Abstract
Background:
A meta-analysis was applied to evaluate the associations between the glutathione-S-transferases (GSTs) M1/T1 gene polymorphisms and male infertility in Chinese populations.
Methods:
A comprehensive search for articles was conducted from PubMed, Web of Science, Embase, China biology medical literature database (CBM), China National Knowledge Infrastructure (CNKI), VIP, and Chinese literature database(Wang fang) up to April 30, 2018. All of the statistical analyses were performed using Review Manager 5.3 and Stata 14.0.
Results:
Ten studies on GSTM1 gene polymorphism involving 3302 cases and 1959 controls, and ten studies on GSTT1 gene polymorphism involving 3048 cases and 1861 controls were included in this meta-analysis. Overall, the null genotype of GSTM1/GSTT1 was significantly related to male infertility risk in Chinese populations (GSTM1, OR = 1.35, 95% CI: 1.02–1.78; GSTT1, OR = 1.40, 95% CI: 1.15–1.70). In subgroup analyses stratified by infertility type, significant association was observed between GSTT1 null genotype and male infertility in both nonobstructive azoospermia (NOA) and oligoasthenozoospermia (OAT). However, the GSTM1 null genotype was associated with OAT, but not NOA in Chinese populations. The sensitivity analysis confirmed the reliability and stability of the meta-analysis.
Conclusion:
Our meta-analysis supports that the GSTM1/GSTT1 null genotype might contribute to individual susceptibility to male infertility in Chinese populations.
Keywords: glutathione-S-transferases, GSTM1, GSTT1, male infertility, meta-analysis
1. Introduction
Male infertility is a complicated disease globally, and is defined as the failure of a couple to achieve pregnancy after 1 year of unprotected, regular sexual intercourse.[1,2] Globally, an estimated 10%–15% of couples suffer from infertility, and it is estimated that the incidence of male infertility accounts for approximately 50% of all infertile couples.[3,4] However, the causes of male infertility is not fully understood. In addition to environmental and lifestyle risk factors, several genetic causes, such as chromosomal and single-gene alterations, are reported to be associated with male infertility.[5–7] Current evidence indicates that GSTM1/T1 gene polymorphism is a potential risk factor.[8,9] Tirumala et al identified an association between the GSTM1 null genotype and idiopathic male infertility in the Indian population.[10] Olshan et al reported that the GSTT1 non-null genotype was associated with reduced sperm count in semen.[11]
Glutathione-S-transferases (GSTs), a family of eukaryotic and prokaryotic phase II metabolic isozymes, play an essential role in cellular detoxification and bioactivation reactions.[12–14] There is a high level of GST in human testis and semen, which serves to protect spermatozoa against the negative effects of oxidative stress.[8,15] Mutations in the GST gene can affect the activity of the glutathione system enzymes and disturb the balance in the detoxification system, resulting in male infertility.[16,17] Of all published genetic association studies regarding male infertility, GST gene polymorphisms M1 and T1 are the most studied.
A number of studies have investigated the relationship between GSTM1/T1 gene polymorphism and male infertility risk in various populations worldwide, including Russian, Brazilian, Iranian, Turkish, and Japanese populations. In the Chinese population, several studies have focused on genetic variation in GSTM1 and GSTT1 in relation to male infertility, but have yielded contradictory results.
Meta-analysis can combine results of different studies to provide an estimate of the major effect with enhanced precision.[18,19] To date, there has been no meta-analysis of studies conducted in Chinese populations. We perform this meta-analysis on all published case–control studies to derive a more precise estimation of the relationship between GSTM1/T1 polymorphism and male infertility risk in Chinese populations.
2. Materials and methods
2.1. Identification of relevant studies
PubMed, Web of Science, Embase, CBM, CNKI, VIP, and Wang fang databases were searched for studies examining the relation between GSTM1/GSTT1 gene polymorphisms and male infertility in Chinese population up to April 30, 2018. The search terms were used as follows: “Glutathione S-transferases,” “GST,” “GSTM1,” “GSTT1,” and “male infertility.” Besides the database search, the references lists of the relevant studies were also screened for the potential articles that may have been missed in the initial search. All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
2.2. Inclusion and exclusion criteria
Studies included in the meta-analysis have to meet the following criteria:
-
(1)
case–control study describing the association of GSTM1/GSTT1 gene polymorphisms and male infertility;
-
(2)
the genotypes in cases and controls were available for the estimation of an odds ratio (OR) with a 95% confidence interval (CI);
-
(3)
the participants were of the Chinese people of all ethnic groups.
