Abstract
Cardiac surgery under cardiopulmonary bypass (CPB) accounts for most consumption of red blood cells (RBCs). Identifying risk factors for massive red blood cell transfusion (MRT) in cardiac surgery may help to reduce this consumption.
We retrospectively analyzed 8238 patients who underwent valve surgery and/or coronary artery bypass grafting (CABG) under CPB at 2 major heart centers in China. Uni- and multivariate logistic regression was carried out to assess whether risk factors for MRT (defined as receiving at least 4 units RBCs) varied with type of cardiac surgery.
A total of 1691 patients (21%) received at least 4 units RBCs (6.77 ± 4.78 units per person). This MRT group consumed 70% of the total units of allogeneic RBCs in the study. MRT incidence was 2-fold higher among patients undergoing CABG with or without valve surgery than among patients undergoing valve surgery alone. Multivariate logistic analysis identified the following MRT risk factors common to valve surgery alone, CABG alone, and their combination: female sex, older age, renal dysfunction, lower body mass index, lower preoperative hemoglobin, and longer CPB. Several independent MRT risk factors were also identified specific to valve surgery: active endocarditis, nonatrial fibrillation, smaller left atrium diameter, abnormal international normalized ratio, and repeat surgery.
Different types of cardiac surgery share several, but not all, MRT risk factors. This study may help guide the prediction and management of patients at higher MRT risk.
Keywords: cardiac surgery, cardiopulmonary bypass, massive red blood cells transfusion, risk factor
1. Introduction
Cardiac surgery under cardiopulmonary bypass (CPB) is a major treatment for heart disease.[1] Around the world, 1 to 1.25 million cardiac surgeries are performed annually,[2] and 20% to 60% of patients receive blood transfusions in hospital.[3–5] In fact, transfusions during cardiac surgeries account for approximately 80% of transfusions for all types of surgery combined,[6] corresponding to 10% to 20% of the national supply of blood products.[7]
Among patients receiving transfusions during cardiac surgery, 14.4% of those receiving so-called massive red blood cell transfusion (MRT) consume 63% of the total transfusions for this type of surgery.[8] MRT exacerbates imbalances between blood supply and demand, and it is associated with dose-dependent increases in mortality and morbidity.[8–11] Among patients receiving MRT, 54% experience at least 1 complication,[12] and 30-day mortality can be as high as 17% or 21.5%.[8,12] Reducing MRT in cardiac surgery can substantially contribute to reducing demand for blood products and improving surgical outcomes.
Studies of red blood cell (RBC) transfusion in general have identified several risk factors[12,13] that include older age, lower body mass index (BMI), female sex, lower preoperative hemoglobin, renal dysfunction, coagulation disorder, endocarditis, operation type, and CPB duration.[3,14–22] Much less, however, is known about the risk factors specifically for MRT, and how these risk factors depend on the type of cardiac surgery.[17,19,23] Risk factors are expected to vary among surgery types because different heart diseases are associated with different pathological changes.[24]
The present retrospective study was undertaken at 2 large heart centers in China in an attempt to identify MRT risk factors in patients with valve disease or coronary heart disease.
2. Methods
2.1. Patients and study design
This retrospective study included patients at least 18 years old with valve disease and/or coronary artery disease who underwent valve surgery and/or coronary artery bypass grafting (CABG) involving CPB at West China Hospital of Sichuan University between January 1, 2011 and June 30, 2017, or at the Second Affiliated Hospital in the School of Medicine of Zhejiang University between September 1, 2013 and June 30, 2017. Patients were excluded from the study if they underwent ascending aortic replacement surgery, cardiac tumor resection, or emergency surgery; they died on the surgery table or within 24 hours after surgery; or their medical records were incomplete.
Medical records were collected from October 15, 2017 to December 20, 2017 at West China Hospital of Sichuan University, and from November 4, 2017 to December 15, 2017 at the Second Affiliated Hospital in the School of Medicine of Zhejiang University. This study was conducted between July 1, 2017 and December 20, 2017, after being approved by the Ethics Committees at West China Hospital (256/2017) and the Second Affiliated Hospital (096/2017). Both committees waived the requirement for individual informed consent because of the retrospective study design. Data that might identify individual patients were removed during analysis.
2.2. Data collection
Data were collected on as many variables as possible that might be related to MRT, based on previous reports.[3,14–22] Data were collected from the Hospital Information System, Laboratory Information System, and the Transfusion System at West China Hospital, as well as from the Electronic Medical Record System and “Do Care” System at the Second Affiliated Hospital. We collected data on patient demographics (sex, age, ethnicity, BMI, history of smoking, and alcohol consumption), perioperative variables and variables previously associated with RBC transfusion during cardiac surgery. The following perioperative data were collected: New York Heart Association class, preoperative comorbidities (hypertension, hyperlipemia, coronary heart disease, myocardial infarction, active endocarditis, atrial fibrillation, history of heart failure, peripheral vascular disease, pulmonary insufficiency, transient ischemic attack, stroke, diabetes mellitus, thyroidism disorder, renal dysfunction, hepatic insufficiency, gastrointestinal hemorrhage), history of medications (β-blockers, antiplatelet drugs, anticoagulants, digitoxin, diuretics), preoperative laboratory tests (routine blood tests, blood biochemistry, coagulation examinations, pulmonary function, cardiac ultrasonography), and surgical characteristics (CPB duration, repeat surgery, operative procedures).
2.3. Anesthesia and CPB
Anesthesia and CPB were performed using procedures standard at the 2 institutions.[25,26] Briefly, anesthesia was induced with midazolam, sufentanil, and a muscle relaxant, which was usually etomidate at the Second Affiliated Hospital. Anesthesia was maintained with a continuous infusion of remifentanil, inhalation of sevoflurane, intermittent muscle relaxant, and sufentanil. During CPB, nasopharyngeal temperature was maintained at 30°C to 33°C, and moderate hemodilution was used. All patients received an initial heparin dose of 375 U/kg to achieve systemic anticoagulation, and additional heparin was intermittently injected to maintain activated clotting time longer than 480 seconds during CPB. After weaning from CPB, heparin was neutralized with protamine in a 1:1 ratio with the initial heparin dose.
