Abstract
Rationale:
Few comparative studies have evaluated the differences between intravenous alendronate (ALN) and ibandronate (IBN) in patients with osteoporosis. This study was to compare the effects of these 2 drugs on bone mineral density (BMD), bone metabolic markers, and adverse events in patients with osteoporosis.
Patient concerns:
Seventy-eight subjects were assigned to the ALN group and 66 to the IBN group.
Diagnoses:
The diagnosis of osteoporosis was based on BMD values of the femoral neck or lumbar spine less than −2.5 SD below the reference values.
Interventions:
This study was designed as a 52-weeks, prospective, non-randomized study involving a parallel-group comparison between intravenous ALN and intravenous IBN in elderly women with osteoporosis.
Outcomes:
The non-switched-IBN subgroup showed significant decrease in serum collagen type I cross-linked telopeptide (NTX) at 6 and 12 months compared with baseline, and the decrease in NTX were significantly greater in the non-switched-IBN subgroup than in the non-swithed-ALN subgroup. BMD in the lumbar spine in the non-switched-IBN subgroup showed a significant increase at 12 months and the increase in BMD were significantly larger than in the non-switched-ALN subgroup.
Lessons:
Intravenou IBN might result in a significantly greater increase of BMD and decrease in NTX, but it had a higher incidence of adverse drug reactions than ALN.
Keywords: alendronate, ibandronate, osteoporosis
1. Introduction
It has been widely recognized that bisphosphonates are a first-choice treatment for osteoporosis, but several concerns with oral intake, such as gastroesophageal reflux disease,[1] poor bioavailability, decreasing adherence,[2] and adverse drug reactions,[3,4] have been raised. To address these problems, intravenous alendronate (ALN) regimens have been developed.[5] We showed in a previous study that intravenous administration of ALN was not inferior to oral ALN for the treatment of osteoporosis.[6] Ibandronate (IBN) (ibandronate sodium hydrate) is available for intravenous administration for the treatment of osteoporosis. Several comparative studies have been conducted to examine the safety of intravenous administration of IBN and oral risedronate in a clinical trial in Japanese patients with osteoporosis.[7–9] These reports showed the non-inferiority of intravenous IBN to oral administration of risedronate with 2 years of follow-up with regard to bone mineral density (BMD), fracture risk, and adverse drug effects.[9] With respect to other studies of oral bisphosphonates, Nakamura et al[10] confirmed that there was non-inferiority of oral IBN (100 mg/month) to intravenous IBN (1 mg/month) in lumbar spine BMD in Japanese osteoporotic patients.
However, no comparative studies have evaluated the differences between intravenous ALN and intravenous IBN in patients with osteoporosis. Therefore, the objective of the present study was to compare the effects of these 2 drugs given intravenously on BMD, bone metabolic markers, and adverse events in patients with osteoporosis.
2. Materials and methods
2.1. Study design
This study was designed as a 52-week, prospective, non-randomized study involving a parallel-group comparison between intravenous ALN (900 μg ALN sodium hydrate, Teijin Pharmaceutical Company, Tokyo, Japan) once monthly and intravenous IBN (1 mg IBN sodium hydrate, Chugai Pharmaceutical Company, Tokyo, Japan) monthly.
2.2. Participants
Study subjects were recruited from among female osteoporotic patients over the age of 70 years who visited our orthopedic clinic. They were diagnosed as having osteoporosis based on BMD values of the femoral neck or lumbar spine less than −2.5 SD below the reference values.[11]
The subjects, who were prospectively selected, included both new patients who had no previous treatment for osteoporosis and existing patients on pharmacological treatment who were non-responding or poorly adhering to their treatment and in whom switching their treatment to intravenous bisphosphonates was considered. The non-responders were defined as having BMD increases of less than 3% at the lumbar spine or less than 0% at the femoral neck from baseline to 1 year.[12] Poor adherence was considered present if the patient was poorly compliant and/or was complaining of adverse drug effects due to the treatment for osteoporosis.
Thus, 200 patients were selected prospectively as participants for this study. Following a comprehensive explanation of this study and expected benefits and risks, a total of 144 women provided their written, informed consent using forms approved by the institutional review board of our institution. All procedures performed in studies involving human participants were in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The ethics committee of our institution approved this study protocol.
Patients were assigned to receive either intravenous drip infusion of ALN (ALN group, n = 78) or IBN (IBN group, n = 66). Assignment to the 2 groups was determined by the doctors, and the analysis included those patients who were not lost to follow-up (Fig. 1). Participants were then sub-classified into 2 subgroups according to their previous treatment, that is, the no previous treatment subgroup (non-switched) and the switched subgroup. Therefore, in the ALN group, there were 30 patients in the non-switched-ALN subgroup and 48 patients in the switched-ALN subgroup, while in the IBN group, there were 28 patients in the non-switched-IBN subgroup and 38 patients in the switched-IBN subgroup.
