Abstract
Background:
Magnetic resonance imaging (MRI) is often used in children for its clear display of body parts. But it is usually hard to acquire high-quality images, for the uncooperative ability of children. It is believed that pre-MRI training could ensure the high quality of images. The current meta-analysis was done to analyze the current evidences in this field.
Methods:
PubMed, Cochrane Library, and Web of Science were systematically searched up to July 2018, for studies assessing the effects of training on pediatric MRI. Data, including image quality, failed scanning rate, and sedation use, were extracted and analyzed using Revman 5.2 software.
Results:
There were 5 studies with 379 subjects in the meta-analysis. Training and control groups were quite comparable when accepted image quality was reviewed (P = .30), but a lower rate of excellent image quality was found in subjects with training (P = .02). The pooling results found no significance between training and control group in sedation use (P = .09) and successful MRI scanning (P = .63).
Conclusions:
It is cautious to conclude that pre-MRI training does not improve the image quality and reduce sedation use among children, for the limited number of studies and sample size. More trials should be encouraged to demonstrate this issue.
Keywords: image quality, magnetic resonance imaging, pediatric, sedation, training
1. Introduction
Magnetic resonance imaging (MRI) is an important diagnostic approach, which is increasingly used in child healthcare.[1,2] For its high spatial resolution, absence of radiation, and various scanning sequences, it helps neurologists and radiologists reveal the structural and functional properties of the scanned parts.[3] But children may have difficulties in completing the scanning process for the fear of motion, anxiety, and an uncontrolled need to escape.[4] It takes much efforts and time to acquire images with high quality from these pediatric patients. They may then have to receive repeated examinations, and sometimes even sedative agents are applied to ensure the successful scanning.[5,6] These increase the unnecessary expenses and potential risk to pediatric neurodevelopment.[7]
Studies have indicated that pre-MRI training methods may help getting high-quality images with no motion or slight motion for diagnosis. The training system includes booklets, audio, video, MRI model, and behavioral interventions.[8–10] These approaches make children familiar with and interested in scanning process. Training then increases the acquirement of high-quality images, the successful completion of MRI.[11] Moreover, Rothman et al[12] and Bharti et al[9] indicated that instruction including simulator practice was associated with a reduced use of anesthesia among children undergoing MRI. And the study by Barnea-Goraly et al[6] indicated a high success rate for obtaining high-quality T1- and diffusion-weighted brain images in subjects between 4 and 10 years old without sedation. However, no meta-analysis is currently available on this topic. It then encourages a summarization of these studies.
2. Materials and methods
2.1. Search strategy
All data in this meta-analysis were extracted from the published articles, so no patient consent and institutional board approval were needed. We searched the electronic databases such as PubMed, Web of Knowledge, Embase, Scopus, and Cochrane Library until July, 2018 without language restriction, according to the recommendations from the Cochrane Collaboration handbook[13] and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[14] Key words were used in various combinations in the following strategy: ((((((((education) OR instruction) OR training) OR mock MRI) OR video) OR movie)) AND ((magnetic resonance imaging) OR MRI)) AND (((pediatric) OR child) OR children).
2.2. Inclusion and exclusion criteria
Clinical trials were enrolled comparing pre-MRI training with those without training among children. To be eligible, the subjects should be below 14 years. Studies with data of image quality assessment were included. Studies with subjects who suffered from any disorder associated with significant intellectual or motor impairments, neurological, neurodevelopment disorder, or any systematic disease that would be reluctant to cooperate with scanning were excluded.
2.3. Data extraction
Two reviewers independently extracted the data. Any disagreement was resolved by a senior author. Variables, including study characteristics (title, authors, publication year, and journal), study details (region, study period, participant number, age, gender, disease, training details, and outcomes) were recorded from the trials. For outcomes, sedation use, successful scanning, and image quality (excellent and accepted) were extracted. Sedation use was the incidence of sedative agent application in the subjects. A successful MRI meant that the scanning could be completed with full cooperation or little motion during scanning. And subjects with high MRI image were also seen with success. An application of sedative agents was seen as a failure. Excellent image quality indicated scanning with full cooperation or little motion and the acquirement of images without or slight motion artifacts. Accepted image quality meant an MRI image with moderate motion artifacts, but the severity would not influence the assessment of images. Then the number of participants with accepted image quality would be more than that of subjects with excellent image quality.