Cell Saver was routinely administered to all patients. During CPB, pericardial blood was salvaged by suction and returned to the CPB circuit. After CPB, residual blood was collected in a bag containing sodium citrate, neutralized by protamine, and transfused into the patient as described.[26,27]
2.4. Red blood cell transfusion
Transfusions were carried out similarly at the 2 hospitals. Transfusion was performed if hemoglobin concentration was <7 g/dL during CPB, <8 g/dL during surgery or <9.5 g/dL in the intensive care unit. Hemoglobin concentration was determined by blood gas analysis using a Cobas b 123 device (Roche, Basel, Switzerland) at West China Hospital or a Cobas b 221 device (Roche) at the Second Affiliated Hospital.
3. Statistical analysis
Categorical variables were expressed as frequencies (percentages), and compared between patient groups using the chi-squared or Fisher exact tests. Shapiro-Wilk tests were used to determine the distribution of continuous variables. Normally distributed data were reported as mean ± standard deviation and compared between groups using Student t test. Skewed data were expressed as median (interquartile range) and compared between groups using Wilcoxon rank sum tests.
In the present study, MRT was defined as receiving at least 4 units of RBCs during hospitalization. The minimum number of RBCs in MRT has been variously set at 4,[3] 5,[17] 6,[9] or 8,[28] and we opted for 4 as our cut-off because morbidity sharply increases beyond this level,[8] and postoperative complications even increase 19.4% (P < .01) when the patients receive ≥4 units of RBCs.[29]
MRT risk factors were identified by first conducting univariate logistic regression. Variables associated with P < .05 in bivariate analysis were considered statistically significant and were included in logistic regression involving backward selection. Variables associated with P < .1 were then included in multiple logistic regression, which also involved variables associated with P < .05 in univariate logistic regression. The following variables were considered potential confounding factors: sex, age, BMI, active endocarditis, atrial fibrillation, hemoglobin, abnormal international normalized ratio (INR), renal dysfunction, left atrium diameter, CPB duration, and operative procedure.
SPSS 20.0 (IBM, Chicago, IL) was used for statistical analysis. All P values were 2 sided, and P < .05 was considered statistically significant. Missing values were addressed by multiple imputation.
4. Results
A total of 8521 adult patients with cardiac disease who underwent cardiac surgery at the 2 sites during the study period were screened. A total of 283 patients were excluded because their medical records were incomplete (n = 32); they died within 24 hours after operation (n = 19); or they underwent ascending aortic replacement surgery (n = 131), cardiac tumor resection (n = 91), or emergency surgery (n = 10) (Fig. 1). In the end, 8238 patients were included in the analysis, of whom 7287 (88.5%) underwent valve surgery, 800 (9.7%) CABG, and 151 (1.8%) the combination of CABG and valve surgery.
Figure 1.
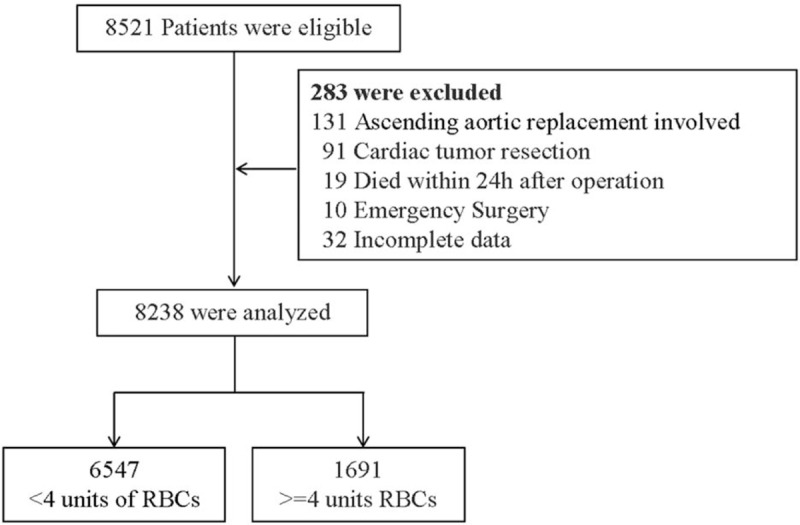
Flow diagram of the study. A total of 8521 adult patients with cardiac disease who underwent cardiac surgery were screened. A total of 283 patients were excluded and 8238 were included in the analysis, of whom 6547 received <4 and 1691 received ≥4 units of red blood cells (RBCs).
No patient in our study received preoperative RBC transfusion, and 4322 (52.5%) patients did not receive any transfusion during hospitalization. The remaining 3916 (47.5%) received a total of 16,414 RBC units, with each patient receiving an average of 4.19 units. Therefore MRT was defined as transfusion involving at least 4 units, similar to thresholds in previous reports.[3,28] Based on this threshold, 1691 (21%) patients in our study received MRT (6.77 ± 4.78 units per person), which accounted for 70% of all units of allogeneic RBCs transfused in our study population. The remaining 6547 patients (79%) received the remaining 30% of the total units (0.76 ± 1.13 units per person; Fig. 2). The demographic, medical, and perioperative data for patients who received MRT or not are summarized in Table 1.
Figure 2.
