Abstract
To investigate the pathological features of metastatic lymph nodes (LN) in pancreatic ductal adenocarcinoma (PDAC) and to determine factors with prognostic implications.
Metastatic LN status is a proven significant factor for predicting postoperative prognosis in pancreatic cancer patients. However, the effective prognostic criteria regarding metastatic LNs for such disease remain unknown.
We retrospectively reviewed 98 patients with R0/1 resection for PDAC. All metastatic LNs were evaluated for the pathomorphological features of metastasis and analyzed in terms of postoperative outcomes. Various morphological patterns of metastasis were assessed in 440 positive LNs and then classified into 4 groups: common type, direct type (continuously invaded by the main tumor), scatter type (multiple tumor clusters among the normal LN tissues), and isolated tumor cell (ITC).
The pathological stage was defined as stage IIA in 10% and IIB in 90% patients. Common-type metastasis was noted in 55% positive LNs of 75% node-positive patients; direct type in 36% LNs of 69% patients; scatter type in 5% LNs of 14% patients; and ITCs in 5% LNs of 18% patients. Significant difference was noted only in recurrence-free survival (RFS) but not in overall survival (OS) in the common-type; only in OS but not in RFS for the scatter type; and neither in RFS nor OS for both direct type and ITC. Multivariate analysis revealed that only LN ratio and curability were independent predictive factors of poor.
The tumor distribution patterns in metastatic LNs are the postoperative prognostic factors in pancreatic cancer.
Keywords: lymph node metastasis, pancreatic cancer, pathomorphological feature, postoperative prognostic factor
1. Introduction
Pancreatic cancer is one of the highly lethal cancers, with a mortality rate of > 90% after 5-years follow-up.[1,2] Approximately 23,000 Japanese die of this disease every year, making it the fourth leading cause of cancer-related deaths in Japan. Recent studies have documented that adjuvant chemotherapy with gemcitabine (GEM) or S-1 can improve RFS as well as OS.[3–5] However, despite curative resection, RFS remains <20%. Therefore, it is important to identify the pathological factors that are associated with recurrence and survival in patients who undergo curative resection for not only prognostication but also potentially guided adjuvant therapy.
LN status is recognized as a significant factor for the determination of therapeutic treatment options or for the prediction of prognosis in pancreatic cancer patients.[6–8] However, the effective prognostic criteria about metastatic LNs in pancreatic cancer remain to be determined.[9] Indeed, the most recent editions of both UICC Classification and General Rules for the Study of Pancreatic Cancer of Japanese Pancreatic Society (JPS7) do not address the histological categorization of LN involved; instead, they address only the number of regional metastatic LNs.
In the present study, we aimed to investigate the pathological features of metastatic LNs in PDAC and to determine the factors with prognostic implications.
2. Materials and methods
With approval from the ethical committee of our institution (approval number: 2867), we conducted this study as a retrospective single-center survey. In total, 258 patients who underwent pancreatic resection between January 2008 and December 2014, including 102 with PDAC, 46 with intraductal papillary mucinous neoplasm, 14 with neuroendocrine neoplasm, 1 with acinar cell tumor, 50 with extrahepatic cholangiocarcinoma, 2 with gallbladder cancer, 22 with ampullary cancer, 1 with duodenal adenocarcinoma, 4 with metastatic pancreatic tumor, and 16 with other diseases, were indicated for surgery. After excluding patients who had undergone incomplete resection (R2) (n = 3) or were diagnosed with para-aortic LN metastasis postoperatively (n = 1), 98 patients were finally analyzed.
A prospectively maintained patient database was used to review the patients’ demographics and perioperative clinicopathological information, including the date of operation, operative procedures (pancreatoduodenectomy (PD), distal pancreatectomy (DP), and total pancreatectomy (TP)), the date of diagnosis of the initial recurrence, and the date of death. The RFS and OS was calculated from the date of surgery until the date of recurrence and death or the date of the last follow-up.