2.4. Quality assessment
Quality of nonrandomized controlled trials (non-RCTs) was assessed according to Newcastle-Ottawa Quality Assessment Scale,[15] including patient selection, comparability of the study groups, and assessment of outcome, with a score of 0–9 for each study. Studies with 6 or more stars were seen as high quality. The Cochrane Risk of Bias Tool[16] was adopted to explore the risk of bias for each RCT, with the following items for test: generation of allocation sequence, allocation concealment, blinding (participants and personnel), blinding (outcome assessment), incomplete outcome data, selective reporting, and other sources of bias.
2.5. Statistical meta-analysis
Data were analyzed using Revman 5.2 software. Risk ratio (RR) was used to compare dichotomous variables, with 95% confidence intervals (CIs). Heterogeneity was quantified by the estimated I2 with a Cochrane Q test. When the level of I2 was ≥ 50% or P ≤ 0.10, it was defined to be with high heterogeneity with an application of the random effects model. Otherwise, it was considered using fixed effects model. Funnel plots were used to examine publication bias if there were more than 10 studies in the comparison. Sensitivity analysis was performed when necessary by excluding one single study before any pooling.
3. Results
3.1. Search results and study characteristics
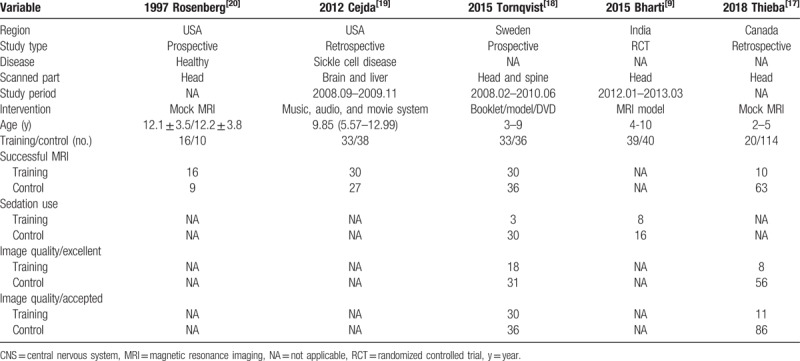
The flow diagram of the meta-analysis was shown in Figure 1. The initial search yielded 817 records after systematic search in PubMed, Web of Knowledge, and Cochrane Library. 411 articles were enrolled by removing duplicates. Review of titles and abstracts excluded 43 papers. Further analysis of whole texts included 5 trials with 379 subjects. The study characteristics were displayed in Table 1. These papers[9,17–20] were published from 1997 to 2018, in the United States, Canada, Sweden, and India. None indicated the disease types, except one with sickle cell disease. The scanned parts included head (3 trials), head/spine (1 trials), and head/liver (1 trial).
Figure 1.