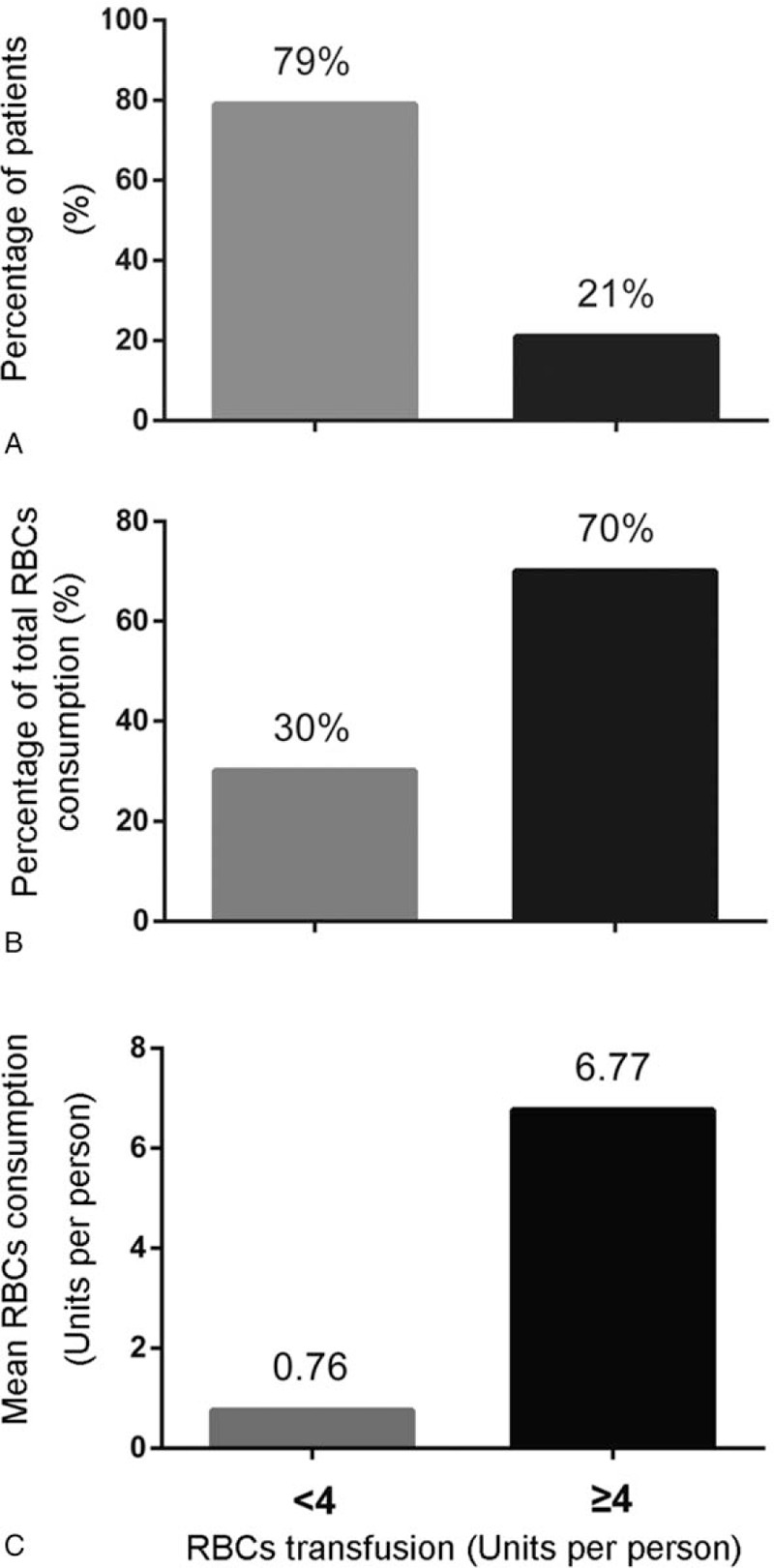
The distribution of red blood cells (RBCs) during hospitalization. Of all study participants, 21% had massive red blood cell transfusion (MRT) (A), and this subgroup accounted for 70% of all units of allogeneic RBCs consumed (B). Mean RBC consumption per person was nearly 10-fold higher among patients receiving MRT than among other patients (C).
Table 1.
Demographic and perioperative characteristics of patients.
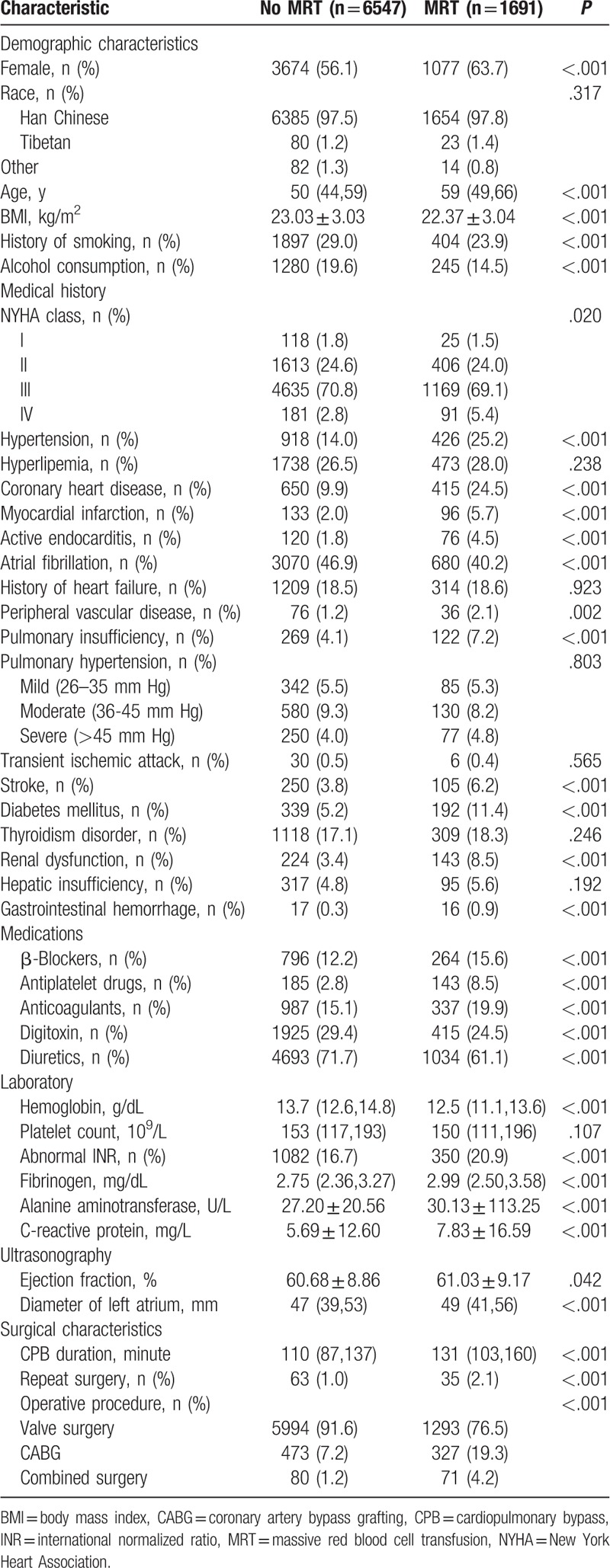
The MRT group showed a greater proportion of women than the non-MRT group (36.3% vs 43.9%), lower proportions of smokers and alcohol drinkers, lower BMI (22.37 ± 3.04 vs 23.03 ± 3.03 kg/m2), and older age (59 vs 50 years). The MRT group also showed higher incidence of comorbidity, including hypertension, coronary heart disease, myocardial infarction, active endocarditis, pulmonary insufficiency, stroke, diabetes mellitus, renal dysfunction, and gastrointestinal hemorrhage. The MRT group showed higher incidence of the use of β-blockers, antiplatelet drugs, and anticoagulants, but lower incidence of the use of digitoxin and diuretics. The MRT group showed lower preoperative hemoglobin levels, higher levels of fibrinogen and C-reactive protein, and higher incidence of abnormal INR. MRT patients also showed smaller left atrium diameter, higher ejection fraction, longer CPB, and higher rates of repeat surgery, CABG surgery, and the combination of valve and CABG surgery (Table 1).
