemia,” and “randomized controlled trial.” The detailed retrieval strategies for the 3 databases are shown in the Table S2.
1.2. Study selection and outcomes
The RCTs from phase 2 or higher that evaluating statins, ezetimibe, and PCSK9 inhibitors monotherapy and comparing them against each other or placebo in adults with hypercholesterolemia were included. Eligible trials should report at least one of the outcomes listed below. Trials with follow-up shorter than 6 weeks or performed on children or adolescents were excluded. No restrictions on language or sample size were applied. Lipid data were percentage change from baseline in LDL-C, high-density lipoprotein (HDL) cholesterol, and total cholesterol (TC) level. Efficacy endpoints were CV events, all-cause mortality, and CV mortality. Safety outcomes were serious adverse events, neurocognitive event, new-onset diabetes, and elevation of serum CK (3 to 10 folds increase) and ALT level (3 to 10 folds increase).
1.3. Data extraction and quality assessment
Two authors of us independently selected studies and extracted data; any discrepancies were resolved after discussing with a third reviewer. Basic characteristics and outcome data of eligible trials were extracted into an Excel (Microsoft Corporation) table. Trials were defined to have baseline characteristic of background statins therapy if statins had been prescribed for patients before enrollment or been prescribed for patients during study for both intervention and control group in equal doses. When more than 1 publication originated from the same registration study, the report with the most complete data would be extracted; if discrepancies remained, then we selected the 1 with the longest follow-up. We extracted the doses of PCSK9 inhibitors that had been tested in phase 3 RCTs. We considered the patients allocated to the intervention or control group if background lipid-lowering agents were administrated to patients in both the intervention and control group of the trial in equivalent doses. For example, if statins were administered to participants in addition to ezetimibe in both the intervention and the control arm of the trial in equivalent dose, we will consider participants as allocated to ezetimibe and control. The recommended tool for assessing the risk of bias in the Cochrane Collaboration guidelines was utilized to assess the quality of the eligible studies.[22]
1.4. Statistical analysis
We conducted a pairwise meta-analysis and a network meta-analysis successively. First, we did a standard pairwise meta-analysis using a random-effects model. We estimated the relative therapeutic effects of pairwise comparisons with odds ratios (OR) for dichotomous outcomes (CV events, all-cause mortality, and CV mortality, serious adverse events, neurocognitive event, new-onset diabetes, and elevation of serum CK and ALT) and standardized mean differences (SMD) for continuous outcomes (LDL-C, HDL, and TC) with 95% confidence interval (CI). We used the percentage change from baseline to calculate the change of lipid levels before and after treatment. Heterogeneity in these analyses was assessed by I2 test and tau2 (τ2) value. Second, we conducted frequentist network meta-analysis to compare different agents.[23,24] Random-effects model was used to pool estimates for both pairwise and network meta-analysis. To obtain the treatment hierarchy for each outcome, we used the surface under the cumulative ranking probabilities, rankograms, mean ranks to estimate the relative ranking probability of each lipid-lowering agent.[25,26]
In this network meta-analysis, we assumed a common heterogeneity variable across different comparisons (τ2 value). τ2 represents the absolute extent of variability beyond sampling error. For all outcomes, the extent of heterogeneity was investigated by comparing magnitude of heterogeneity variance for the network with the quartiles of empirical distributions.[27] The extent of heterogeneity was judged as low heterogeneity if the τ2 was disturbed below 25% of that outcome or comparison, as moderate heterogeneity if the τ2 between 25% and 75%, and as high heterogeneity if the τ2 above 75%. Inconsistency was assessed using 3 methods. First, we used the loop-specific approach to assess the difference between direct and indirect estimates of specific treatment comparison (inconsistency factor) in each closed loop.[28] The loops were identified as inconsistent if the 95% CI did not include zero. Second, we used the node-splitting method, which divided evidence of a specific comparison into direct and indirect and estimated the indirect therapeutic effect of the specific comparison after splitting the direct 1.[29] Third, we utilized the design-by-treatment interaction model of Higgins and colleagues to check the consistency of the entire network.[30] Network meta-regression was used to further evaluate the possible existence of small-study effects in network of interventions.[31]
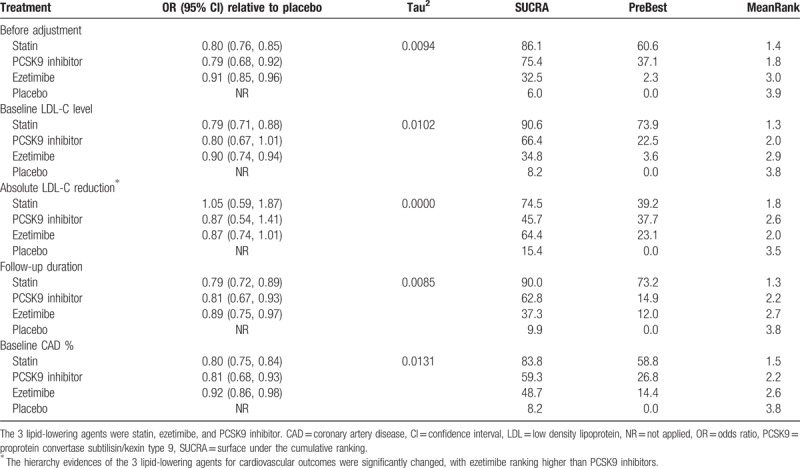
We performed meta-regression analysis in order to adjust the effects of covariates (confounding factors: the baseline LDL-C levels, the absolute LDL-C reduction, follow-up duration. Sensitivity analyses were performed by limiting trials to have following characteristics: with background statin therapy, without background statin therapy, adjusted follow-up, follow-up longer than 1 year, secondary prevention, reports published after 2008, and baseline LDL-C level lower than 130 mg/L.
All procedures were performed and analyzed using Stata version 13 (Stata Corp, College Station, TX) with the mvmeta commands[32–34] and the previously described self-programmed Stata routines.[25]
2. Results
In this study, 4863 citations (2026 in Embase, 1449 in PubMed, 1336 in the Cochrane Central Register of randomized trials, and 52 in manual search) met our inclusion and exclusion criteria. After removal of duplicates, 2418 citations remained. Of these, 2072 citations were excluded based on the title or abstract. Three hundred forty-six citations were retrieved for full-text screening; among them, 262 citations were excluded for various reasons (not RCT, wrong population, wrong intervention, with no predefined outcome, ongoing trials without published results, repeated publication). Finally, 84 studies were eligible for the criteria and all of them were included in meta-analysis. The full selection process is shown in Figure 1. Generally, all eligible studies were generally judged as low risk of bias as assessed by tool for assessing the risk of bias in the Cochrane Collaboration guidelines (Fig S1).