Exclusion criteria:
-
(1)
study with incomplete data;
-
(2)
duplicate publications with overlapping data;
-
(3)
editorial articles, review articles, case reports, and meeting abstracts.
2.3. Data extraction and quality assessment
Two investigators extracted the data using a standardized data extraction form independently. Discrepancies were resolved by discussion with a third investigator. The following information was extracted from each study: first author, year of publication, sample size, geographical location, and genotype frequencies of GSTM1 and GSTT1.
The Newcastle-Ottawa Scale (NOS) was used to assess the quality of included studies by two authors.[20] A star rating system was used to judge methodological quality. Scores range from 0 stars (worst) to 9 stars (best), and studies with a score ≥7 were defined as high quality.
2.4. Statistical analysis
Odds ratios (ORs) with 95% CIs were used to assess the strength of association between GSTM1/GSTT1 gene polymorphisms and male infertility. The significance of the pooled OR was analyzed by the Z test, and P < .05 was considered statistically significant. The heterogeneity among eligible studies was calculated by the Chi-square-based Q-test and I2 statistics. A fixed effect model was used when the Q test was P > .05 or I2 < 50%, which indicated a statistically significant degree of heterogeneity among the included studies. Otherwise, the random-effects model was used. The Hardy-Weinberg equilibrium (HWE) test could not be conducted, for there was no distribution of null/present heterozygote in each single study included. All statistical analyses were conducted by using Review Manager 5.3 and Stata 14.0. Publication bias was investigated with the funnel plot, Begg's test, and Egger's test. Sensitivity analysis was performed to assess the stability of the results by sequentially omitted individual studies. P > .05 was considered to indicate statistical significance.
3. Results
3.1. Description of included studies
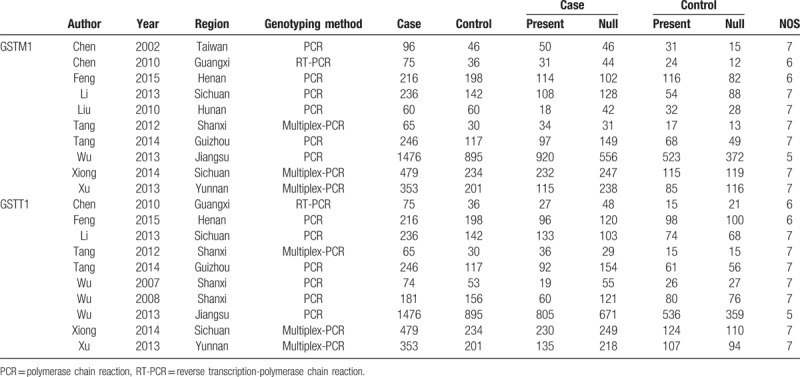
A flow diagram of the search process is shown in Figure 1. Two hundred and ninety-six related studies were retrieved through database searching. After applying the inclusive and exclusive criteria, 12 case–control studies considering 3557 cases and 2168 controls were included in the meta-analysis.[15,17,21–32] The publication years of the assessed studies ranged from 2002 to 2015. Of these, 10 case–control studies involving 3302 cases and 1959 controls addressed the GSTM1 gene polymorphism, and 10 case–control studies involving 3048 cases and 1861 controls addressed the GSTT1 gene polymorphism. The characteristics of each of the included studies are shown in Table 1.
Figure 1.
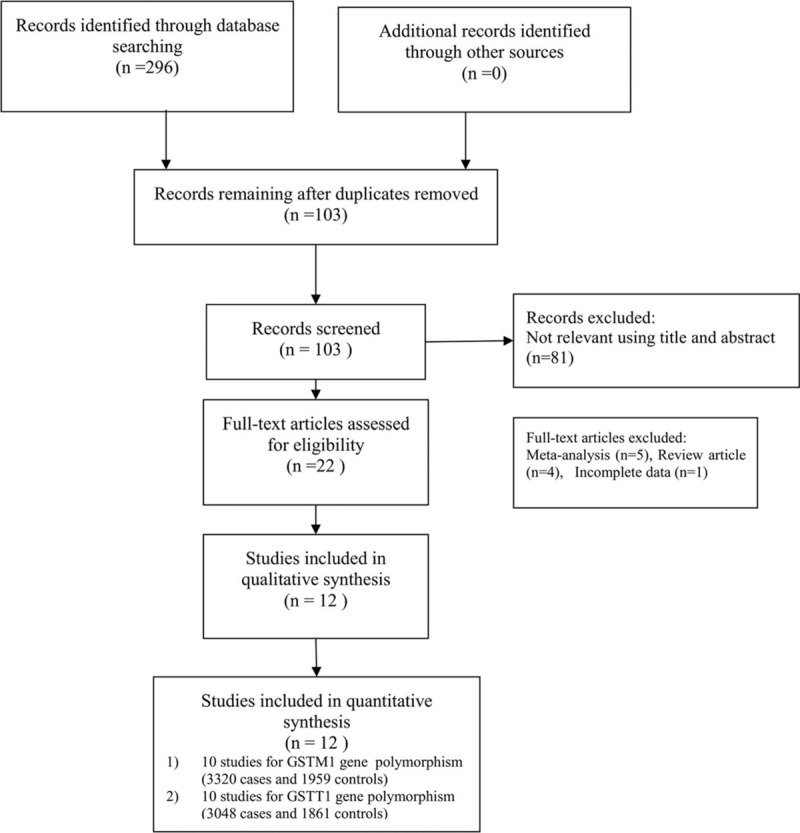
Flowchart showing the study selection.