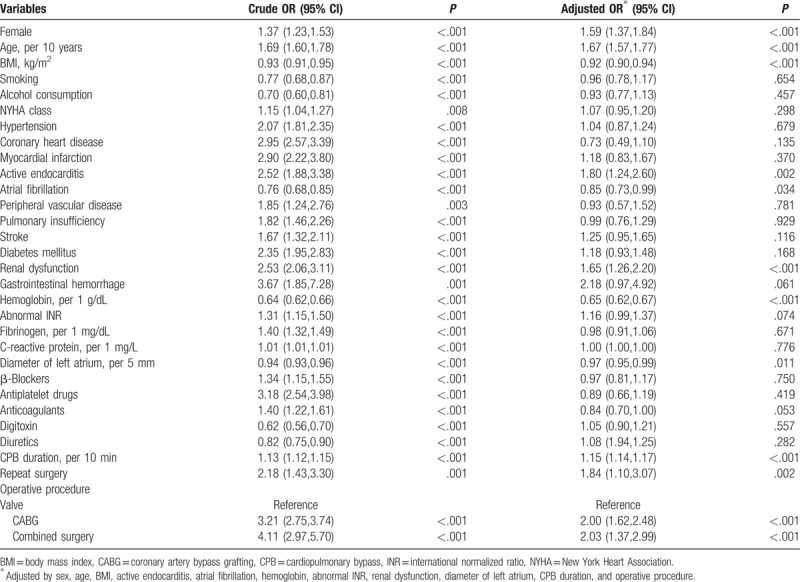
Univariate regression identified 30 variables associated with MRT, and 11 remained significant after adjusting by sex, age, BMI, active endocarditis, atrial fibrillation, preoperative hemoglobin level, abnormal INR, renal dysfunction, diameter of left atrium, CPB duration, and operative procedures (Table 2).
Table 2.
Risk factors of massive red blood cell transfusion.
MRT incidence increased with increasing age (Fig. 3A). Incidence was below 20% among patients younger than 60 years, but it increased sharply from 17% among patients younger than 60 to 31% among those aged 60 to 69. It again increased sharply from 31% among patients aged 60 to 69 to 52% among those at least 70 years old.
Figure 3.
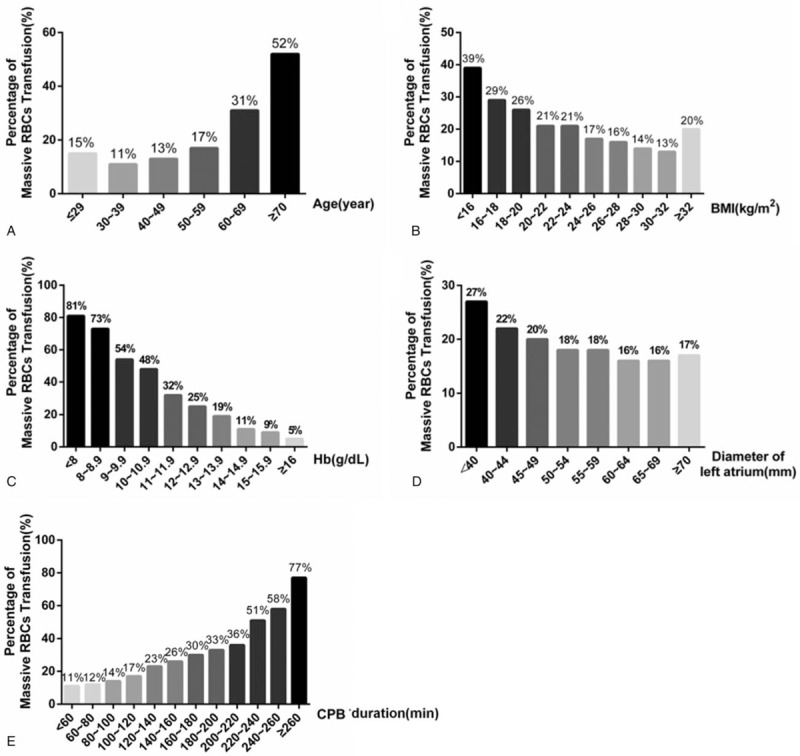
Effect of (continuous) risk factors on massive red blood cell transfusion (MRT). Incidence of (MRT) increased with older age (A), lower body mass index (BMI) (B), lower preoperative hemoglobin (Hb) level (C), smaller left atrium diameter (D), and longer cardiopulmonary bypass (CPB) (E).
MRT incidence generally decreased with increasing BMI, similar to previous reports.[17] MRT incidence, however, increased with BMI at BMIs >32 kg/m2 (Fig. 3B).
Lower preoperative hemoglobin level was associated with increased MRT incidence, as reported previously.[17] Incidence of MRT increased from 2% at 16 g/dL hemoglobin to 7% at 11 g/dL hemoglobin, with a stepwise increase occurring for each 1-g/dL decrease in hemoglobin level. A large jump in MRT incidence occurred when hemoglobin level decreased from 11 (32%) to 10 g/dL (48%). A second large jump in MRT incidence occurred from 9 (54%) to 8 g/dL (73%). Among patients with hemoglobin levels <8 g/dL, 81% received MRT (Fig. 3C).
Larger left atrium diameter was associated with lower MRT risk. MRT incidence fell approximately 2% with each 5-mm increase in diameter, but this effect disappeared at diameters >60 mm (Fig. 3D).
Each 20-minute prolongation of CPB increased MRT incidence by 1% to 5%. MRT incidence increased sharply from 36% to 51% when CPB duration exceeded 220 minutes. The incidence increased again sharply from 58% to 77% when it exceeded 260 minutes (Fig. 3E).