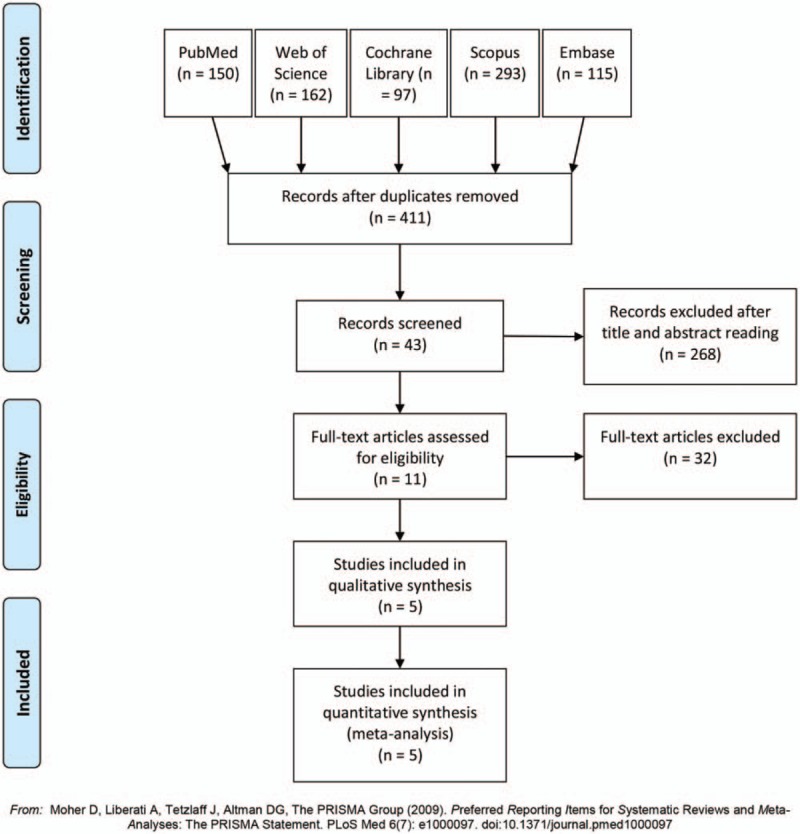
The flow diagram of study selection in the meta-analysis.
Table 1.
Study characteristics of the included studies.
3.2. Quality assessment
The quality of the studies was shown in Table 2 (non-RCTs) and Table 3 (RCTs). Most of the non-RCT trials (3 studies, 75.00%) were with high quality, with a mean score of 6.25. The single RCT[9] kept a good control of blinding (participants and personnel and outcome assessment), incomplete outcome data, and selective reporting. Other sources of bias were unclear.
Table 2.
Quality assessment of the included non-RCTs.
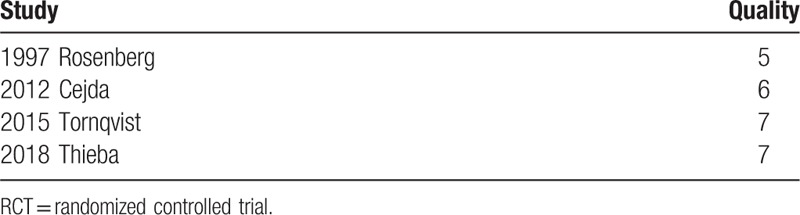
Table 3.
Assessment of bias risk of RCTs in this meta-analysis.

3.3. Outcome of interest
The pooling results were displayed in Table 4. Although the rate of sedation use was lower in training (15.28%), compared with control group (60.53%), but no significance was indicated (P = .09, I2 = 83%) (Fig. 2). There was a lower rate of excellent image quality in subjects with training (Training vs. Control: 49.06% vs. 58.00%; P = .02, I2 = 0%) (Fig. 3). When data of accepted image quality were pooled, the two groups were quite comparable (P = .30, I2 = 59%) (Fig. 4). We also reported a similar rate of successful MRI scanning in subjects with (84.31%) and without training (68.18%) (P = .63, I2 = 65%) (Fig. 5).
Table 4.
Meta-analysis results of the included studies.
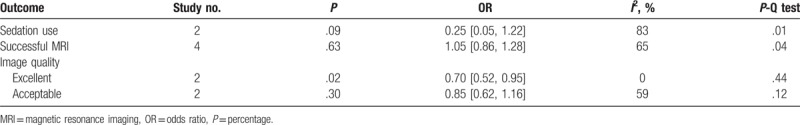
Figure 2.
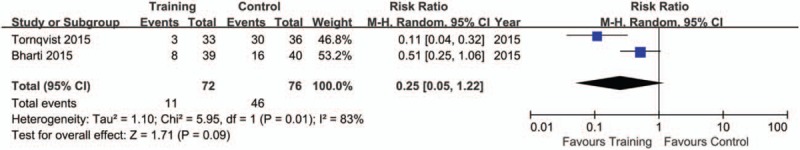
Forest plot of sedation use between training and control groups in the included studies.
Figure 3.