2.1. Operative procedures
For lymphadenectomy, all pancreatic resections for PDAC were conducted with D2 LN dissection. The LN stations in the standard D2 lymphadenectomy were defined according to JPS7. LN dissection in case of PD included LN around the head of the pancreas, along the common hepatic artery, in the lower half of the hepatoduodenal ligament, and on the right side of the superior mesenteric artery (SMA) and celiac axis (CA). The involvement of the portal or superior mesenteric vein was treated with en bloc resection of the vein. The standard procedure of DP with splenectomy included LN dissection along the left aspect of the CA and SMA.
2.2. Assessment of resected specimen and lymph node
The resected specimens were fixed with formalin, sliced into 3-mm thick sections, embedded with paraffin, and stained with hematoxylin and eosin (HE). N-staging in the JPS7 was defined as same as that in the 8th UICC Classification, depending on the number of metastatic regional LNs. Patients without any metastatic LNs were staged as N0 in both the classifications, those with 1 to 3 positive LNs were classified into N1/N1a, and those with more than 4 metastatic LNs were staged into N2/N1b in the 8th UICC Classification/JPS7. The specimens were re-examined for the assessment of the mode of LN metastasis by the first author (M.H.) and an experienced pathologist (S.O.) in all cases. The mode of LN involvement was classified as follows (Fig. 1):
Figure 1.
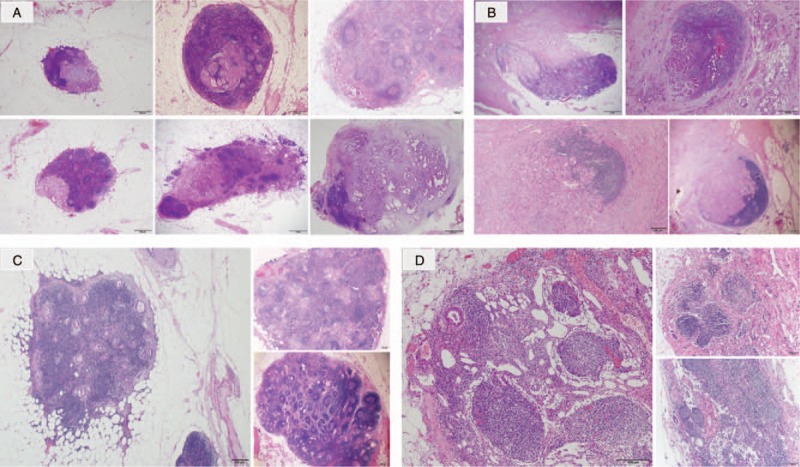
Classification of lymph node (LN) metastasis. (a): Common type, (b): Direct type, (c): Scatter type, (d): Isolated tumor cell (ITC). Common-type metastasis was noted in 55% positive LNs of 75% node-positive patients; direct type in 36% LNs of 69% patients; scatter type in 5% LNs of 14% patients; and ITCs in 5% LNs of 18% patients.
-
1.
the common type—when metastases was present as a partial or total destruction of the normal trabecular structure of the node without continuous extension from the main tumor;
-
2.
the direct type—when the node was adjacent to and invaded by the main tumor;
-
3.
the scatter type—when multiple tumor clusters occurred among the normal trabecular structure of LN; and
-
4.
the isolated tumor cell (ITC) type—in case of an isolated cancer cell foci of <0.2-mm diameter of the nodes.
2.3. Assessment of the survival and statistical analysis
The OSs and RFSs were assessed for all recruited cases. Data were described using their main distribution parameters. The survival rates were studied by the Kaplan–Meier method and compared by the log-rank test. Univariate Cox model was used to calculate the hazard-ratio (HR), and multivariate analysis using Cox proportional hazards regression model was used to identify the independent factors for the OS or RFS. All analyses were conducted by using the JMP Pro version 13.0 (SAS). Non-parametric continuous variables were compared by using the Wilcoxon test, while the nominal variables were compared using Fisher exact test. Statistical significance was set at P < .05. On multivariate analysis, the medians were used as the cut-off values of the number of evaluated LNs and lymph node ratio (LNR).