Figure 1.
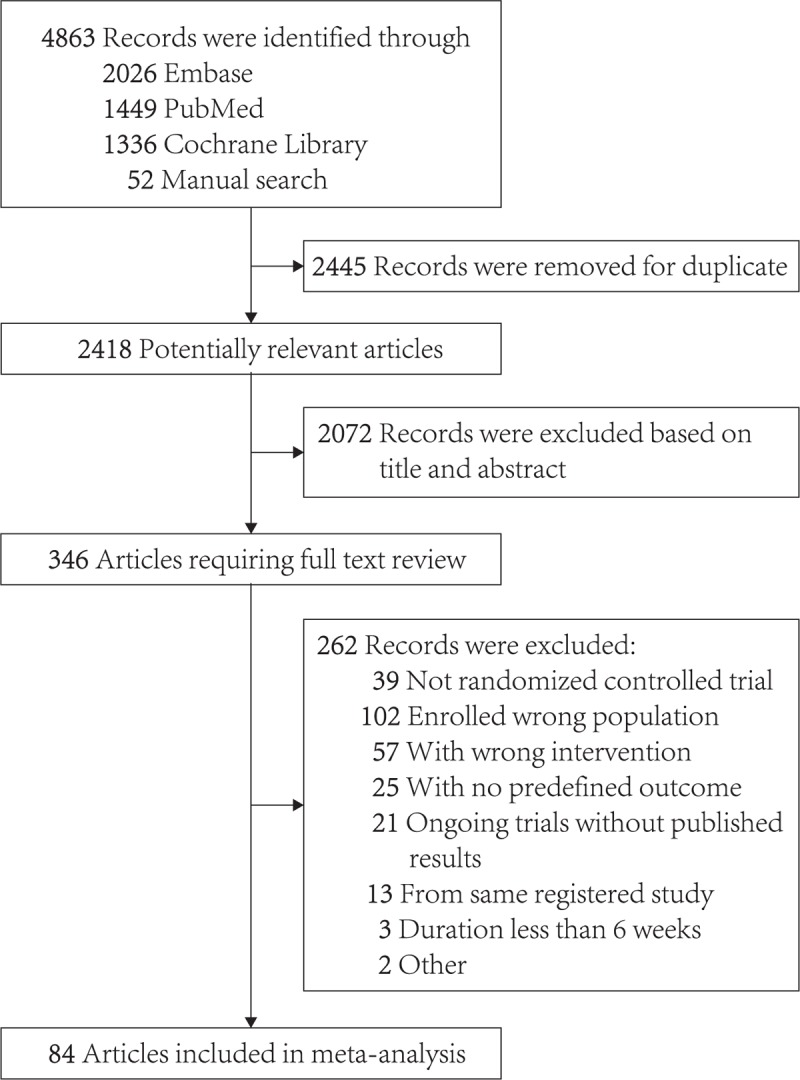
The process of study selection.
2.1. Basic characteristics of included trials
The baseline characteristics of the included studies are summarized in Table S3. Eighty-four RCTs enrolled 246,706 patients were included. Statin-related trials had the largest number (33 trials with 152,037 patients) compared with those of ezetimibe (22 trials with 27,567 patients), and PCSK9 inhibitors (29 trials with 67,102 patients). All the included studies were conducted on multicenter except that 4 statin-related and 3 ezetimibe-related studies were conducted on single center. The median duration of statin-related trials was 3.2 years, longer than those of ezetimibe (0.3 years) and PCSK9 inhibitor (0.5 years). Statins and ezetimibe group were both the most common intervention groups, followed by PCSK9 inhibitors group. The mean ages (61.3, 60.1, and 60.0 years), baseline LDL-C (141.7, 156.2, and 144.0 mg/dL), baseline HDL cholesterol (47.3, 48.9, and 49.0 mg/dL), and baseline triglyceride levels (163.3, 168.5, and 156.5 mg/dL) of participants, and the percentage of participants with history of hypertension (54.5%, 43.6%, and 53.1%), diabetes mellitus (27.9%, 17.7%, and 22.9%), and coronary artery disease (62.5%, 48.1%, and 50.1%) across statins, ezetimibe, and PCSK9 inhibitors-related trials were all approximately the same.
2.2. Lipid levels
Seventy-one trials involving 210,068 participants provided the outcome data for LDL-C, 60 with 204,432 participants for HDL cholesterol, and 60 with 204,432 participants for TC. The network estimates for LDL-C, HDL cholesterol, and TC are respectively presented in Figure 2. Compared with placebo, PCSK9 inhibitor ranked first (probabilities of ranking first for lipid levels were all 100%) for improving LDL-C (SMD −50.76, 95% CI −58.26 to −43.26), HDL cholesterol (7.73, 6.11–8.63), and TC levels (−35.81, −39.53 to −32.09), followed by statin that ranked second for improving LDL-C (−34.03, −44.21 to −23.84), HDL cholesterol (4.17, 2.78–5.57), and TC levels (-24.75, -28.66–-20.85) and ezetimibe that ranked last for improving LDL-C (−18.70, −25.80 to −11.59), HDL cholesterol (2.43, 1.32–3.53), and TC levels (−13.75, −16.73 to −10.78). (Fig S2A–S2C)
Figure 2.