MRT incidence was 5% lower among men than women (Fig. 4A), and it was 5% lower among patients with atrial fibrillation than among those without it (Fig. 4D). MRT incidence was nearly 2-fold higher among patients with renal dysfunction (Fig. 4B) or active endocarditis (Fig. 4C) than among those without these conditions. MRT incidence was significantly higher among those undergoing repeat surgery (26%) than among those undergoing primary surgery (20%, P < .001; Fig. 4F).
Figure 4.
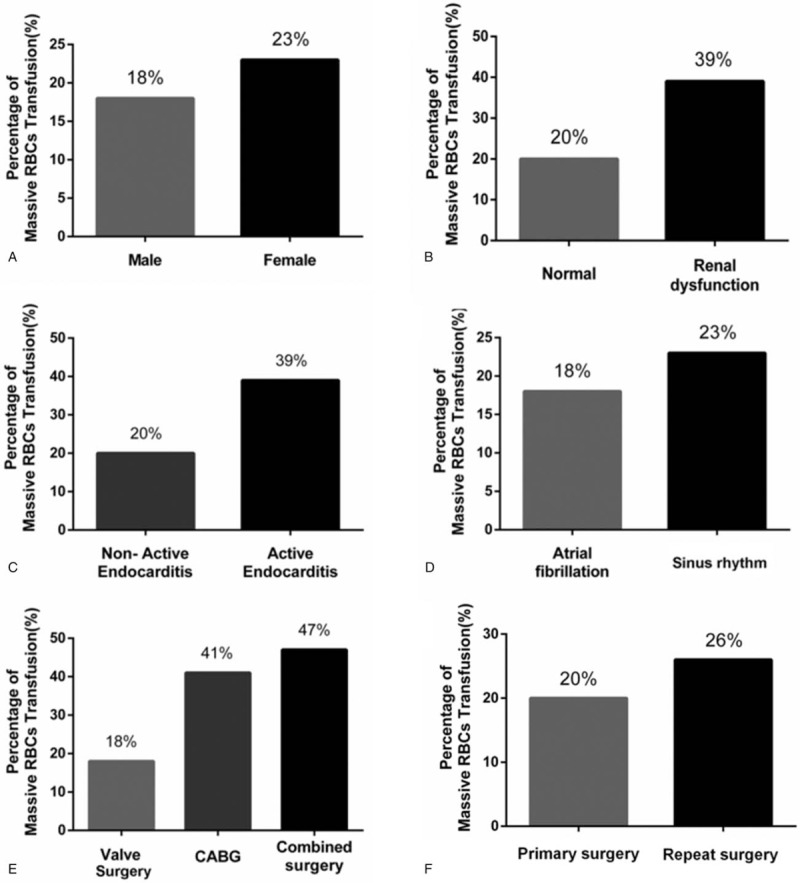
Effect of (categorical) risk factors on massive red blood cell transfusion (MRT). Incidence of MRT was 5% lower among men than women (A) and 5% lower among patients with atrial fibrillation than among those without it (B). MRT incidence nearly doubled in the presence of renal dysfunction (C), in the presence of active endocarditis (D), or when the surgery involved coronary artery bypass grafting (CABG) or the combination of CABG and valve surgery (E). MRT incidence was higher among patients undergoing repeat surgery than among those undergoing primary surgery (F).
MRT incidence was similar between patients undergoing CABG surgery and those undergoing the combination of CABG and valve surgery. This incidence was more than 2-fold the incidence among patients undergoing valve surgery alone (Fig. 4E).
Because MRT incidence was similar between patients undergoing CABG or combination surgery, these 2 groups were merged and then compared with the group of patients undergoing valve surgery alone. This comparison identified the following independent risk factors for MRT, independent of the type of cardiac surgery: female sex, older age, renal dysfunction, lower BMI, lower preoperative hemoglobin, and longer CBP. In addition, this comparison identified the following MRT risk factors specific to valve surgery: active endocarditis, nonatrial fibrillation, smaller left atrium diameter, abnormal INR, and repeat surgery (Fig. 5).
Figure 5.
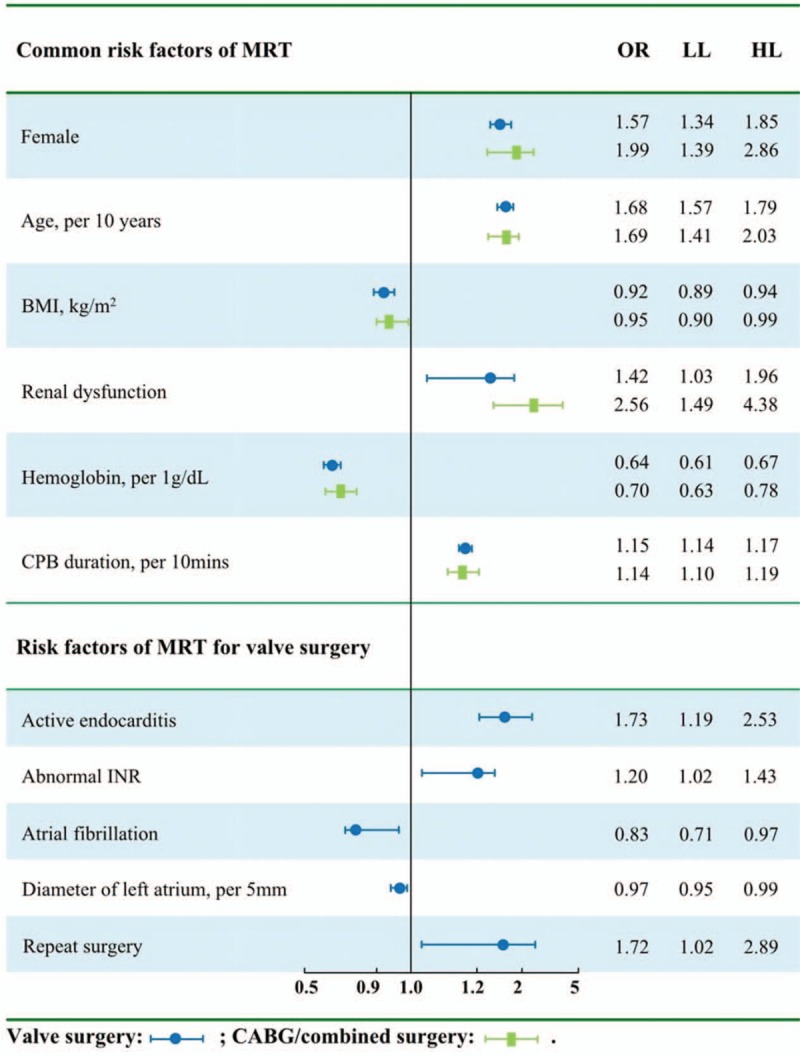
Subgroup analysis for risk factors of MRT. Six risk factors of massive red blood cell transfusion (MRT) were found to be shared between patients undergoing valve surgery alone and patients undergoing coronary artery bypass grafting (CABG) or combined surgery. Five risk factors were found to be specific to patients undergoing valve surgery.