Table 1.
Characteristics of studies included in meta-analysis.
3.2. Meta-analysis of GSTM1 null genotype in male infertility susceptibility
Ten studies involving a total of 5261 individuals evaluated the influence of the GSTM1 null genotype on the risk of male infertility. The I2 value was 76%, which suggested a statistically significant degree of heterogeneity among the studies. Thus, the random effect model was used to synthesize the data. Overall, the results revealed a significant association between the GSTM1 null genotype and Chinese male infertility (null type vs. present type, OR = 1.35, 95% CI = 1.02–1.78, P = .03 in Fig. 2). Subgroup analyses on male infertility type showed that significant association was observed in OAT (null type vs. present type, OR = 1.54, 95% CI = 1.04–2.29, P = .03 in Fig. 3), but not in NOA (null type vs. present type, OR = 0.90, 95% CI = 0.63–1.30, P = .57 in Fig. 3).
Figure 2.
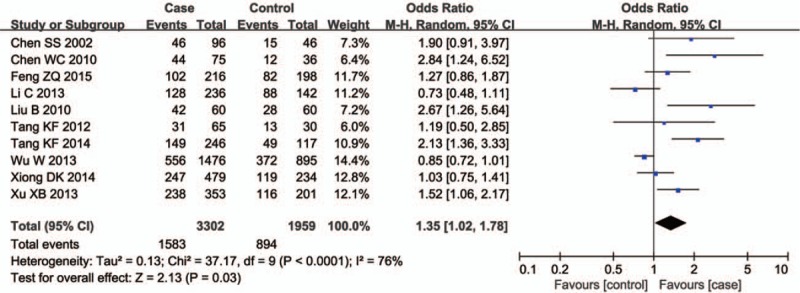
Forest plots of all selected studies on the association between GSTM1 polymorphism and male infertility risk in Chinese.
Figure 3.
Forest plots of all selected studies on the association between GSTM1 polymorphism and male infertility risk in Chinese (subgroup analyses for the OAT and NOA). OAT = oligoasthenozoospermia, NOA = nonobstructive azoospermia.
3.3. Meta-analysis of GSTT1 null genotype in male infertility susceptibility
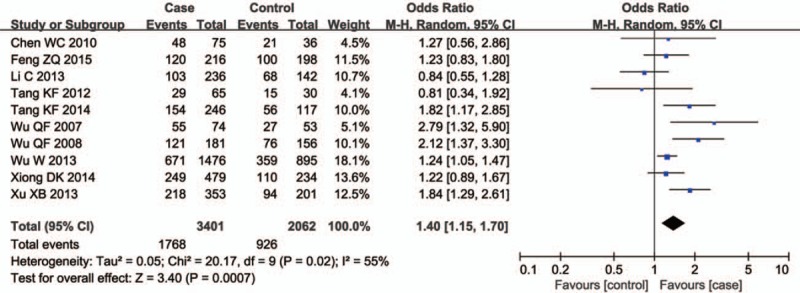
There were ten studies including 3048 cases and 1861 controls evaluating the influence of the GSTT1 null genotype on the male infertility. The I2 value was 55% and the random effect model was applied. Overall, the results revealed a significant association between the GSTT1 null genotype and Chinese male infertility (null type vs. present type, OR = 1.40, 95% CI = 1.15–1.70, P = .0007 in Fig. 4). In the subgroup analysis stratified by male infertility type, a significant association was observed for both NOA (null type vs. present type, OR = 1.52, 95% CI = 1.25–1.84, P < .0001 in Fig. 5) and OAT patients (null type vs. present type, OR = 1.55, 95% CI = 1.10–2.18, P = .01 in Fig. 5).