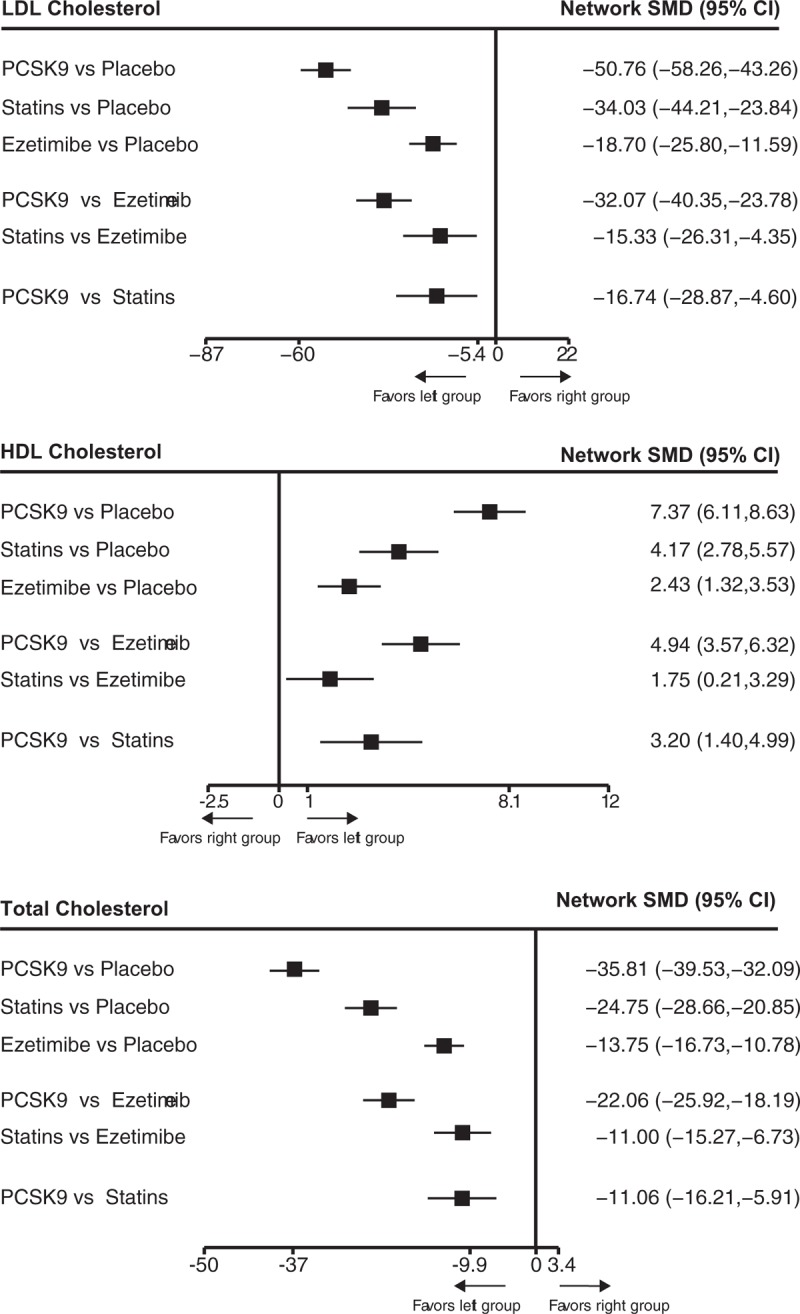
The relative efficacies of PCSK9 inhibitors, statins, ezetimibe, and placebo for improving LDL cholesterol, HDL cholesterol, and total cholesterol level in patients with hypercholesterolemia. Data are presented with standard mean difference and 95% confidence intervals. HDL = high-density lipoprotein, LDL = low-density lipoprotein, PCSK9 = proprotein convertase subtilisin-kexin type 9 serine protease.
2.3. CV events
Fifty-eight trials with 226,368 participants for CV events were included in the analysis of CV events. The network map of eligible comparisons for CV events is presented in Fig S3, which has no direct comparisons between statin and PCSK9 inhibitor. The detailed analyses of pairwise comparisons for CV events are listed in Table 1. The network estimates of CV events among the 3 lipid-lowering agents and placebo are presented in Figure 3. No firm ranking results could be made based on rankograms, but generally statin ranked first (OR 0.80, 95% CI 0.76–0.85; probability of ranking first = 60.6%), PCSK9 inhibitor second (0.82, 0.74–0.92; probability of ranking first = 37.1%), and ezetimibe (0.88, 0.76–1.01; probability of ranking first = 2.3%) last for decreasing CV events as compared with placebo. (Fig S4) Statins (0.98, 0.87–1.11) were equal to PCSK9 inhibitors for decreasing CV events.
Table 1.
Pairwise and network estimates of the 3 lipid-lowering agents on major clinical outcomes.
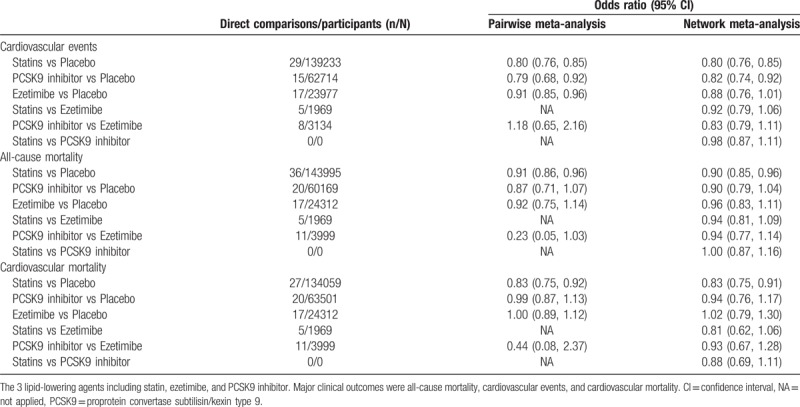
Figure 3.