3. Results
3.1. Clinicopathological background
Forty-nine male and 49 female patients with PDAC were included in this study. The clinicopathological characteristics of the patients are summarized in Table 1. The median age at surgery was 69 years (age range: 42–92 years). The performed operative procedures were PD in 62 patients (63%), DP in 29 patients (30%), and TP in 7 patients (7%). According to the final pathology, 10 patients (10%) had stage IIA disease and 88 (90%) had stage IIB disease. Regarding the grade of metastatic LN, 10 patients (10%) were N0, 43 (44%) were N1, and 45 (46%) were N2. In addition, 73 patients (74%) underwent R0 resection, while 25 (26%) underwent R1 resection. Preoperative neoadjuvant therapy such as GEM, S-1, erlotinib, FOLFOX, or radiation was performed in 15 patients (15%), and postoperative adjuvant therapy such as GEM, S-1 and erlotinib were performed in 70 patients (71%). The median RFS was 374 days (range: 78–2491 days), and the median OS was 778 days (range: 157–2491 days).
Table 1.
Clinicopathologic characteristics of 98 pancreatic cancer patients.
3.2. Status and pattern of LN metastasis
A total of 3225 nodes were examined, and the median number of LNs examined for each patient was 31 (range: 10–77 LNs). Moreover, 90 patients (90%) had a total of 440 positive LNs. The median number of metastatic LNs was 4 (range: 1–19 LNs). The common-type mode was demonstrated by 240 LNs (55% of positive LNs) in 66 patients (75% of node-positive patients). As for the 3 other types of LN metastases, direct type was noted in 157 LNs (36% of positive LNs) of 61 patients (69% of node-positive patients); scatter type in 22 LNs (5% of positive LNs) of 12 patients (14% of node-positive patients), and ITCs in 21 LNs (5% of positive LNs) of 16 patients (18% of node-positive patients).
3.3. Relationship among the pattern of LN metastases and conventional pathological factors
Patients with any of the metastases types showed significantly larger number of positive LNs and higher LNR. The presence of common-type metastasis showed an increased incidence of advanced venous invasion. The patients with the scatter-type metastasis showed a larger tumor and more frequent positive surgical margin. The patients with LNs of the ITCs showed no relationship with any of the other pathological factors (Table 2).
Table 2.
Relationship between T/N factors and metastatic pattern.
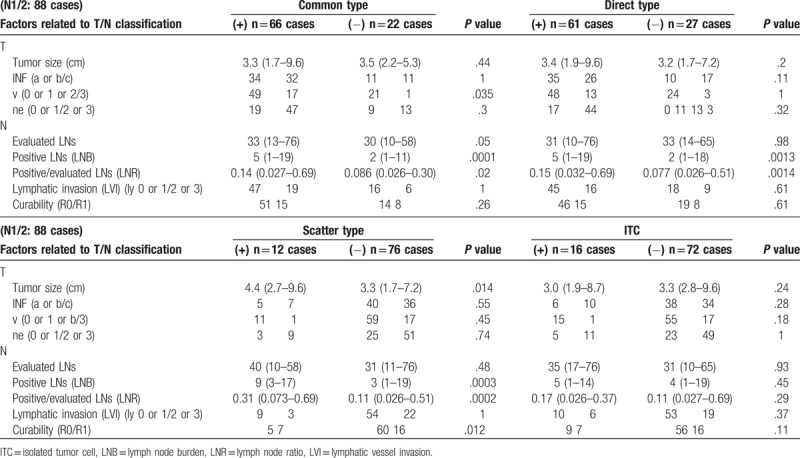
Regarding interrelation among the different types of LN metastasis, the patients with the scatter-type metastasis showed more frequent direct-type metastasis (P = .028) and ITCs (P = .024) than those without it. The common-type metastasis was not interrelated to any of the other types of node metastasis.