Figure 4.
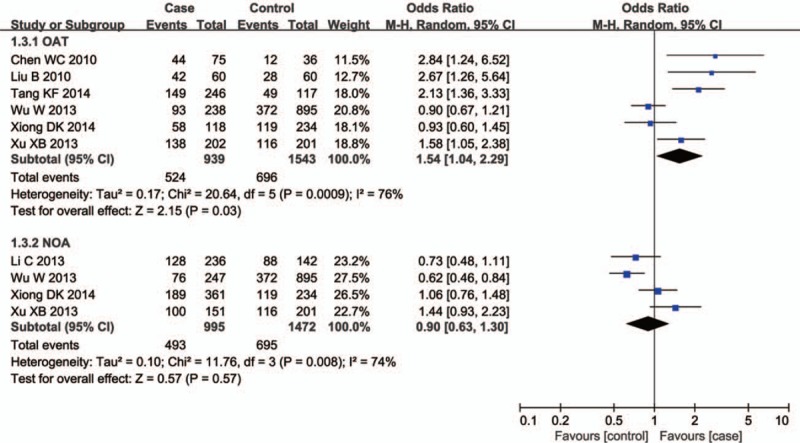
Forest plots of all selected studies on the association between GSTT1 polymorphism and male infertility risk in Chinese.
Figure 5.
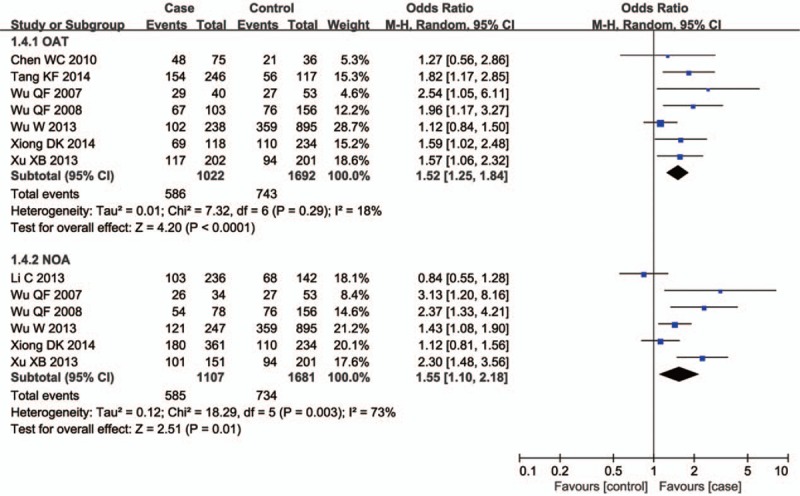
Forest plots of all selected studies on the association between GSTT1 polymorphism and male infertility risk in Chinese (subgroup analyses for the OAT and NOA). OAT = oligoasthenozoospermia, NOA = nonobstructive azoospermia.
3.4. Sensitivity and publication bias
The sensitivity analyses were performed to calculate the pooled ORs through sequentially excluding individual studies, and the results showed no individual study influenced the overall pooled ORs (Figs. 6 and 7), indicating that the results of this meta-analysis are relatively stable. The publication bias was assessed by funnel plots, Begg's test, and Egger's test. There was no publication bias for GSTT1 gene polymorphism (Table 2, Fig. 8). However, some publication bias was observed for GSTM1 gene polymorphism according to Egger's test and funnel plots (Table 2, Fig. 9).
Figure 6.
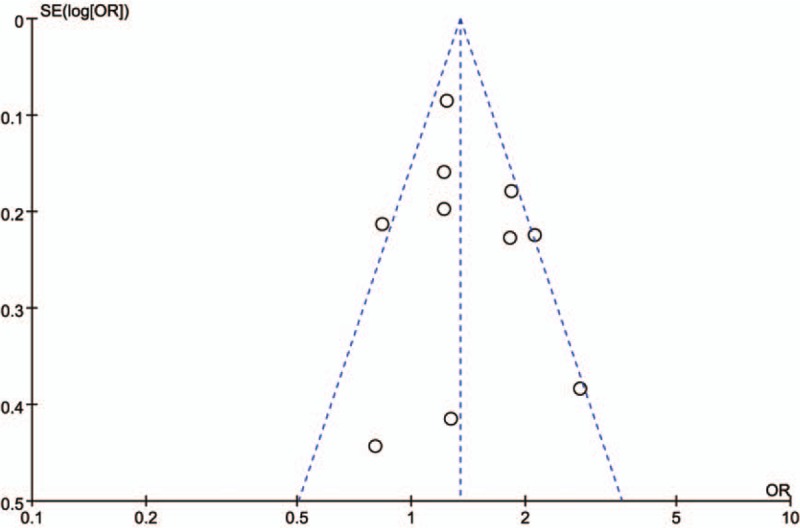
Sensitivity analysis diagram for each study used to assess the relative risk estimates for the GSTM1 polymorphism and male infertility risk in Chinese.