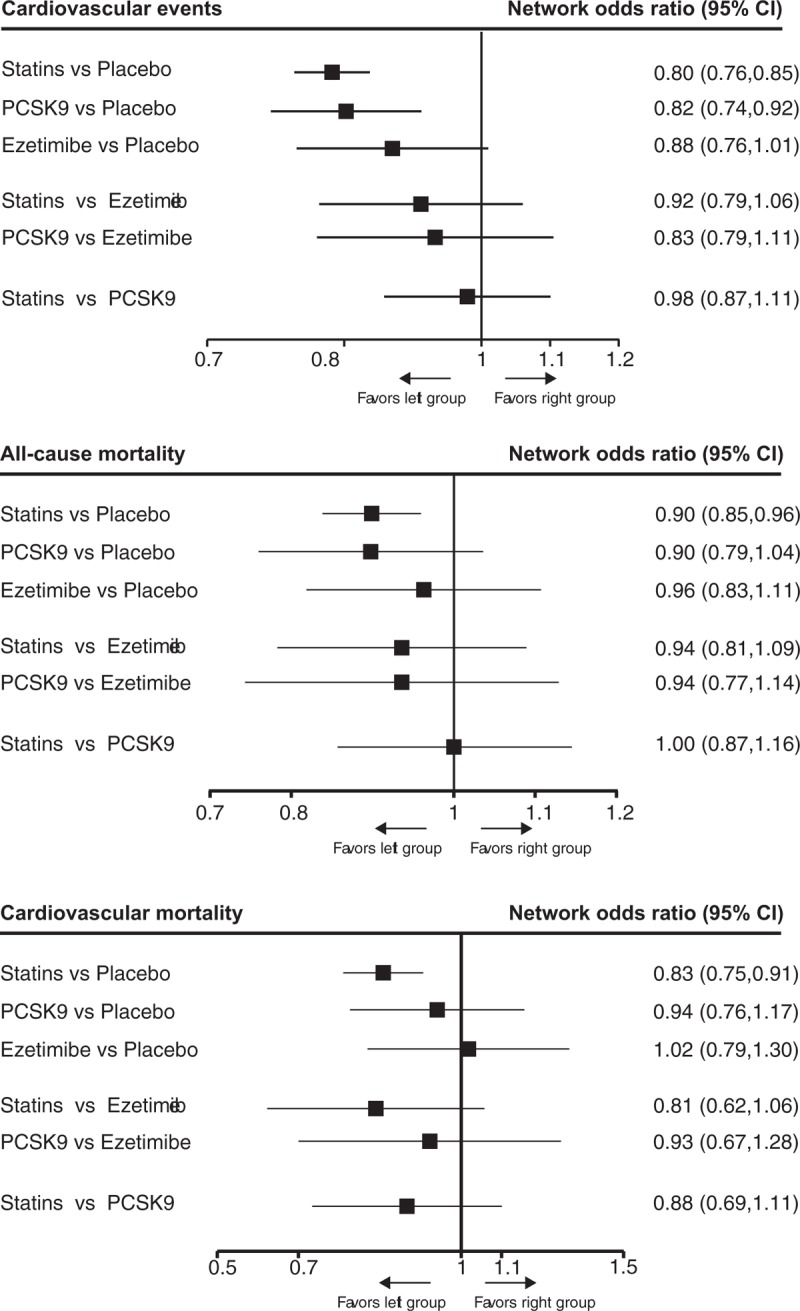
The relative benefits of statins, PCSK9 inhibitors, ezetimibe, and placebo against cardiovascular events, all-cause mortality and cardiovascular mortality in patients with hypercholesterolemia. Data are presented with odds ratios and 95% confidence intervals. PCSK9 = proprotein convertase subtilisin-kexin type 9 serine protease.
2.4. All-cause and CV mortality
Seventy-two trials involving 228,992 participants provided the outcome data for all-cause mortality, and 61 with 223,356 participants for CV mortality. The network map of all-cause and CV mortality was similar with CV events. The detailed pairwise comparisons for all-cause and CV mortality are provided in Table 1. The network estimates for all-cause and CV mortality are respectively presented in Fig. 3. Statins (0.90, 0.85–0.96) ranked first, PCSK9 inhibitors (0.90, 0.79–1.04) second and ezetimibe (0.96, 0.83–1.11) last for improving all-cause mortality compared with placebo. (Fig S5A) A similar finding was also observed in analysis of CV mortality. (Fig S5B) However, statins were not superior to PCSK9 inhibitors for improving all-cause mortality (1.00, 0.87–1.16) and CV mortality (0.88, 0.69–1.11).
2.5. Serious adverse events and neurocognitive events
Clinical data for the 3 agents were lacking in analysis of neurocognitive adverse event, especially for ezetimibe. The network estimates of safety outcomes were provided in Figure 4. Although ezetimibe ranked first in reducing serious adverse events (Fig S6A), none of the PCSK9 inhibitors (0.98, 0.94–1.03), statins (0.99, 0.94–1.03), or ezetimibe (0.87, 0.67–1.13) were associated with significant increase of serious adverse events as compared with placebo. However, compared with placebo, ezetimibe (3.94, 1.18–13.12) was associated with increased rate of neurocognitive adverse events while PCSK9 inhibitors (1.26, 0.80–2.00) and statin were not (0.97, 0.51–1.86). Statins ranked first in reducing neurocognitive adverse events compared with other interventions. (Fig S6B)
Figure 4.
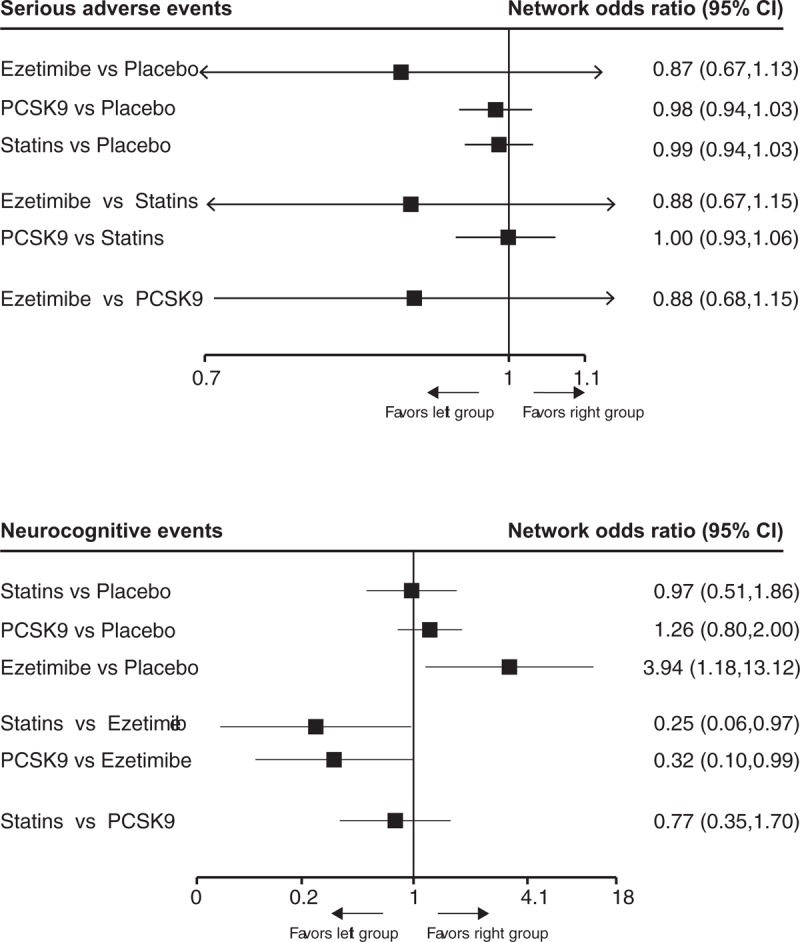
The relative benefit of statins, PCSK9 inhibitors, ezetimibe, and placebo against adverse events and neurocognitive events in patients with hypercholesterolemia. Data are presented with odds ratios and 95% confidence intervals. PCSK9 = proprotein convertase subtilisin-kexin type 9 serine protease.