Figure 1.
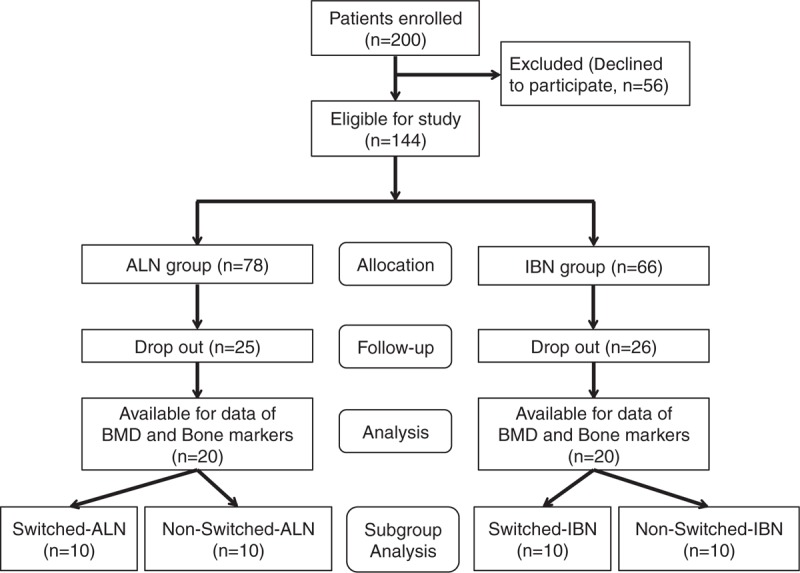
Participant selection process. Participants were divided into 2 groups, the ALN group (n = 80) and the IBN group (n = 70), and then subgroup analysis was conducted: switched-ALN (n = 10), non-switched-ALN (n = 10), switched-IBN (n = 10), and non-switched-IBN (n = 10). ALN = alendronate, IBN = ibandronate.
The previous drug treatment for osteoporosis was unknown in 1 patient in the IBN group (Table 1). The previous drugs for osteoporosis of both switched groups were elcatonin (n = 12), selective estrogen receptor modulators (SERMs) (n = 11), and oral ALN (n = 15) in the switched-ALN subgroup (total number 38) and elcatonin (n = 8), SERMs (n = 5), and oral ALN (n = 11) in the switched-IBN subgroup (total number 24).
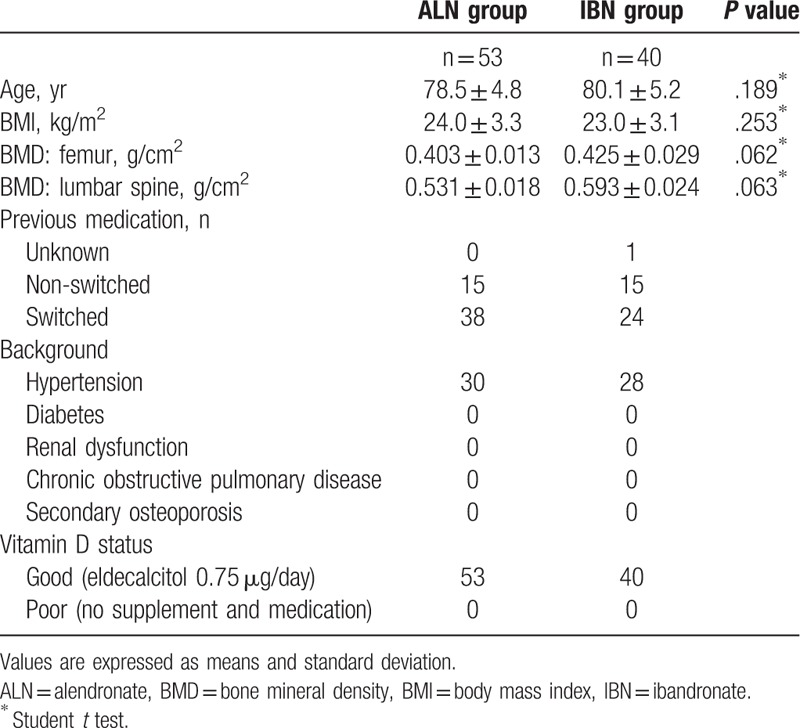
Table 1.
Baseline characteristics of the 2 groups.