5. Discussion
In this large study of patients undergoing cardiac surgery, 21% of patients received MRT and accounted for 70% of the total units of allogeneic RBCs consumed. These findings provide strong support for the idea that reducing MRT can contribute substantially to reductions in overall demand on blood products. The large, multisite nature of our study, combined with our use of an MRT threshold similar to that in previous work,[3,28] suggests that our results can be generalized to many medical centers. In order to help predict and manage patients at higher risk of MRT, we analyzed MRT risk factors in patients undergoing different types of cardiac surgery. We found that several, but not all, risk factors depended on the type of surgery.
MRT risk factors differed between valve disease and coronary heart disease. Although MRT incidence was 2-fold higher in CABG surgery than in valve surgery, patients in the 2 groups shared 6 risk factors: female sex, older age, renal dysfunction, lower BMI, lower preoperative hemoglobin level, and longer CPB. These risk factors have been reported previously.[17,30] We further identified MRT risk factors that may be specific to patients with valve disease: active endocarditis, nonatrial fibrillation, smaller left atrium diameter, abnormal INR, and repeat surgery.
We found that MRT incidence was 2-fold higher among patients who underwent CABG or combination surgery than among those who underwent isolated valve surgery. This suggests an association between coronary heart disease and higher MRT risk.[23] This may reflect greater blood volume in patients with valve disease, in whom congestion is common.[36] It may also reflect that compared to patients undergoing valve surgery alone, those undergoing CABG or combined surgery experience longer CPB (140 ± 49 vs 117 ± 41 minutes, P < .001) and tend to be older (62 ± 9 vs 51 ± 11 year, P < .001).
The differences and overlap in MRT risk factors between surgical groups in our study may be influenced by the fact that 88.5% of our patients underwent valve surgery. Therefore our results should be confirmed in larger studies with similarly large numbers in different surgical groups. Even so, the present results make a strong case that MRT risk factors should be assessed based on the heart disease involved.
Regardless of the type of cardiac surgery, MRT risk was higher among women in our study than among men, as reported previously.[3] Similarly, older age was an independent risk factor of MRT in our study, as in previous work.[16,17,30] This may reflect the lower blood volume[31] and higher incidence of comorbidities among older patients.[33] We found a 68% increase in MRT risk for each 10-year increase in age, even higher than the 22% increase reported previously.[17] MRT risk was 2.92-fold higher among patients older than 60 than among younger patients, and it was 3.24-fold higher among patients older than 70 than among younger patients, similar to results reported previously.[5] These results suggest that efforts to reduce MRT should target older patients.
We found that lower BMI was associated with greater MRT incidence, as reported previously.[3,17,30] This likely reflects that lower weight usually means lower blood volume.[32] Interestingly, BMI had no protective effect on MRT when it was ≥32 kg/m2.
Preoperative hemoglobin level has previously been reported to be a strong predictor of RBC transfusion generally[18,22] and of MRT in particular.[3] In our study, MRT risk increased by 50% for each 1-g/dL decrease in hemoglobin level. MRT risk increased sharply by 16% when the level dropped <11 g/dL, and it rose sharply by 19% when the level dropped <9 g/dL. Among patients with preoperative hemoglobin levels <8 g/dL, MRT incidence reached 81%. These results suggest that substantially reducing RBC consumption will require increasing preoperative hemoglobin levels >9 g/dL, preferably above 11 g/dL.[23]
Patients with renal dysfunction in our study had lower preoperative hemoglobin levels than those with normal function (12.9 ± 2.3 vs 13.4 ± 1.8 g/dL). This may explain why renal dysfunction was identified as an independent MRT risk factor in our study, in accordance with previous work.[3] Renal dysfunction may lead to erythropoietin insufficiency and therefore preoperative anemia.[34] We found that renal dysfunction increased MRT risk by 2-fold, whereas other work showed it to increase risk of transfusion by 1.4-fold.[19] Yet another study showed that preoperative creatinine >100 μmol/L increased RBC requirement by 4.6-fold.[35]
In our study, MRT risk increased by 15% per 10-minute prolongation of CPB, similar to previous reports.[17] As many as 28% of patients for whom CPB lasted longer than 120 minutes required MRT; in contrast, only 14% of patients with CPB shorter than 120 minutes required MRT. We observed sharp increases in MRT incidence for CPB lasting longer than 220 or 260 minutes. Our findings suggest that CPB should be as short as possible, certainly within 4 hours.
Our analysis identified 5 MRT risk factors that appeared to be specific to patients who underwent valve surgery, although we caution that this may be biased by the small number of patients who presented with these risk factors and who underwent CABG or combined surgery (repeat surgery, n = 0; active endocarditis, 5; abnormal INR, 48; atrial fibrillation, 83).
Previous studies[36,37] identified active endocarditis as an independent predictor of MRT, and we found that it increased risk of MRT by 1.80-fold. Patients with this condition usually have lower preoperative hemoglobin levels; in our study, the level was 11.4 ± 2.2 g/dL among those with active endocarditis and 13.5 ± 1.8 g/dL among those without it (P < .001). Patients with active endocarditis also usually have numerous comorbidities[38,39] and they may have complex valve lesions, which increases the complexity of surgical procedures.[39] All these factors may increase the requirement for RBC transfusion in general and for MRT in particular.