2.6. Statin-related side effects and new-onset diabetes
Seventy-one trials involving 210,068 participants provided the outcome data for outcome of CK, 60 with 204,432 participants for outcome of ALT, and rare data were available for analyzing outcome of new-onset diabetes. The network estimates of safety outcomes are provided in Figure 5. Generally, statin ranked first in increasing the incidences of statin-related side-effects and new-onset diabetes as compared with other interventions. (Figure S7A–S7C) Compared with placebo, only statins were associated with elevation of ALT (1.89, 1.42–2.51) and CK levels (1.45 1.09–1.93), and increase of new-onset diabetes (1.13, 1.02–1.26), while PCSK9 inhibitor and ezetimibe were not.
Figure 5.
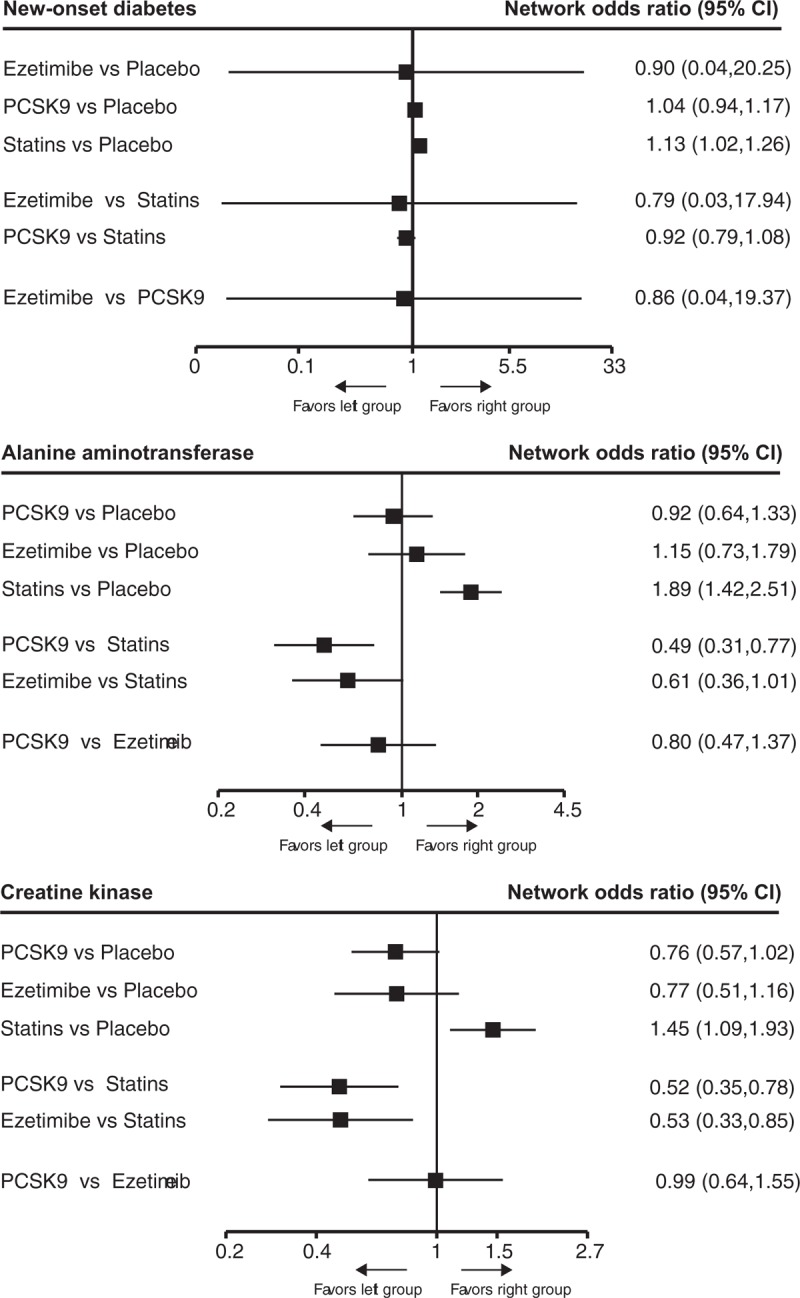
The relative benefits of statins, PCSK9 inhibitors, ezetimibe, and placebo against new-onset diabetes, and the elevation of alanine aminotransferase and creatine kinase levels in patients with hypercholesterolemia. Data are presented with odds ratios and 95% confidence intervals. PCSK9 = proprotein convertase subtilisin-kexin type 9 serine protease.
2.7. Additional analyses
Our main results for CV events were not significantly changed in predefined sensitivity analyses (Table 2). In meta-regression analyses, the intervention effects and ranking results for CV outcome were substantially unchanged after correcting baseline LDL-C level, follow-up duration, and baseline CAD percentage, while the intervention effects, heterogeneity, and ranking results were probably changed after the adjustment of the absolute LDL-C reduction. (Table 3). No significantly visual tendency suggested that active treatment effects of CV events (Fig S8A), all-cause mortality (Fig S8B), and CV mortality (Fig S8C) were affected by small-study, findings that were further confirmed by network meta-regression analysis. The extents of heterogeneities for network meta-analysis of major outcomes (CV events, all-cause mortality and CV mortality) were all low heterogeneity. (Table S4) Generally, no inconsistency was detected by the loop-specific approach (Table S5), design-by-treatment interaction model (Table S6), and node-splitting method (Table S7) in analyzing all the efficacy and safety outcomes.
Table 2.
Sensitivity analyses for cardiovascular ∗events.
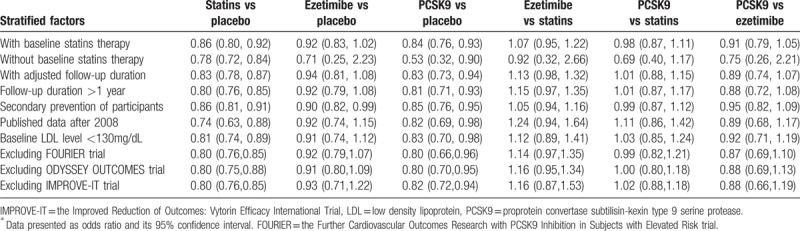
Table 3.