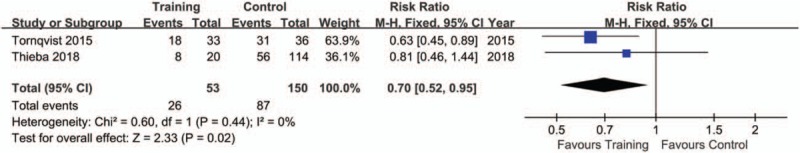
Forest plot of excellent image quality between training and control groups in the included studies.
Figure 4.
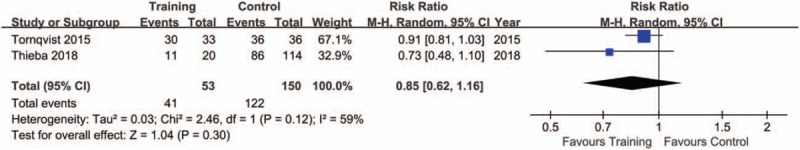
Forest plot of accepted image quality between training and control groups in the included studies.
Figure 5.
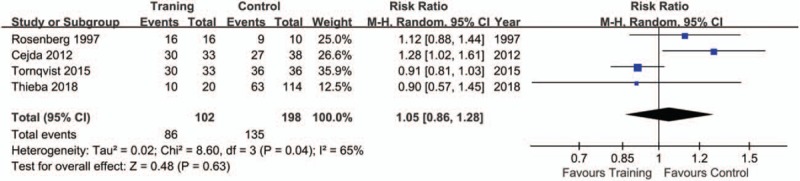
Forest plot of successful MRI between training and control groups in the included studies.
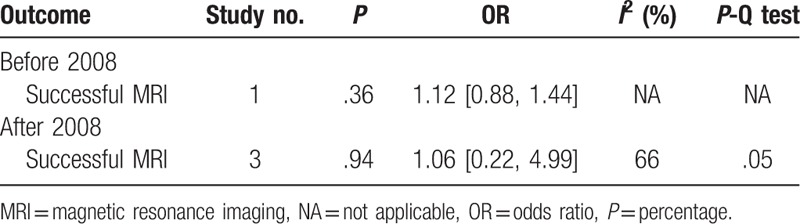
Comparisons were done for successful MRI scanning based on publication year (Table 5). The respective analysis of studies before and after 2008 revealed no differences in successful MRI.
Table 5.
Meta-analysis results of the included studies based on year and age.
3.4. Publication bias and sensitivity analysis
Sensitivity analysis was done in the comparisons with successful MRI scanning (with more than 2 studies) by excluding one single study. No significant changes of results were found in sedation use, but a robust change of heterogeneity was generated when removing any single study. Since there were less than 10 studies in each comparison, no publication bias analysis was done.
4. Discussion
This is the first meta-analysis to demonstrate the efficacy of pre-MRI training among children. Most of the studies had a relatively high quality. This strengthened the evidence level of the results. It indicated that pre-scanning training did not improve MRI image quality. Sedation use and the rate of successful scanning were not affected.
MRI is a widely applied diagnostic approach for pediatric patients.[21] However, it is sometimes hard to acquire high-quality images from children. They may be afraid of noises and be inpatient with long process, leading to a failed scanning.[22] This makes repeated scanning and even sedation use among them. Although sedation improves the examination, it is criticized for the potential risk on children's neurodevelopment.[23] Studies have indicated that pre-MRI training reduced the use of sedatives and increased the scanning process.[24,25,22] But no differences of sedation use and successful MRI rate were found in our results. This is quite different from the findings in the previous studies. We believed that it could be attributed that training increases anxiety and fear among these children. And the inconsistent results may also be caused by the limited number of studies. More trials should be included in the future. The methods of training included booklet, audio, video and toy model, and some researchers also established a mock MRI system to simulate the scanning environment.[26] Although these training methods make children familiar with, relaxed and interested in MRI machine, the variety of training methods in the included studies can influence the pooling results, inducing the inconsistent findings with previous studies.