We found that the presence of atrial fibrillation and larger left atrium diameter decreased MRT risk among patients undergoing valve surgery. This may reflect the indirect relationship of blood volume with left atrium diameter and atrial fibrillation.[36] The presence of mitral regurgitation, mitral stenosis, or tricuspid regurgitation may increase pressure in the right and left atria, inducing pulmonary and systemic congestion, enlarging the atrium, and triggering atrial fibrillation.[40,41]
Interestingly, atrial fibrillation and larger left atrium diameter reduced MRT risk among patients undergoing valve surgery, but not among patients undergoing CABG or combined surgery. This may reflect the fact that our patients with valve disease had larger left atrium diameter when atrial fibrillation was present than when it was absent (60 ± 17 vs 45 ± 11 mm, P < .001), which differs from the pathophysiology of atrial fibrillation in patients with coronary heart disease.[42–44]
This analysis of MRT risk factors may provide insights for how to reduce MRT in cardiac surgery and thereby improve outcomes. Official guidelines[45] already recommend several evidence-based measures for reducing risk of blood transfusion, including effective treatment of perioperative anemia, avoidance of CPB circuits with reduced priming volumes (minicircuits), and modifications during ultrafiltration. Our results may help extend the measures that can be implemented to reduce blood transfusion risk.
There are several potential limitations to this study. The fact that it was a retrospective study means that we could not avoid selection bias. We did not include all potential MRT risk factors in our analysis, such as platelet function, although we did collect data on as many potentially relevant variables as we could. Our small proportion of patients who underwent CABG or combined surgery meant that we could not examine in these patients whether active endocarditis (n = 5) or abnormal INR (n = 48) were associated with MRT risk. Future work should include larger patient samples for each type of cardiac surgery.
6. Conclusions
We provide evidence that MRT risk factors vary with the type of heart disease being treated. Six risk factors were common among all patients undergoing cardiac surgery in our study: sex, age, BMI, renal dysfunction, preoperative hemoglobin level, and CPB duration. Another 5 factors appeared to be specific to patients undergoing valve surgery: atrial fibrillation, active endocarditis, abnormal INR, left atrium diameter, and repeat surgery. Our results suggest the need to assess MRT risk based on the heart disease involved, and they provide insights useful for identifying and managing patients at higher MRT risk. Reducing MRT may lead to substantial savings of blood products.
Acknowledgments
The authors thank Chaonan Liu (Department of Laboratory Medicine, West China Hospital, Sichuan University) for help with statistical analysis.
Author contributions
Conceptualization: Min Yan, Lei Du.
Formal analysis: Dou Huang, Changwei Chen, Li Zhou.
Funding acquisition: Min Yan, Lei Du.
Investigation: Dou Huang, Changwei Chen, Yue Ming, Jing Liu, Fengjiang Zhang.
Methodology: Li Zhou, Min Yan, Lei Du.
Project administration: Min Yan, Lei Du.
Writing – original draft: Dou Huang, Changwei Chen, Yue Ming.
Writing – review and editing: Lei Du.
Footnotes
Abbreviations: BMI = body mass index, CABG = coronary artery bypass grafting, CPB = cardiopulmonary bypass, INR = international normalized ratio, IQR = interquartile range, MRT = massive red blood cell transfusion, RBC = red blood cell.
DH and CC contributed equally to this work
Funding: This work was supported by grant 81570374 from the National Natural Science Foundation of China (to LD) and grant 2018272998 from the National and Zhejiang Health and Family Planning Commission (to MY).
The authors declare that they have no competing interest.
References
- [1].Pearse RM, Moreno RP, Bauer P, et al. Mortality after surgery in Europe: a 7 day cohort study. Lancet (London, England) 2012;380:1059–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dixon B, Santamaria JD, Reid D, et al. The association of blood transfusion with mortality after cardiac surgery: cause or confounding? Transfusion 2013;53:19–27. [DOI] [PubMed] [Google Scholar]
- [3].Goudie R, Sterne JA, Verheyden V, et al. Risk scores to facilitate preoperative prediction of transfusion and large volume blood transfusion associated with adult cardiac surgery. Br J Anaesth 2015;114:757–66. [DOI] [PubMed] [Google Scholar]
- [4].Alghamdi AA, Davis A, Brister S, et al. Development and validation of Transfusion Risk Understanding Scoring Tool (TRUST) to stratify cardiac surgery patients according to their blood transfusion needs. Transfusion 2006;46:1120–9. [DOI] [PubMed] [Google Scholar]
- [5].Magruder JT, Blasco-Colmenares E, Crawford T, et al. Variation in red blood cell transfusion practices during cardiac operations among centers in Maryland: results from a state quality-improvement collaborative. Ann Thorac Surg 2017;103:152–60. [DOI] [PubMed] [Google Scholar]
- [6].Yende S, Wunderink RG. Effect of clopidogrel on bleeding after coronary artery bypass surgery. Crit Care Med 2001;29:2271–5. [DOI] [PubMed] [Google Scholar]
- [7].Moskowitz DM, Klein JJ, Shander A, et al. Predictors of transfusion requirements for cardiac surgical procedures at a blood conservation center. Ann Thorac Surg 2004;77:626–34. [DOI] [PubMed] [Google Scholar]
- [8].Koch CG, Li L, Duncan AI, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med 2006;34:1608–16. [DOI] [PubMed] [Google Scholar]
- [9].Delaney M, Stark PC, Suh M, et al. Massive transfusion in cardiac surgery: the impact of blood component ratios on clinical outcomes and survival. Anesth Analg 2017;124:1777–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chelemer SB, Prato BS, Cox PM, Jr, et al. Association of bacterial infection and red blood cell transfusion after coronary artery bypass surgery. Ann Thorac Surg 2002;73:138–42. [DOI] [PubMed] [Google Scholar]
- [11].Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304:1559–67. [DOI] [PubMed] [Google Scholar]
- [12].Turan A, Yang D, Bonilla A, et al. Morbidity and mortality after massive transfusion in patients undergoing non-cardiac surgery. Can J Anesth 2013;60:761–70. [DOI] [PubMed] [Google Scholar]
- [13].Hébert PC, Yetisir E, Martin C, et al. Is a low transfusion threshold safe in critically ill patients with cardiovascular diseases? Crit Care Med 2001;29:227–34. [DOI] [PubMed] [Google Scholar]