Figure 7.
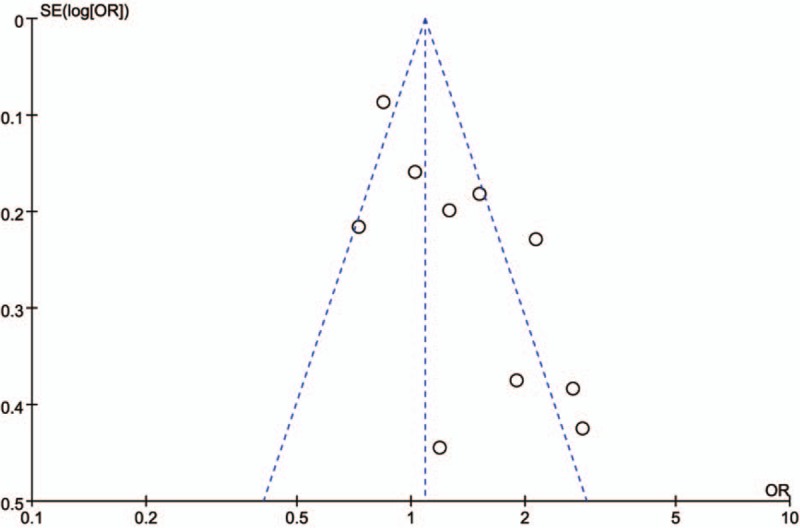
Sensitivity analysis diagram for each study used to assess the relative risk estimates for the GSTT1 polymorphism and male infertility risk in Chinese.
Table 2.
Publication bias test for GSTM1/GSTT1 polymorphisms.
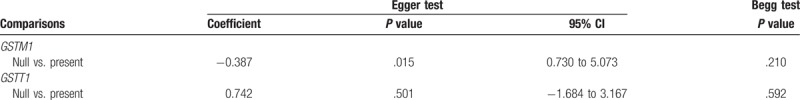
Figure 8.
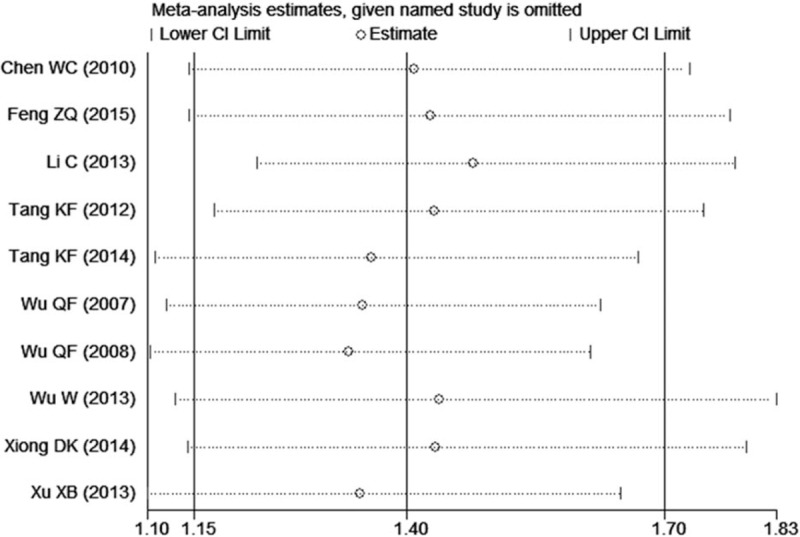
Funnel plot of the studies assessing the association between GSTT1 polymorphism and male infertility risk in Chinese.
Figure 9.