The development of MRI technics should be taken into account. The improved technology in scanning and training can cause the inconsistency. MRIs in recent years are done with new machines costing less time, and the technics to minimize artifacts are used in new scanning sequences. Considering the development of MRI scanning technology, we here did subgroup analysis by analyzing studies before 2008 and after 2008. It indicated that training system did not increased scanning success both subgroups. But there were too limited studies, encouraging more outcomes to be evaluated.
Images with no motion or slight motion are seen with high quality in this meta-analysis. Those with moderate motion artifacts dose not influence diagnosis and are seen as acceptable images for analysis. This meta-analysis found that training did not improve image quality obviously. But it seemed that more images with excellent quality were produced in control, indicating training system might not improve the acquirement of images suitable for diagnosis. We speculated that this came from the higher sedation rate in controls. Patients with sedation cooperate with scanning process quite well with tolerable motion. But it should be cautious in clinical practice. The results in our study were quite different from the previous trials.
Some limitations existed in the meta-analysis. First, there were a limited number of studies with a small sample size in each outcome. This might hinder the credibility of the results. Second, the training details and age were not so consistent in all the studies, increasing the potential risk of bias. Third, all the comparisons were with high heterogeneity. Only one outcome of successful MRI was deducted from more than 2 studies. The publications ranged from 1997 to 2018. Both scanning and training methods may improve in recent years. This might be the cause of high heterogeneity and results variation existing in most of the comparisons. Fourth, the included studies only head or head/liver or head/spine. The MRI length and sequences may vary by scanning region. Therefore, these outcomes, including image quality and sedation use, may alter consequently. Further studies should be done with other scanning parts among children. Also, not all studies in the meta-analysis were RCT studies. RCTs with large sample size should be included in the future to increase the quality of the study. And age may also have an influence on the outcomes. We assumed that younger children may benefit from training. But most studies did not give clear data of scanning details of each age group or individual. We failed to analyze this aspect in the current study.
5. Conclusion
It is cautious to conclude that pre-MRI training does not improve the image quality and reduce sedation use among children. More studies were needed for further analysis.
Author contributions
Conceptualization: Jie Li, Xiuhong Dai, Xinxian Zhang.
Data curation: Qiancheng Li.
Formal analysis: Jie Li, Qiancheng Li, Jiong Li.
Investigation: Jiong Li.
Methodology: Qiancheng Li, Jiong Li.
Project administration: Qiancheng Li, Xiuhong Dai, Jiong Li, Xinxian Zhang.
Resources: Xiuhong Dai.
Supervision: Xiuhong Dai.
Validation: Qiancheng Li.
Visualization: Xiuhong Dai, Xinxian Zhang.
Writing – original draft: Jie Li, Xinxian Zhang.
Writing – review & editing: Xinxian Zhang.
Footnotes
Abbreviations: CIs = confidence intervals, MRI = magnetic resonance imaging, OR = odds ratio, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RCT = randomized controlled trial.
The authors have no conflicts of interest to disclose.