3.4. Survival analysis
No in-hospital mortality was noted. The N0 grade patients tended to survive longer than node-positive patients, with significantly less frequent recurrence after surgery (MST: 32.6 vs 24.8 months, P = .14; MRFT: 20.5 vs 11.5 months, P = .0145). The following survival analysis was performed only for patients with LN metastasis. The patients were stratified into groups according to the presence or absence of each morphological type of LN metastasis. The patients with the common-type metastatic nodes showed more frequent recurrence than those without it, albeit without any significant survival difference (Fig. 2a). The presence of scatter-type metastasis had a negative impact on the OS, but not on RFS (Fig. 2c). Neither the direct type nor ITCs showed significant prognostic effect on either RFS or OS (Fig. 2b, d).
Figure 2.

Recurrence-free survival (RFS) and overall survival (OS) in patients with (blue) /without (red) 4 types of lymph node metastasis. Common type (MRFT: 17.6 vs 10.3 months, P = .022; MST: 31.1 vs 21.2 months, P = .45). Direct type (MRFT: 13.4 vs 11 months, P = .96; MST: 28.0 vs 24.7 months, P = .57). Scatter type (MRFT: 12.8 vs 7.1 months, P = .13; MST: 28 vs 9.3 months, P = .0033). Isolated tumor cell (MRFT: 11.9 vs 9.9 months, P = .63; MST: 27 vs 18.1 months, P = .75).
As can be seen in Table 3, the factors tumor size (TS 3 or 4, P = .0036), LNB (≥ 4, P = .0017), LNR (> 0.1076, P < .001), scatter-type LNs (P = .0062), and curability (R1, P < .001) influenced the OS. On multivariate analysis, only LNR (P = .042) and curability (P = .0007) were found to be independent factors of poor OS. LNs metastases of the scatter type were not found to be independent prognostic factors. In the patients with R0 resection, lymph node burden (LNB) (≥ 4, P = .00102) and LNR (> 0.1076, P = .0006) significantly influenced the OS. On multivariate analysis, only LNR (P = .0142) was found to be independent factors of poor OS.
Table 3.
Impact of demographic, surgical and histopathological variables on OS.

4. Discussion
Surgical resection is currently the only curative treatment of pancreatic cancer. Recently, the evaluation of the pathological LN status was proven to be useful when determining the therapy selection or predicting prognosis not only in pancreatic cancer patients but also in other cancer patients. In the breast or colon and rectum cancers, N classifications are subdivided in order to more accurately predict the outcome after the surgery according to the 8th UICC Classification. Meanwhile, effective prognostic significance of LN metastases in PDAC remains un-established, and its N classification is still simple.
In pancreatic cancer, several parameters that specifically address the LN involvement have been reported to be prognostic, such as lymph node disease (LND), lymph node burden (LNB), lymph node examined (LNE), and LNR. LND is defined as the confirmed presence of metastatic tumor cells in more than one LN. LNB represents the total number of pathologically confirmed positive LNs. LNE is the total number of examined LNs. LNR is the ratio of the number of positive nodes to the total number of nodes evaluated,[10] which has been reported to be an effective parameter to further stratify the TNM stage N1 patient population for outcome prediction while simultaneously decreasing the likelihood of understaging and stage migration.[11–13] However, the results reported so far are controversial, warranting additional research before the application of these metrics in the general clinical setting.
Additional parameters related to the pathological features of lymphatic metastatic lesion have been researched and discussed recently in pancreatic cancer.[14–28] In this study, we classified 440 positive LNs into 4 types, according to the pathomorphological features of metastatic foci stained with HE. The common-type metastatic nodes represent cancerous foci, showing partial or total destruction of the normal trabecular structure of a node without the continuous extension from the main tumor. Such patterns are most frequently observed in more than half of the positive nodes examined and in three-fourth of the patients. It thus seems evident that the presence of this node metastasis type has a negative impact on the postoperative survival, because it is strongly correlated with the representative LN-related metrics such as LNB and LNR. More frequent presentation of this type of metastasis in patients with primary tumors manifesting microscopic venous invasion suggests a possibility of such LN metastasis via the blood vessels. In fact, node-positive patients with the common-type metastasis significantly reoccur at the distant sites (such as the lung, liver, or skin) (P = .0298).