Meta-regression analysis of cardiovascular events adjusted by predefined effect modifiers.
3. Discussion
The current network meta-analysis (comprising 84 trials with 246,706 patients) provides unified hierarchies of evidence for statins, ezetimibe, and PCSK9 inhibitors in patients with hypercholesterolemia, overcoming the lack of comparative data in head to head trials. Our findings were summarized as follows:
-
1)
of the 3 lipid-lowering agents, PCSK9 inhibitors were the most effective agent in improving lipid levels;
-
2)
furthermore, PCSK9 inhibitors were associated with similar decreased risk of CV events as statins;
-
3)
however, no significant CV protective effect of ezetimibe was identified as compared with placebo;
-
4)
only statins were associated with reduced risks of all-cause and CV death;
-
5)
compared with placebo, all the lipid-lowering agents conferred an equal risk profile regarding safety outcomes, except that statins significantly increase the levels of ALT and CK and incidence of new-onset diabetes.
Generally, our results suggested that treatments with statins and PCSK9 inhibitors were associated with nearly identical decrease of CV events in patients with hypercholesterolemia although PCSK9 inhibitors reduced LDL-C level by a greater extent than statins. The strength of the present study is that our findings were consistent after taking into account the possible confounding factors (the background statins therapies, initial LDL-C level, follow-up duration, and publication date). First, a part of trials included in the present study was conducted on patients who were already on background statins therapy that was thought to have possible synergistic effect with PCSK9 inhibitor.[35] However, the similarly hierarchical evidences as our main results for analyzing CV events were presented between statins and PCSK9 inhibitors in patient subgroup analyses stratified according to with or without background statins use, in line with the results of recent FOURIER trial[10] in which a consistent CV benefit was achieved with PCSK9 inhibitors therapy across different intensity of background statins use. The results of current subgroup analyses were also supported by the recent Mendelian randomization study[36] in which the effects of variants in PCSK9 and variants in HMGCR were found to be independent and additive. Second, our analyses did not show that the CV outcome in our study was influenced by baseline LDL-C level, for similar comparative effects of CV outcome among the 3 agents were observed when using baseline LDL-C level as a covariate in meta-regression analysis. Likewise, previous Cholesterol Treatment Trialists’ Collaboration meta-analysis,[37] and recent RCTs of IMPROVE-IT[18] and FOURIER[10] all did not find that CV benefits obtained by lipid-lowering therapy were varied across the range of baseline LDL-C levels. Third, in view of the fact that the follow-up duration of included trials in our study was varied, we accounted for this fact by using person-year instead of the total number of participants to estimate network OR. Moreover, we performed sensitivity analysis based on trials with follow-up duration longer than 1 year in order to test the robustness of our findings in long-term follow-up. As a result, both analyses were consistent with our main finding for analyzing CV events. Finally, the present study replaced RCTs published before 2000 with the latest large RCTs of statin and ezetimibe such as primary prevention trial of HOPE3,[38] and second prevention trial of IMPROVE-IT[18] and HIJ-PROPER.[39] Through this replacement, we not only kept a contemporaneity across the included trials, but also guaranteed a balance of CV risk profile, lifestyles, and the rate of use of evidence-based CV pharmacotherapies among the 3 categories of trials. To sum up, the 4 aspects mentioned above may indicate that our view of no significant difference of CV benefit between patients who received PCSK9 inhibitors and those received statins therapies was reasonable and robust.
Moreover, our network estimates showed that ezetimibe was not associated with significant reduction of CV events as compared with placebo. This result may attribute to the inclusion/exclusion criteria of the current analysis. In the present study, major clinical outcomes with respect to ezetimibe focused on the efficacy of ezetimibe relative to placebo rather than ezetimibe plus statins relative to placebo. Thus, 2 large scale ezetimibe-related RCTs, SEAS,[40] and SHARP[41] studies, both of which have sufficient power and consistent views to comment on events but investigated the efficacy of ezetimibe plus statins relative to placebo, were excluded. In addition, treatment with ezetimibe alone lowered LDL-C level by a small extent, 19% reduction from baseline as showed by our lipid outcomes, which, according to previous meta-analysis,[42] yielded a slight decrease in CV risk. A case in point is the 2 large scale RCTs, ENHANCE,[43] and HIJ-PROPER,[39] in which both ezetimibe group reduced LDL-C by about 16% while failed to show a significant decrease in rate of CV events as compared with placebo. Collectively, given the uncertain CV benefit of ezetimibe in the absence of statins and its low intensity lipid-lowering effect, combination data from previous SEAS[40] and SHARP[41] trials and current analyses, in accordance with recent 2016 ESC/EAS guideline[44] for the management of dyslipidaemia, would again suggest that ezetimibe is appropriately served as an adjuvant agent in combination with statins rather than used alone.
Additionally, present study, consistent with previous systemic reviews,[1,45] showed that statins were associated with significant reduction of all-cause mortality and CV mortality in adults with hypercholesterolemia. However, treatments with ezetimibe and PCSK9 inhibitor did not result in improvement of all-cause and CV mortality, though they showed potential or certain benefits for prevention of CV diseases. This finding was possibly related to the low number of death in participants with ezetimibe and PCSK9 inhibitor therapy, as compared with 5176 deaths occurred in statin group during follow-up, there are only 1268 and 470 deaths respectively occurred in ezetimibe group and PCSK9 inhibitor group. In addition, a delay between the onset of LDL-C reduction and the improvement of survival and CV mortality had been well documented by previous statin-related trials.[46–49] Generally, significant reduction of all-cause death occurred 2 years after the initiation of lipid-lowering therapy. However, available trials for analysis of ezetimibe and PCSK9 inhibitors were shorter than 2 years except IMPROVE-IT,[18] HIJ-PROPER,[39] FOURIER,[10] OSLER-1extension,[50] and ODYSSEY OUTCOMES trial.[20] Finally, statins had been showed significant benefits on decreasing mortality of multiple cancer types, including lung, prostate, breast, and colorectal cancer,[51,52] while no anti-tumor effects of ezetimibe and PCSK9 inhibitors have been reported. In this way, survival of patients under ezetimibe and PCSK9 inhibitors therapy might not obtain additional antitumor effects beyond these benefit achieved with LDL-C reduction. In summary, due to the limit of clinical data and the lack of relevant reports, the present study was still too premature to make determined conclusions that ezetimibe and PCSK9 inhibitors therapies have no benefits on all-cause and CV mortality, which warrants further studies in future.