References
- [1].Wielandner A, Mlczoch E, Prayer D, et al. Potential of magnetic resonance for imaging the fetal heart. Semin Fetal Neonatal Med 2013;18:286–97. [DOI] [PubMed] [Google Scholar]
- [2].Pareek A, Muehe AM, Theruvath AJ, et al. Whole-body PET/MRI of pediatric patients: the details that matter. J Vis Exp 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Qiao Y, Zeiler SR, Mirbagheri S, et al. Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology 2014;271:534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cox AD, Virues-Ortega J, Julio F, et al. Establishing motion control in children with autism and intellectual disability: applications for anatomical and functional MRI. J Appl Behav Anal 2017;50:8–26. [DOI] [PubMed] [Google Scholar]
- [5].Waitayawinyu P, Wankan P. The success of MRI without sedations in 6-15 years old pediatric patients after watching MRI introductory video. J Med Assoc Thai 2016;99:596–601. [PubMed] [Google Scholar]
- [6].Barnea-Goraly N, Weinzimer SA, Ruedy KJ, et al. High success rates of sedation-free brain MRI scanning in young children using simple subject preparation protocols with and without a commercial mock scanner—the Diabetes Research in Children Network (DirecNet) experience. Pediatr Radiol 2014;44:181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Natarajan G, Shankaran S, Laptook AR, et al. Association between sedation-analgesia and neurodevelopment outcomes in neonatal hypoxic-ischemic encephalopathy. J Perinatol 2018;38:1060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Szeszak S, Man R, Love A, et al. Animated educational video to prepare children for MRI without sedation: evaluation of the appeal and value. Pediatr Radiol 2016;46:1744–50. [DOI] [PubMed] [Google Scholar]
- [9].Bharti B, Malhi P, Khandelwal N. MRI customized play therapy in children reduces the need for sedation—a randomized controlled trial. Indian J Pediatr 2016;83:209–13. [DOI] [PubMed] [Google Scholar]
- [10].Tyc VL, Leigh L, Mulhern RK, et al. Evaluation of a cognitive-behavioral intervention for reducing distress in pediatric cancer patients undergoing magnetic resonance imaging procedures. Int J Rehabil Health 1997;3:267–79. [Google Scholar]
- [11].de Bie HM, Boersma M, Wattjes MP, et al. Preparing children with a mock scanner training protocol results in high quality structural and functional MRI scans. Eur J Pediatr 2010;169:1079–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rothman S, Gonen A, Vodonos A, et al. Does preparation of children before MRI reduce the need for anesthesia? Prospective randomized control trial. Pediatr Radiol 2016;46:1599–605. [DOI] [PubMed] [Google Scholar]
- [13].John Wiley & Sons, Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. 2011. [Google Scholar]
- [14].Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg 2011;39:91–2. [DOI] [PubMed] [Google Scholar]
- [15].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [16].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Thieba C, Frayne A, Walton M, et al. Factors associated with successful MRI scanning in unsedated young children. Front Pediatr 2018;6:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Törnqvist E, Månsson Å, Hallström IK. Children having magnetic resonance imaging: a preparatory storybook and audio/visual media are preferable to anesthesia or deep sedation. J Child Health Care 2015;19:359–69. [DOI] [PubMed] [Google Scholar]
- [19].Cejda KR, Smeltzer MP, Hansbury EN, et al. The impact of preparation and support procedures for children with sickle cell disease undergoing MRI. Pediatr Radiol 2012;42:1223–8. [DOI] [PubMed] [Google Scholar]
- [20].Rosenberg DR, Sweeney JA, Gillen JS, et al. Magnetic resonance imaging of children without sedation: preparation with simulation. J Am Acad Child Adolesc Psychiatry 1997;36:853–9. [DOI] [PubMed] [Google Scholar]
- [21].Arthurs OJ, Thayyil S, Pauliah SS, et al. Diagnostic accuracy and limitations of post-mortem MRI for neurological abnormalities in fetuses and children. Clin Radiol 2015;70:872–80. [DOI] [PubMed] [Google Scholar]
- [22].Greene DJ, Koller JM, Hampton JM, et al. Behavioral interventions for reducing head motion during MRI scans in children. Neuroimage 2018;171:234–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Daud YN, Carlson DW. Pediatric sedation. Pediatr Clin North Am 2014;61:703–17. [DOI] [PubMed] [Google Scholar]
- [24].de Amorim e Silva CJ, Mackenzie A, Hallowell LM, et al. Practice MRI: reducing the need for sedation and general anaesthesia in children undergoing MRI. Australas Radiol 2006;50:319–23. [DOI] [PubMed] [Google Scholar]
- [25].Munn Z, Jordan Z. The effectiveness of interventions to reduce anxiety, claustrophobia, sedation and non-completion rates of patients undergoing high technology medical imaging. JBI Libr Syst Rev 2012;10:1122–85. [DOI] [PubMed] [Google Scholar]
- [26].Hallowell LM, Stewart SE, de Amorim E Silva CT, et al. Reviewing the process of preparing children for MRI. Pediatr Radiol 2008;38:271–9. [DOI] [PubMed] [Google Scholar]