Notably, direct-type metastasis, which is the second-most frequent pattern of node metastasis, had no prognostic impact after surgery in the present analysis. Konstantinidis et al[29] were the first to mention direct-invaded LNs in pancreatic cancer with no survival difference for patients with direct versus regional LN invasion. They also reported that node involvement by metastasis or by direct invasion was an equally significant predictor of reduced survival. Unfortunately, the authors limited their analysis to patients with 1 or 2 positive nodes, of whom the isolated direct invasion occurred in only 20% of the patients, and patients who had both direct and regional LN involvement were excluded from further analysis. On the other hand, Pai et al[24] reported that patients with isolated direct-invaded nodes had a comparable OS to those with node-negative primary tumor, but a superior OS to those with metastatic nodes apart from the primary tumor. Their study included 31 cases classified with direct extension into 1 or 2 nodes in the absence of metastatic nodes, apart from the primary tumor. In addition, Williams et al[26] reported that patients with LNs involving direct extension showed similar survival to those with node-negative diseases. However, the low proportion of patients with direct invasion (14 in 385 patients; 3.6%) limited their statistical analysis. On the other hand, Buc et al[16] classified metastatic nodes into 3 types, such as the standard lymphatic metastasis, standard metastasis with extracapsular invasion, and contiguous metastasis from the main tumor (C-type). Their study revealed that the presence of C-type metastasis was predictive of reduced survival. In our study, however, the direct-type metastatic nodes accounted for 36% of all positive LNs (157/440), and 69% of all patients had this metastasis. Moreover, of the 22 node-positive patients without the common-type metastasis, only 14 patients showed directly invaded nodes, 4 showed ITCs, and another 4 showed directly invaded nodes or scatter types or ITCs.
According to the 8th UICC Classification, the direct extension of the primary tumor into LNs is classified as the LN metastasis. The 14 node-positive patients with only direct-type metastasis had 34 metastatic nodes with a median number of 2 positive nodes (range: 1–7 nodes). Similar to that reported by Pai et al,[24] our patients with exclusively the direct-type metastatic nodes showed a comparable OS and RFS to the node-negative patients (data not shown). Direct invasion was not a prognostic factor for the node-positive patients. Because the pancreas arises and organizes in the embryonic mesentery, almost all peripancreatic nodes about the pancreas parenchyma. Thus, the direct invasion may frequently occur irrespective of the metastatic ability of the tumor. Overall, a large multi-institutional study on the direct invasion is warranted considering the infrequency of direct LN extension and the conflicting findings reported by smaller studies, such as the present study.
The scatter-type metastasis is an uncommon metastatic mode of distinct pathomorphology. A node with such a metastatic pattern includes numerous scattered foci of tumor clusters that retain the normal trabecular structure of LN, unlike in the common types. The metastatic lesions of the scatter-type LNs were distributed separately without desmoplastic reaction. Although the nodes presenting with the scatter patterns accounted for only 5% of the positive nodes and were detected in 14% of the node-positive patients, the presence of such metastatic nodes has an apparent negative impact on the OS among node-positive patients. As for the recurrence, such a negative effect did not reach significance due to its small number (when compared with the Wilcoxon test results, the median RFS was 7.1 months in the scatter-type group and 12.8 months in the other groups, reaching a statistical significance at P = .022). As assumed from the fact that the tumor size and curability of pancreatic resection are significantly larger and higher, respectively, in patients with the scatter-type metastasis, such pattern of LN metastasis may indicate the aggressive feature of pancreatic cancer. Further studies would be necessary to clarify the significance of this type of node metastasis in pancreatic cancer and also to determine the factors that induce such pathological features.