2.3. BMD measurements
BMD was measured at the proximal femur (femoral neck) and lumbar spine (anteroposterior, L2-4) using dual-energy X-ray absorptiometry (DXA, Hologic QDR Discovery W type; Toyo Medic., Tokyo, Japan) at baseline and every 6 months. The non-responders to intravenous treatment were defined in the same manner as described above for the previous treatment before intravenous ALN or IBN.[12]
2.4. Vertebral fractures and adverse events
Incidence rates of vertebral fractures were evaluated with plain radiographs of the thoracic and lumbar spines on lateral views every 6 months. Vertebral fractures were determined as grade 1 or progressed by more than 1 grade by a semi-quantitative method.[13,14] Adverse drug reactions such as bone and muscle pain, pyrexia, myalgia, fatigue, or lymphopenia were recorded.
2.5. Bone turnover markers and serum calcium (Ca)
Serum collagen type 1 cross-linked N telopeptide (NTX, nmol BCE/L), serum bone-specific alkaline phosphatase (BAP, μg/L), and serum Ca (mg/L) were measured at baseline, 6 months, and 12 months. The least significant change of each bone turnover marker was 14.1%, 23%, and 5% respectively.
2.6. Statistical analyses
Statistical analysis was performed using Microsoft Office Excel and the Statcel 3 program (OMS, Inc., Hyogo, Japan). All data are presented as means and standard deviation. Both BMDs, bone turnover markers, serum Ca, and their changes were analyzed by Student t test to compare differences between the groups. The changes of BMD (g/cm2) and bone turnover markers in each group were analyzed by repeated measures analysis of variance (ANOVA) with the Bonferroni test. For the incidences of fractures and adverse drug reactions, the Chi-squared test was used to evaluate the significance of differences. Statistical tests were regarded as significant at P <.05.
3. Results
The baseline characteristics of both groups are shown in Table 1. There were no significant differences in age, body mass index (BMI), BMD, previous fractures, and distribution of previous treatment.
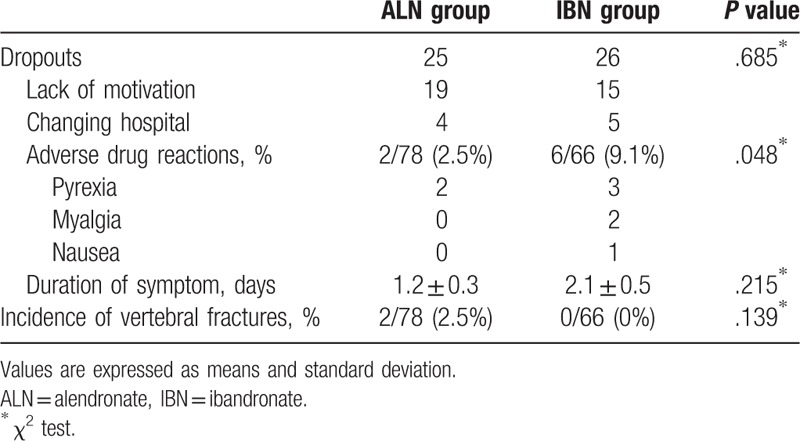
In the ALN group, 25 subjects dropped out because of discontinuation at the beginning stage within 6 months. In the IBN group, 26 subjects dropped out due to discontinuation within 6 months (Fig. 1). The causes of discontinuation were lack of motivation (ALN = 19, IBN = 15), changing hospital (ALN = 4, IBN = 5), and adverse drug reactions (ALN = 2, IBN = 6). Two adverse drug reactions (both pyrexia) occurred in the ALN group, and 6 adverse drug reactions occurred in the IBN group (3 pyrexia, 2 myalgia, and 1 nausea). There was a significant difference in the incidence of adverse drug reactions between the groups (P = .048) (Table 2). They all recovered quickly with non-steroidal anti-inflammatory drug or antiemetic drug treatment, although they resulted in discontinuation of the study drugs. Vertebral fractures at follow-up were found in 2 of 78 (2.5%) patients in the ALN group and in no patients in the IBN group, with no significant difference between the groups (Table 2).
Table 2.
Comparison of dropouts and events between groups.
The BMD measurements at follow-up were available for 53 patients in the ALN group and 40 patients in the IBN group after excluding the patients lacking the data (Table 1). There were 2 non-responders in the ALN group and 4 in the IBN group, with no significant difference between the groups, and 31 patients in the ALN group and 16 patients in the IBN group were ineligible for data analysis.
At the follow-up, bone turnover markers and Ca were available in 20 patients of each group for analysis. Therefore, 20 patients in both groups with all data for BMD and bone markers obtained from baseline to follow-up were analyzed.
The non-switched-IBN subgroup showed significant decreases in NTX at 6 and 12 months compared with baseline. The decreases in NTX were significantly greater in the non-switched-IBN subgroup at 12 months than in the non-switched-ALN subgroup (Fig. 2). There were no significant changes over time in NTX in both the non-switched-ALN and switched-ALN subgroups (Figs. 2 and 3). For BAP and Ca, there were no significant changes in both groups over time, and no significant differences were found between the groups.