Finally, treatments with statins, ezetimibe, and PCSK9 inhibitors did not lead to increase of serious adverse events, conferring an equal risk profile among the agents. However, some challenges with respect to other side-effects that might threaten the future application of the 3 agents in clinical practice should be noted. First, the current meta-analysis, consistent with previous systematic reviews,[53–55] population-based study,[56] and basic research,[57] further demonstrated that statins therapy resulted in increased risk of new-onset diabetes; in addition, statins were associated with elevated level of ALT and CK, but ezetimibe and PCSK9 inhibitors were not. Second, although the present study, different from recent meta-analysis,[15] did not bear out that a higher incidence of neurocognitive adverse events was associated with PCSK9 inhibitor therapy, we noted that ezetimibe therapy led to increased risk of neurocognitive adverse events. However, this finding should be interpreted with caution because of scarce available data for analyzing neurocognitive adverse events regarding ezetimibe. At last, although PCSK9 inhibitor conferred a safety profile with respect to side effects, it should be noted that a higher incidence of injection-site reaction had not been addressed by previous RCTs.
Some limitations associated with current network meta-analysis warrant acknowledgment. First, the present study was based on trial level rather than individual level, thus detailed subgroup analyses stratified according to confounding factors such as initial LDL-C level, CV risk, and medication were unavailable; however, the hierarchy of the 3 agents regarding CV outcomes were not changed by these factors as shown by additional meta-regression analyses. Second, the follow-up duration of included trials was varied; nevertheless, the comparative ORs of major outcomes among the 3 agents were generally consistent after adjusting follow-up duration. Third, the components of CV events were not uniformly defined among the included trials. Moreover, specific CV outcome was unavailable in the trials that only reported composite CV endpoints, thus, the comparative benefits of the 3 agents on a specific outcome were lacked. Fourth, although most of the clinical data of PCSK9 inhibitors was derived from FOURIER trial, the exclusion of which did not result in significant change of network estimates for CV events. Fifth, we did not control dose in our study, and the dose of the 3 agents was allowed to titrate by investigators, which, as a consequence, may lead to slight fluctuation in LDL-C level. However, meta-regression analyses addressed this problem by weighting CV outcomes with absolute LDL-C reduction.
4. Conclusions
PCSK9 inhibitors were the most effective agents in improving lipid levels, and simultaneously they were as effective as statins against CV events in patients with hypercholesterolemia. Moreover, ezetimibe alone was not associated with decrease of CV events, which might inform the appropriateness of using ezetimibe in combination with other lipid-lowering agents rather than using ezetimibe alone in management of dyslipidemia. Only statins prolonged survival and reduced CV mortality, whereas for PCSK9 inhibitors and ezetimibe these were not the case. However, unlike statins, any benefits of PCSK9 inhibitors did not need to be balanced against the harms of elevated levels of ALT and CK, and increased risk of new-onset diabetes, which might make PCSK9 inhibitor an alternative lipid-lowering therapy for patients with hypercholesterolemia, especially for these with statin intolerance.
Acknowledgments
We thank Shifei Wang (Department of Cardiology, Nanfang Hospital, Southern Medical University) very much for his great assistance in statistical method and analysis.
Author contributions
Conceptualization: Zonglei Zhao, Lixia Wang.
Data curation: Zonglei Zhao, Song Du, Shuxin Shen.
Formal analysis: Zonglei Zhao, Song Du, Ping Luo.
Methodology: Shoukun Ding.
Supervision: Guanggong Wang, Lixia Wang.
Writing – original draft: Zonglei Zhao, Lixia Wang.
Writing – review & editing: Zonglei Zhao, Song Du, Lixia Wang.
Supplementary Material
Footnotes
Abbreviations: τ2 = Tau2, ALT = alanine aminotransferase, CI = confidence interval, CK = creatine kinase, CV = cardiovascular, HDL = high density lipoprotein, LDL-C = low-density lipoprotein cholesterol, OR = odds ratio, PCSK9 = proprotein convertase subtilisin/kexin type 9, RCT = randomized controlled trials, SMD = standardized mean difference, TC = total cholesterol.
None of the authors claim any conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013;31: doi: 10.1002/14651858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Koskinas KC, Siontis GCM, Piccolo R, et al. Effect of statins and non-statin LDL-lowering medications on cardiovascular outcomes in secondary prevention: a meta-analysis of randomized trials. Eur Heart J 2017;39:1172–80. [DOI] [PubMed] [Google Scholar]
- [3].Silva MA, Swanson AC, Gandhi PJ, et al. Statin-related adverse events: a meta-analysis. Clin Ther 2006;28:26–35. [DOI] [PubMed] [Google Scholar]
- [4].Simic I, Reiner Z. Adverse effects of statins—myths and reality. Curr Pharm Design 2015;21:1220–6. [DOI] [PubMed] [Google Scholar]
- [5].Gudzune KA, Monroe AK, Sharma R, et al. Effectiveness of combination therapy with statin and another lipid-modifying agent compared with intensified statin monotherapy: a systematic review. Ann Inter Med 2014;160:468–76. [DOI] [PubMed] [Google Scholar]
- [6].Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. New Engl J Med 2007;357:2109–22. [DOI] [PubMed] [Google Scholar]
- [7].Group AS, Ginsberg HN, Elam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. New Engl J Med 2010;362:1563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Investigators A-H, Boden WE, Probstfield JL, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. New Engl J Med 2011;365:2255–67. [DOI] [PubMed] [Google Scholar]
- [9].Artenstein AW, Opal SM. Proprotein convertases in health and disease. New Engl J Med 2011;365:2507–18. [DOI] [PubMed] [Google Scholar]
- [10].Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. New Engl J Med 2017;376:1713–22. [DOI] [PubMed] [Google Scholar]
- [11].Raal FJ, Stein EA, Dufour R, et al. RUTHERFORD-2 PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet 2015;385:331–40. [DOI] [PubMed] [Google Scholar]
- [12].Blom DJ, Hala T, Bolognese M, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. New Engl J Med 2014;370:1809–19. [DOI] [PubMed] [Google Scholar]
- [13].Reiner Z. PCSK9 inhibitors—past, present and future. Expert Opin Drug Metab Toxicol 2015;11:1517–21. [DOI] [PubMed] [Google Scholar]