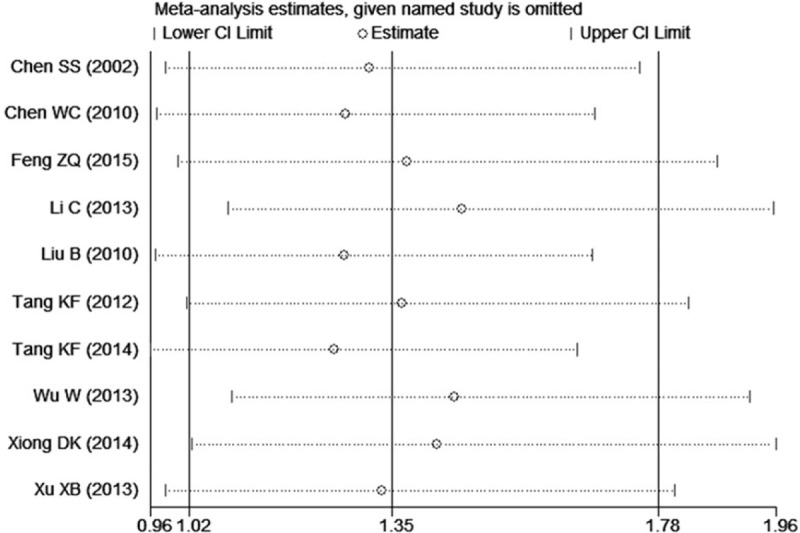
Funnel plot of the studies assessing the association between GSTM1 polymorphism and male infertility risk in Chinese.
4. Discussion
Oxidative stress induced by reactive oxygen species (ROS) has been widely recognized as one of the major causes of male infertility.[33] Low physiological concentrations of ROS plays an essential role for sperm capacitation, hyperactivation, and spermatozoon-oocyte fusion.[34] GSTs, a major group of detoxification and antioxidant enzymes, are considered to play protective roles against toxic xenobiotic and ROS in tissue.[8] Polymorphisms in the GST gene may impact the ability of protection against oxidative stress and lead to the development of male infertility.[35] In the present study, we examined the association between the polymorphisms of GSTM1/GSTT1 and the male infertility risk, the overall results showed that the GSTM1/GSTT1 null genotype might contribute to individual susceptibility to male infertility in Chinese populations. To our knowledge, this is the first meta-analysis to investigate the association between these polymorphisms and the development of male infertility in Chinese population.
There is an increasing evidence investigating the association between GSTM1/GSTT1 gene polymorphism and risk of male infertility, however, the results remain inconclusive rather than consistent. In 2012, Tang et al conducted a meta-analysis to evaluate the association between GSTM1/GSTT1 gene polymorphism and idiopathic male infertility risk; they have suggested that the frequency of GSTM1 null genotype was significantly associated with susceptibility to idiopathic male infertility in Caucasians, but not in Asians, however, no significant association was found between GSTT1 null genotype and male infertility in both Caucasians and Asians.[36] In 2013, a meta-analysis conducted by Wu et al showed that the frequency of GSTM1 null genotype was significantly associated with male infertility risk in both Caucasians and Asians. However, the frequency of GSTT1 null genotype was associated with male infertility risk in Asians other than Caucasians. Due to the differences in the number of participants and different genetic backgrounds, the results provided by each study is not sufficient to draw a convincing conclusion.[17] In our meta-analysis, ten case–control studies (3302 cases and 1959 controls) for the GSTM1 polymorphism, and ten case–control studies (3048 cases and 1861 controls) for the GSTM1 polymorphism were included to evaluate the relationship of GSTM1/GSTT1 gene polymorphisms and male infertility risk. The overall results showed that the GSTM1 null genotype could increase the risk of male infertility in Chinese population (null type vs. present type, OR = 1.35, 95% CI = 1.02–1.78, P = .03). It reveals that individuals with the null genotype may have a higher risk for male infertility than those carrying present genotype. Subgroup analysis based on infertility type showed the consistent result. As regard to the GSTT1 polymorphism, significant association was found with male infertility in the Chinese population (null type vs. present type, OR = 1.40, 95% CI = 1.15–1.70, P = .0007), however, subgroup analysis based on infertility type, we have observed that the GSTT1 null genotype is associated with OAT, but not NOA in Chinese populations. In the present study, Chinese database were searched to more comprehensively assess studies in Chinese populations and more recently-published studies were included in the present meta-analysis, which may underscore the reliability of our findings.
When interpreting the results of the current study, some limitations should be taken with cause. First, the number of included studies was relatively small; therefore, limited data were available. Second, we were unable to analyze gene–gene and gene–environment interactions, due to the lack of information available in the original studies. Third, other factors such as the age, obesity, life-style that may affect the interaction of GSTM1/GSTT1 gene polymorphism with male infertility could not be analyzed due to the lack of original data.
5. Conclusion
In summary, this meta-analysis provides evidence that the null genotype of GSTT1 may contribute to genetic susceptibility to the risk of male infertility in Chinese population. The null genotype of GSTM1 is associated with risk for OAT, but not NOA in Chinese population. Nevertheless, more large sample and representative population-based cases and well-matched controls are needed to validate our results.