Regarding the extremely small metastatic deposits in the LN of the pancreatic cancer, several studies define “micrometastasis” as a single cell or a small clusters of tumor cells in LNs that remain undetected by the routine histopathological staining techniques, but can be detected by immunohistochemical (IHC) or molecular techniques such as epithelial cell adhesion molecule (EpCAM/Ber-EP4),[7,14,23] cytokeratin staining,[18,19,21] CK-19 staining,[20,22] and polymerase chain reaction for mutant K-RAS[15,25,27] (Table 4). On the other hand, the UICC TNM Classification defines “micrometastasis” as a lesion of size 0.2 mm to 0.2 cm and an ITC as a lesion of size < 0.2 mm. We meticulously evaluated the HE-stained tissue sections for tiny LNs in routine testing and found numerous lesions in the size range of 0.2 mm to 0.2 cm. The number of examined nodes per patient was approximately 1.5–2-times more than that of other series, and the ratios of N0 grade patients was only 10% as compared to 21% to 53% reported in previous studies (Table 4). In the present study, we followed the UICC definition and addressed ITC as cancerous cell cluster of size < 0.2 mm.
Table 4.
Summary of the publications (“micrometastasis” defined by IHC, excluding studies of paraaortic LN metastasis alone).
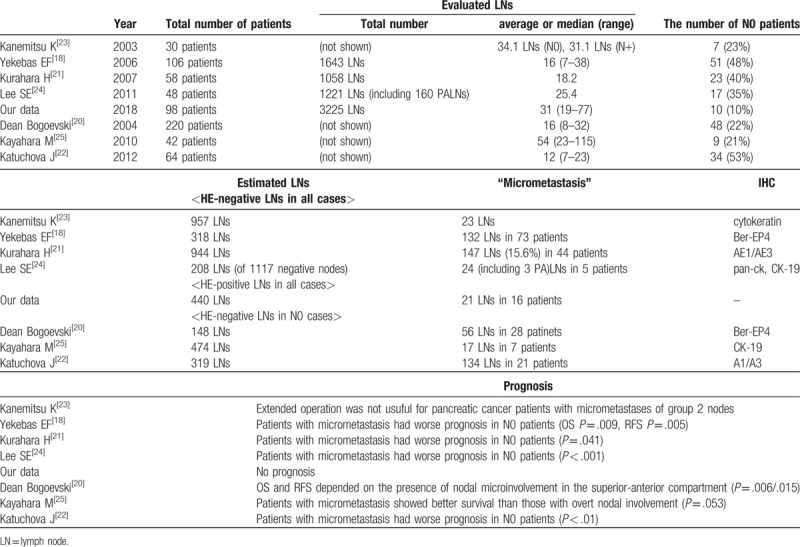
As for the prognosis, Choi et al reported that the presence of IHC/molecular-identified metastasis of LN is associated with poorer survival and is applicable to stratify the risk of recurrence and the need for adjuvant therapy in post-resection patients with pancreatic adenocarcinoma in the conventional HE LN-negative patients.[17] Previous studies have reported that patients without overt and IHC/molecular-identified metastasis of LNs have better prognosis than those with only such metastasis.[19,21,22,28] In our study, ITCs were detected in 18% of the node-positive patients, with no significant prognostic impact on either DFS or OS in node-positive patients (Fig. 2b). In the present series, the numbers of patients with only ITCs were too few (n = 4) to compare with patients without LN metastasis. As for breast cancer, Houvenaeghel et al reported that patients with micrometastases show increased RFS and shorter OS when axillary LN dissection (ALND) is not performed.[30] However, the general consensus today is that ALND can be safely omitted in patients with micrometastases or ITCs in sentinel LNs, given that appropriate adjuvant therapy is undertaken.[31,32] Until date, no study has reported about the size of LN metastasis in pancreatic cancer. We found that the metastatic lesions in the nodes of size 0.2 mm to 0.2 cm were quite frequent.
This study was limited by its retrospective design; its performance at a single center; and the small number of patients enrolled. A larger collective study on this research subject is needed to evaluate the prognostic effects of the discussed metrics about LN metastasis, with due consideration to the contradictory conclusions of previous smaller studies.
In conclusion, the tumor distribution pattern in metastatic LNs could act as predictors for postoperative prognosis in pancreatic cancer. Elaborate investigation in the future will possibly elucidate the prognostic importance of pathomorphological LN features.
Author contributions
Conceptualization: Mayumi Hoshikawa, Sho Ogata, Hideki Ueno, Junji Yamamoto.