- [14].Pecin I, Hartgers ML, Hovingh GK, et al. Prevention of cardiovascular disease in patients with familial hypercholesterolaemia: The role of PCSK9 inhibitors. Eur J Prev Cardiol 2017;24:1383–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lipinski MJ, Benedetto U, Escarcega RO, et al. The impact of proprotein convertase subtilisin-kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta-analysis. Eur Heart J 2016;37:536–45. [DOI] [PubMed] [Google Scholar]
- [16].Toth PP, Worthy G, Gandra SR, et al. Systematic review and network meta-analysis on the efficacy of evolocumab and other therapies for the management of lipid levels in hyperlipidemia. J Am Heart Assoc 2017;6: doi: 10.1161/JAHA.116.005367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Khan SU, Talluri S, Riaz H, et al. A Bayesian network meta-analysis of PCSK9 inhibitors, statins and ezetimibe with or without statins for cardiovascular outcomes. Eur J Prev Cardiol 2018;25:844–53. [DOI] [PubMed] [Google Scholar]
- [18].Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. New Engl J Med 2015;372:2387–97. [DOI] [PubMed] [Google Scholar]
- [19].Taguchi I, Iimuro S, Iwata H, et al. High-dose versus low-dose pitavastatin in japanese patients with stable coronary artery disease (REAL-CAD): a randomized superiority trial. Circulation 2018;137:1997–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. New Engl J Med 2018;379:2097–107. [DOI] [PubMed] [Google Scholar]
- [21].Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–84. [DOI] [PubMed] [Google Scholar]
- [22].Higgins JPT AD, Sterne JAC. Higgins JPT, Green S. Chapter 8: assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5. 1. 0 2011. [Google Scholar]
- [23].Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cipriani A, Higgins JP, Geddes JR, et al. Conceptual and technical challenges in network meta-analysis. Ann Intern Med 2013;159:130–7. [DOI] [PubMed] [Google Scholar]
- [25].Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PloS One 2013;8: doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–71. [DOI] [PubMed] [Google Scholar]
- [27].Turner RM, Davey J, Clarke MJ, et al. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol 2012;41:818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Veroniki AA, Vasiliadis HS, Higgins JP, et al. Evaluation of inconsistency in networks of interventions. Int J Epidemiol 2013;42:332–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932–44. [DOI] [PubMed] [Google Scholar]
- [30].Higgins JP, Jackson D, Barrett JK, et al. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 2012;3:98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Res Synth Methods 2012;3:161–76. [DOI] [PubMed] [Google Scholar]
- [32].White IR, Barrett JK, Jackson D, et al. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods 2012;3:111–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chaimani A, Salanti G. Visualizing assumptions and results in network meta-analysis: the network graphs package. Stata J 2015;15:905–50. [Google Scholar]
- [34].White IR. Network meta-analysis. Stata J 2015;15:951–85. [Google Scholar]
- [35].Zhang XL, Zhu QQ, Zhu L, et al. Safety and efficacy of anti-PCSK9 antibodies: a meta-analysis of 25 randomized, controlled trials. BMC Med 2015;13: doi: 10.1186/s12916-015-0358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ference BA, Robinson JG, Brook RD, et al. Variation in PCSK9 and HMGCR and Risk of Cardiovascular Disease and Diabetes. New Engl J Med 2016;375:2144–53. [DOI] [PubMed] [Google Scholar]
- [37].Cholesterol Treatment Trialists C, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yusuf S, Bosch J, Dagenais G, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. New Engl J Med 2016;374:2021–31. [DOI] [PubMed] [Google Scholar]
- [39].Hagiwara N, Kawada-Watanabe E, Koyanagi R, et al. Low-density lipoprotein cholesterol targeting with pitavastatin + ezetimibe for patients with acute coronary syndrome and dyslipidaemia: the HIJ-PROPER study, a prospective, open-label, randomized trial. Eur Heart J 2017;38:2264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rossebo AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. New Engl J Med 2008;359:1343–56. [DOI] [PubMed] [Google Scholar]
- [41].Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011;377:2181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA 2016;316:1289–97. [DOI] [PubMed] [Google Scholar]
- [43].Kastelein JJ, Akdim F, Stroes ES, et al. enhance Simvastatin with or without ezetimibe in familial hypercholesterolemia. New Engl J Med 2008;358:1431–43. [DOI] [PubMed] [Google Scholar]
- [44].Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999–3058. [DOI] [PubMed] [Google Scholar]
- [45].Tonelli M, Lloyd A, Clement F, et al. Efficacy of statins for primary prevention in people at low cardiovascular risk: a meta-analysis. CMAJ: Canadian Med Assoc J 2011;183:E1189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ridker PM, Danielson E, Fonseca FA, et al. JUPITER Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. New Engl J Med 2008;359:2195–207. [DOI] [PubMed] [Google Scholar]
- [47].Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. New Engl J Med 1995;333:1301–7. [DOI] [PubMed] [Google Scholar]
- [48].Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383–9. [PubMed] [Google Scholar]
- [49].LIPID Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. New Engl J Med 1998;339:1349–57. [DOI] [PubMed] [Google Scholar]
- [50].Koren Michael J, Sabatine Marc S, Giugliano Robert P, et al. Long-term low-density lipoprotein cholesterol-lowering efficacy, persistence, and safety of evolocumab in treatment of hypercholesterolemia: results up to 4 years from the open-label OSLER-1 extension study. JAMA Cardiol 2017;2:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wang A, Aragaki AK, Tang JY, et al. Statin use and all-cancer survival: prospective results from the Women's Health Initiative. Br J Cancer 2016;115:129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bansal D, Undela K, D’Cruz S, et al. Statin use and risk of prostate cancer: a meta-analysis of observational studies. PloS One 2012;7: doi: 10.1371/journal.pone.0046691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Preiss D, Sattar N. Statins and the risk of new-onset diabetes: a review of recent evidence. Curr Opin Lipidol 2011;22:460–6. [DOI] [PubMed] [Google Scholar]
- [54].Rajpathak SN, Kumbhani DJ, Crandall J, et al. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care 2009;32:1924–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010;375:735–42. [DOI] [PubMed] [Google Scholar]
- [56].Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population based study. BMJ 2013;346: doi: 10.1136/bmj.f2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Henriksbo BD, Lau TC, Cavallari JF, et al. Fluvastatin causes NLRP3 inflammasome-mediated adipose insulin resistance. Diabetes 2014;63:3742–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