Author contributions
Conceptualization: Chun-Yan Hu, Tao Zhang.
Data curation: Tao Wu.
Methodology: Shu-Lin Cheng.
Software: Dong-Liang Lu, Tao Wu, Tian-tian Wu.
Writing – original draft: Chun-Yan Hu, Dong-Liang Lu.
Writing – review & editing: Tian-tian Wu, Shu Wang, Tao Zhang.
Footnotes
Abbreviations: CBM = China biology medical literature database, CIs = confidence intervals, CNKI = China National Knowledge Infrastructure, GSTs = glutathione-S-transferases, HWE = Hardy-Weinberg equilibrium, NOA = nonobstructive azoospermia, NOS = Newcastle-Ottawa Scale, OAT = oligoasthenozoospermia, ORs = odds ratios, ROS = reactive oxygen species.
C-YH and D-LL contributed equally to this work and should be considered co-first authors.
Funding: There is no funding for this study.
Conflict of interest: The authors have no conflicts of interest to disclose.
References
- [1].Coutton C, Fissore RA, Palermo GD, et al. Male infertility: genetics, mechanism, and therapies. Biomed Res Int 2016;2016:7372362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kamel RM. Management of the infertile couple: an evidence-based protocol. Reprod Biol Endocrinol 2010;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Agarwal A, Mulgund A, Hamada A, et al. A unique view on male infertility around the globe. Reprod Biol Endocrinol 2015;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Poongothai J, Gopenath TS, Manonayaki S. Genetics of human male infertility. Singapore Med J 2009;50:336–47. [PubMed] [Google Scholar]
- [5].Navarro-Costa P, Gonçalves J, Plancha CE. The AZFc region of the Y chromosome: at the crossroads between genetic diversity and male infertility. Hum Reprod Update 2010;16:525–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Li Y, Pan KJ, Wang L, et al. [Association of gr/gr deletion in the AZFc region of Y chromosome with male infertility: a meta-analysis]. Zhonghua Nan Ke Xue 2011;17:546–52. [PubMed] [Google Scholar]
- [7].Lend AK, Belousova A, Haller-Kikkatalo K, et al. Follicle-stimulating hormone receptor gene haplotypes and male infertility in Estonian population and meta-analysis. Syst Biol Reprod Med 2010;56:84–90. [DOI] [PubMed] [Google Scholar]
- [8].Safarinejad MR, Shafiei N, Safarinejad S. The association of glutathione-S-transferase gene polymorphisms (GSTM1, GSTT1, GSTP1) with idiopathic male infertility. J Hum Genet 2010;55:565–70. [DOI] [PubMed] [Google Scholar]
- [9].Jaiswal D, Sah R, Agrawal NK, et al. Combined effect of GSTT1 and GSTM1 polymorphisms on human male infertility in north Indian population. Reprod Sci 2012;19:312–6. [DOI] [PubMed] [Google Scholar]
- [10].Tirumala Vani G, Mukesh N, Siva Prasad B, et al. Role of glutathione S-transferase Mu-1 (GSTM1) polymorphism in oligospermic infertile males. Andrologia 2010;42:213–7. [DOI] [PubMed] [Google Scholar]
- [11].Olshan AF, Luben TJ, Hanley NM, et al. Preliminary examination of polymorphisms of GSTM1, GSTT1, and GSTZ1 in relation to semen quality. Mutat Res 2010;688:41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jancova P, Anzenbacher P, Anzenbacherova E. Phase II drug metabolizing enzymes. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2010;154:103–16. [DOI] [PubMed] [Google Scholar]
- [13].Tew KD, Townsend DM. Glutathione-s-transferases as determinants of cell survival and death. Antioxid Redox Signal 2012;17:1728–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Qi Z-P, Zhao E-J, Li B, et al. Glutathione S-transferase M1 polymorphism and bladder cancer risk: a meta-analysis involving 50 studies. Int J Clin Exp Pathol 2017;10:3209–18. [Google Scholar]
- [15].Xu XB, Liu SR, Ying HQ, et al. Null genotype of GSTM1 and GSTT1 may contribute to susceptibility to male infertility with impaired spermatogenesis in Chinese population. Biomarkers 2013;18:151–4. [DOI] [PubMed] [Google Scholar]
- [16].Salehi Z, Gholizadeh L, Vaziri H, et al. Analysis of GSTM1, GSTT1, and CYP1A1 in idiopathic male infertility. Reprod Sci 2012;19:81–5. [DOI] [PubMed] [Google Scholar]