Data curation: Mayumi Hoshikawa, Sho Ogata, Makoto Nishikawa, Akifumi Kimura, Takahiro Einama, Takuji Noro, Suefumi Aosasa, Kazuo Hase, Hironori Tsujimoto, Hideki Ueno, Junji Yamamoto.
Formal analysis: Mayumi Hoshikawa, Hideki Ueno, Junji Yamamoto.
Investigation: Mayumi Hoshikawa, Sho Ogata, Makoto Nishikawa, Akifumi Kimura, Takahiro Einama, Takuji Noro, Suefumi Aosasa, Kazuo Hase, Hironori Tsujimoto, Hideki Ueno, Junji Yamamoto.
Methodology: Mayumi Hoshikawa, Sho Ogata, Hideki Ueno, Junji Yamamoto.
Project administration: Mayumi Hoshikawa, Sho Ogata, Hideki Ueno, Junji Yamamoto.
Supervision: Hideki Ueno, Junji Yamamoto.
Validation: Junji Yamamoto.
Visualization: Sho Ogata.
Writing – original draft: Mayumi Hoshikawa.
Writing – review & editing: Hideki Ueno, Junji Yamamoto.
Footnotes
Abbreviations: DP = distal pancreatectomy, ITC = isolated tumor cell, LN = lymph node, LNB = lymph node burden, LND = lymph node disease, LNE = lymph node examined, LNR = lymph node ratio, PD = pancreatoduodenectomy, PDAC = pancreatic ductal adenocarcinoma, TP = total pancreatectomy.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin 2008;58:71–96. [DOI] [PubMed] [Google Scholar]
- [2].Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet (London, England) 2004;363:1049–57. [DOI] [PubMed] [Google Scholar]
- [3].Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267–77. [DOI] [PubMed] [Google Scholar]
- [4].Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473–81. [DOI] [PubMed] [Google Scholar]
- [5].Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet (London, England) 2016;388:248–57. [DOI] [PubMed] [Google Scholar]
- [6].Liu Z, Luo G, Guo M, et al. Lymph node status predicts the benefit of adjuvant chemoradiotherapy for patients with resected pancreatic cancer. Pancreatology 2015;15:253–8. [DOI] [PubMed] [Google Scholar]
- [7].Nathanson SD, Shah R, Rosso K. Sentinel lymph node metastases in cancer: causes, detection and their role in disease progression. Semin Cell Dev Biol 2015;38:106–16. [DOI] [PubMed] [Google Scholar]
- [8].Kawada K, Taketo MM. Significance and mechanism of lymph node metastasis in cancer progression. Cancer Res 2011;71:1214–8. [DOI] [PubMed] [Google Scholar]
- [9].Fink DM, Steele MM, Hollingsworth MA. The lymphatic system and pancreatic cancer. Cancer Lett 2016;381:217–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].John BJ, Naik P, Ironside A, et al. Redefining the R1 resection for pancreatic ductal adenocarcinoma: tumour lymph nodal burden and lymph node ratio are the only prognostic factors associated with survival. HPB (Oxford) 2013;15:674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Berger AC, Watson JC, Ross EA, et al. The metastatic/examined lymph node ratio is an important prognostic factor after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg 2004;70:235–40. discussion 240. [PubMed] [Google Scholar]
- [12].Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol 2008;15:165–74. [DOI] [PubMed] [Google Scholar]
- [13].Pawlik TM, Gleisner AL, Cameron JL, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery 2007;141:610–8. [DOI] [PubMed] [Google Scholar]
- [14].Pai RK, Beck AH, Mitchem J, et al. Pattern of lymph node involvement and prognosis in pancreatic adenocarcinoma: direct lymph node invasion has similar survival to node-negative disease. Am J Surg Pathol 2011;35:228–34. [DOI] [PubMed] [Google Scholar]
- [15].Williams JL, Nguyen AH, Rochefort M, et al. Pancreatic cancer patients with lymph node involvement by direct tumor extension have similar survival to those with node-negative disease. J Surg Oncol 2015;112:396–402. [DOI] [PubMed] [Google Scholar]