- [14].Likosky DS, Paugh TA, Harrington SD, et al. Prediction of transfusions after isolated coronary artery bypass grafting surgical procedures. Ann Thorac Surg 2017;764–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Al-Khabori M, Al-Riyami AZ, Mukaddirov M, et al. Transfusion indication predictive score: a proposed risk stratification score for perioperative red blood cell transfusion in cardiac surgery. Vox Sang 2014;269–75. [DOI] [PubMed] [Google Scholar]
- [16].Ad N, Massimiano PS, Burton NA, et al. Effect of patient age on blood product transfusion after cardiac surgery. J Thorac Cardiovasc Surg 2015;150:209–14. [DOI] [PubMed] [Google Scholar]
- [17].Karkouti K, O’Farrell R, Yau TM, et al. Reducing Bleeding in Cardiac Surgery Research Group. Prediction of massive blood transfusion in cardiac surgery. Can J Anesth 2006;53:781–94. [DOI] [PubMed] [Google Scholar]
- [18].De Boer WJ, Visser C, Ganushchak YM. Preoperative hemoglobin level: the best predictor of transfusion of packed red cells. Perfusion 2016;31:691–8. [DOI] [PubMed] [Google Scholar]
- [19].Stone GW, Clayton TC, Mehran R, et al. Impact of major bleeding and blood transfusions after cardiac surgery: analysis from the Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial. Am Heart J 2012;163:522–9. [DOI] [PubMed] [Google Scholar]
- [20].Robich MP, Koch CG, Johnston DR, et al. Trends in blood utilization in United States cardiac surgical patients. Transfusion 2015;55:805–14. [DOI] [PubMed] [Google Scholar]
- [21].Williams ML, Trivedi JR, Doughtie C, et al. Is female sex an independent risk factor for perioperative transfusion in coronary artery bypass graft surgery? J Am Coll Surg 2011;212:362–6. [DOI] [PubMed] [Google Scholar]
- [22].Covin R, O’Brien M, Grunwald G, et al. Factors affecting transfusion of fresh frozen plasma, platelets, and red blood cells during elective coronary artery bypass graft surgery. Arch Pathol Lab Med 2003;415–23. [DOI] [PubMed] [Google Scholar]
- [23].Craver C, Belk KW, Myers GJ. Measurement of total hemoglobin reduces red cell transfusion in hospitalized patients undergoing cardiac surgery: a retrospective database analysis. Perfusion 2017;1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Drexler H. Endothelial dysfunction: clinical implications. Prog Cardiovasc Dis 1997;39:287–324. [DOI] [PubMed] [Google Scholar]
- [25].Guo Y, Tang J, Du L, et al. Protamine dosage based on two titrations reduces blood loss after valve replacement surgery: a prospective, double-blinded, randomized study. Can J Cardiol 2012;28:547–52. [DOI] [PubMed] [Google Scholar]
- [26].Zhou ZF, Jia XP, Sun K, et al. Mild volume acute normovolemic hemodilution is associated with lower intraoperative transfusion and postoperative pulmonary infection in patients undergoing cardiac surgery– a retrospective, propensity matching study. BMC Anesthesiol 2017;17:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tan Z, Zhou L, Qin Z, et al. Low-dose sevoflurane may reduce blood loss and need for blood products after cardiac surgery: a prospective, randomized pilot study. Medicine (Baltimore) 2016;95:e3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Isil CT, Yazici P, Bakir I. Risk factors and outcome of increased red blood cell transfusion in cardiac surgical patients aged 65 years and older. Thorac Cardiovasc Surg 2015;63:39–44. [DOI] [PubMed] [Google Scholar]
- [29].Mazzeffi M, Galvagno S, Gammie JS, et al. Impact of aspirin use on morbidity and mortality in massively transfused cardiac surgery patients: a propensity score matched cohort study. J Anesth 2016;30:817–25. [DOI] [PubMed] [Google Scholar]
- [30].Karkouti K, Wijeysundera DN, Beattie WS, et al. Variability and predictability of large-volume red blood cell transfusion in cardiac surgery: a multicenter study. Transfusion 2007;47:2081–8. [DOI] [PubMed] [Google Scholar]
- [31].Messerli FH, Sundgaard-Riise K, Ventura HO, et al. Essential hypertension in the elderly: haemodynamics, intravascular volume, plasma renin activity, and circulating catecholamine levels. Lancet (London, England) 1983;2:983–6. [DOI] [PubMed] [Google Scholar]
- [32].Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038–47. [DOI] [PubMed] [Google Scholar]
- [33].Boer P. Estimated lean body mass as an index for normalization of body fluid volumes in humans. American Physiological Society 1984;247:F632–6. [DOI] [PubMed] [Google Scholar]
- [34].Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006;355:2085–98. [DOI] [PubMed] [Google Scholar]
- [35].Elmistekawy EM, Errett L, Fawzy HF. Predictors of packed red cell transfusion after isolated primary coronary artery bypass grafting—the experience of a single cardiac center: a prospective observational study. J Cardiothorac Surg 2009;4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ranucci M, Castelvecchio S, Frigiola A, et al. Predicting transfusions in cardiac surgery: the easier, the better: the Transfusion Risk and Clinical Knowledge score. Vox Sang 2009;96:324–32. [DOI] [PubMed] [Google Scholar]
- [37].Bidstrup BP, Royston D, Taylor KM, et al. Effect of aprotinin on need for blood transfusion in patients with septic endocarditis having open-heart surgery. Lancet 1988;1:366–7. [DOI] [PubMed] [Google Scholar]
- [38].Buyukasyk NS, Ileri M, Alper A, et al. Increased blood coagulation and platelet activation in patients with infective endocarditis and embolic events. Clin Cardiol 2004;27:154–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Seeburger J, Groesdonk H, Borger MA, et al. Quadruple valve replacement for acute endocarditis. J Thorac Cardiovasc Surg 2009;137:1564–5. [DOI] [PubMed] [Google Scholar]
- [40].White CW, Kerber RE, Weiss HR, et al. The effects of atrial fibrillation on atrial pressure-volume and flow relationships. Circ Res 1982;51:205–15. [DOI] [PubMed] [Google Scholar]
- [41].Chamberlain AM, Agarwal SK, Folsom AR, et al. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol 2011;107:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Andrade J, Khairy P, Dobrev D, et al. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res 2014;114:1453–68. [DOI] [PubMed] [Google Scholar]
- [43].Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol 2008;1:62–73. [DOI] [PubMed] [Google Scholar]
- [44].Nattel S, Maguy A, Le Bouter S, et al. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev 2007;87:425–56. [DOI] [PubMed] [Google Scholar]
- [45].Task F, Ferraris VA, Brown JR, et al. Society of Thoracic Surgeons Blood Conservation Guideline. 2011 Update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg 2011;91:944–82. [DOI] [PubMed] [Google Scholar]