- [17].Wu W, Lu J, Tang Q, et al. GSTM1 and GSTT1 null polymorphisms and male infertility risk: an updated meta-analysis encompassing 6934 subjects. Sci Rep 2013;3:2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chaimani A, Mavridis D, Salanti G. A hands-on practical tutorial on performing meta-analysis with Stata. Evid Based Ment Health 2014;17:111–6. [DOI] [PubMed] [Google Scholar]
- [19].Cheng Y, Zhu Y, Huang X, et al. Association between TLR2 and TLR4 gene polymorphisms and the susceptibility to inflammatory bowel disease: a meta-analysis. PLoS One 2015;10:e0126803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [21].Xiong DK, Chen HH, Ding XP, et al. Association of polymorphisms in glutathione S-transferase genes (GSTM1, GSTT1, GSTP1) with idiopathic azoospermia or oligospermia in Sichuan, China. Asian J Androl 2015;17:481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chen SS, Chang LS, Chen HW, et al. Polymorphisms of glutathione S-transferase M1 and male infertility in Taiwanese patients with varicocele. Hum Reprod 2002;17:718–25. [DOI] [PubMed] [Google Scholar]
- [23].Tang K, Xue W, Xing Y, et al. Genetic polymorphisms of glutathione S-transferase M1, T1, and P1, and the assessment of oxidative damage in infertile men with varicoceles from northwestern China. J Androl 2012;33:257–63. [DOI] [PubMed] [Google Scholar]
- [24].Wu QF, Xing JP, Tang KF, et al. Genetic polymorphism of glutathione S-transferase T1 gene and susceptibility to idiopathic azoospermia or oligospermia in northwestern China. Asian J Androl 2008;10:266–70. [DOI] [PubMed] [Google Scholar]
- [25].Liu B, Wang X. Analysis the pertinence between the genomic polymorphism of two metabolic enzymes “CYP 1A1” & “GSTM1” and the genetic predisposition of teratospermia which caused by smoking. Changsha: Central South University; 2010. [Google Scholar]
- [26].Zhou Q, Xia Y. Study on the associations between the CYP1A1 and GST gene polymorphisms and male infertility. Jiangsu: Jiangsu University; 2016. [Google Scholar]
- [27].Li C, Ding XP, Fu L, et al. Association between glutathione-S-transferase gene polymorphisms (GSTMI, GSTTl GSTPl) and idiopathic azoospermia [in Chinese]. Chin J Med Genet 2013;30:102–5. [DOI] [PubMed] [Google Scholar]
- [28].Feng Z, Jing Z, Liu H, et al. [Association of SPO11 and GST gene polymorphisms with idiopathic male infertility in ethnic Han Chinese]. Chin J Med Genet 2015;32:866–70. [DOI] [PubMed] [Google Scholar]
- [29].Tang K, Xu S, Zou T, et al. Correlative analysis of between glutathione S-transferase polymorphisms and idiopathic male infertility. Nat J Androl 2014;28:3–7. [Google Scholar]
- [30].Lu J, Wang X. GST gene polymorphisms and idiopathic male factor infertility. Nanjing: Nanjing Medical University; 2013. [Google Scholar]
- [31].Wu QF, Xing JP, Sun JH, et al. [Genetic polymorphism of glutathione S-transferase T1 associated with idiopathic azoospermia and oligospermie]. Zhonghua Nan Ke Xue 2007;13:407–10. [PubMed] [Google Scholar]
- [32].Chen W, Kang X, Wei Y, et al. The research on GSTTl and GSTMl gene polymorphisms of the patients with oligospermous infertility of Zhuang population in Guangxi area. Chin J Immunol 2010;26:425–7. [Google Scholar]
- [33].Benedetti S, Tagliamonte MC, Catalani S, et al. Differences in blood and semen oxidative status in fertile and infertile men, and their relationship with sperm quality. Reprod Biomed Online 2012;25:300–6. [DOI] [PubMed] [Google Scholar]
- [34].de Lamirande E, O’Flaherty C. Sperm activation: role of reactive oxygen species and kinases. Biochim Biophys Acta 2008;1784:106–15. [DOI] [PubMed] [Google Scholar]
- [35].Dusinská M, Ficek A, Horská A, et al. Glutathione S-transferase polymorphisms influence the level of oxidative DNA damage and antioxidant protection in humans. Mutat Res 2001;482:47–55. [DOI] [PubMed] [Google Scholar]
- [36].Tang M, Wang S, Wang W, et al. The glutathione-S-transferase gene polymorphisms (GSTM1 and GSTT1) and idiopathic male infertility risk: a meta-analysis. Gene 2012;511:218–23. [DOI] [PubMed] [Google Scholar]