- [16].Buc E, Couvelard A, Kwiatkowski F, et al. Adenocarcinoma of the pancreas: does prognosis depend on mode of lymph node invasion? Eur J Surg Oncol 2014;40:1578–85. [DOI] [PubMed] [Google Scholar]
- [17].Yekebas EF, Bogoevski D, Bubenheim M, et al. Strong prognostic value of nodal and bone marrow micro-involvement in patients with pancreatic ductal carcinoma receiving no adjuvant chemotherapy. World J Gastroenterol 2006;12:6515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Milsmann C, Fuzesi L, Werner C, et al. Significance of occult lymphatic tumor spread in pancreatic cancer. Chirurg 2005;76:1064–72. [DOI] [PubMed] [Google Scholar]
- [19].Bogoevski D, Yekebas EF, Schurr P, et al. Mode of spread in the early phase of lymphatic metastasis in pancreatic ductal adenocarcinoma: prognostic significance of nodal microinvolvement. Ann Surg 2004;240:993–1000. discussion 1000-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kurahara H, Takao S, Maemura K, et al. Impact of lymph node micrometastasis in patients with pancreatic head cancer. World J Surg 2007;31:483–90. discussion 491-482. [DOI] [PubMed] [Google Scholar]
- [21].Katuchova J, Bober J, Katuch V, et al. Significance of lymph node micrometastasis in pancreatic cancer patients. Eur Surg Res 2012;48:10–5. [DOI] [PubMed] [Google Scholar]
- [22].Kanemitsu K, Hiraoka T, Tsuji T, et al. Implication of micrometastases of lymph nodes in patients with extended operation for pancreatic cancer. Pancreas 2003;26:315–21. [DOI] [PubMed] [Google Scholar]
- [23].Lee SE, Jang JY, Kim MA, et al. Clinical implications of immunohistochemically demonstrated lymph node micrometastasis in resectable pancreatic cancer. J Korean Med Sci 2011;26:881–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kayahara M, Funaki K, Tajima H, et al. Surgical implication of micrometastasis for pancreatic cancer. Pancreas 2010;39:884–8. [DOI] [PubMed] [Google Scholar]
- [25].Tamagawa E, Ueda M, Takahashi S, et al. Pancreatic lymph nodal and plexus micrometastases detected by enriched polymerase chain reaction and nonradioisotopic single-strand conformation polymorphism analysis: a new predictive factor for recurrent pancreatic carcinoma. Clin Cancer Res 1997;3:2143–9. [PubMed] [Google Scholar]
- [26].Yamada T, Nakamori S, Ohzato H, et al. Outcome of pancreatic cancer patients based on genetic lymph node staging. Int J Oncol 2000;16:1165–71. [DOI] [PubMed] [Google Scholar]
- [27].Brown HM, Ahrendt SA, Komorowski RA, et al. Immunohistochemistry and molecular detection of nodal micrometastases in pancreatic cancer. J Surg Res 2001;95:141–6. [DOI] [PubMed] [Google Scholar]
- [28].Choi SB, Han HJ, Park P, et al. Systematic review of the clinical significance of lymph node micrometastases of pancreatic adenocarcinoma following surgical resection. Pancreatology 2017;17:342–9. [DOI] [PubMed] [Google Scholar]
- [29].Konstantinidis IT, Deshpande V, Zheng H, et al. Does the mechanism of lymph node invasion affect survival in patients with pancreatic ductal adenocarcinoma? J Gastrointest Surg 2010;14:261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Houvenaeghel G, Boher JM, Reyal F, et al. Impact of completion axillary lymph node dissection in patients with breast cancer and isolated tumour cells or micrometastases in sentinel nodes. Eur J Cancer 2016;67:106–18. [DOI] [PubMed] [Google Scholar]
- [31].Yi M, Giordano SH, Meric-Bernstam F, et al. Trends in and outcomes from sentinel lymph node biopsy (SLNB) alone vs SLNB with axillary lymph node dissection for node-positive breast cancer patients: experience from the SEER database. Ann Surg Oncol 2010;17Suppl 3:343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol 2013;14:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]