Figure 2.
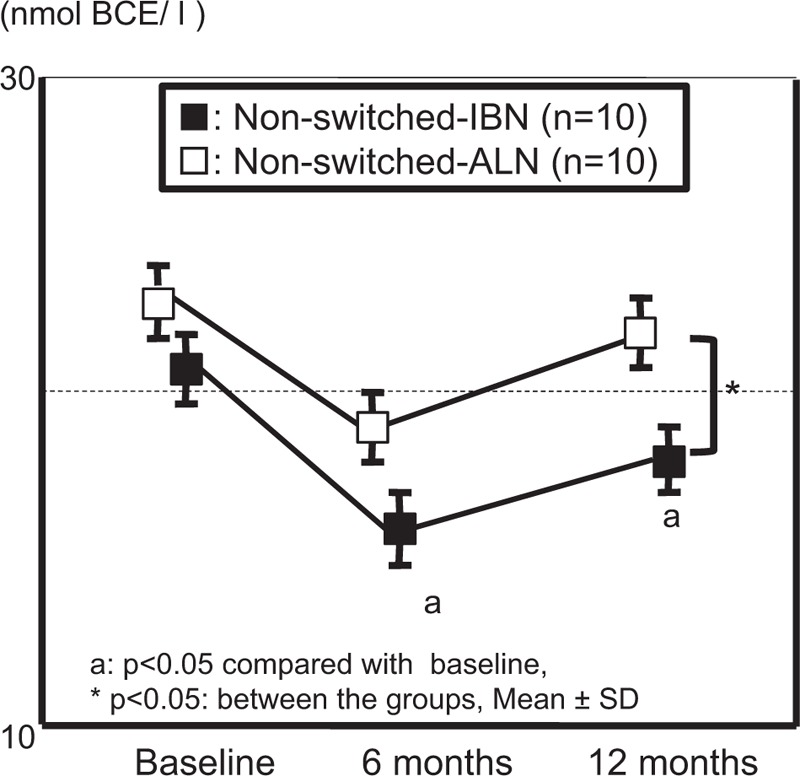
Comparison of changes in serum NTX from the starting phase in the non-switched group between IBN and ALN. ALN = alendronate, IBN = ibandronate, NTX = serum collagen type I cross-linked telopeptide.
Figure 3.
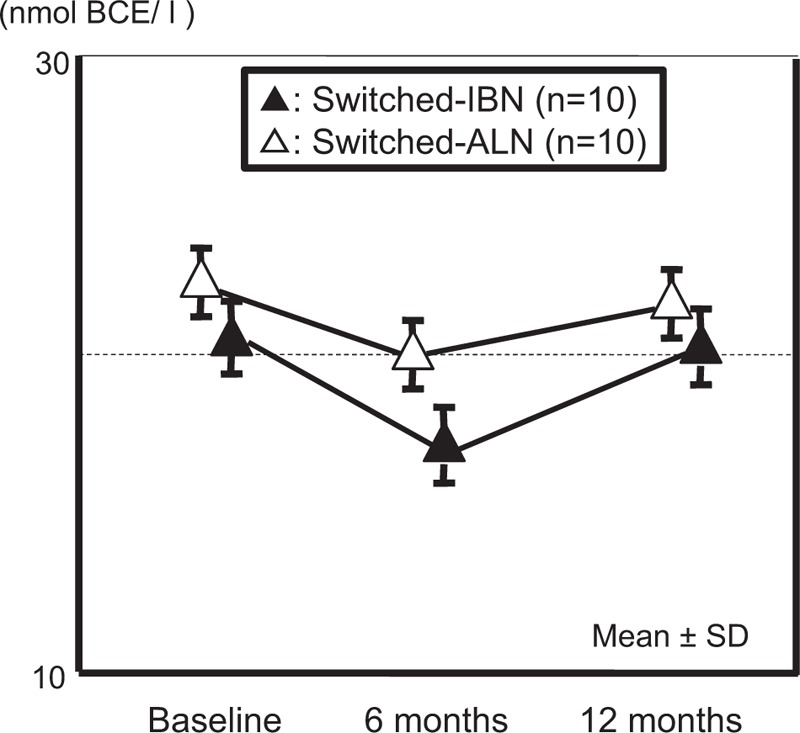
Comparison of changes in serum NTX from the starting phase in the switched group between IBN and ALN. ALN = alendronate, IBN = ibandronate, NTX = serum collagen type I cross-linked telopeptide.
The BMD in the lumbar spine in the non-switched-IBN subgroup showed a significant increase at 12 months compared with baseline. The increase in BMD was significantly larger in the non-switched-IBN subgroup than in the non-switched-ALN subgroup at 12 months (Fig. 4). The increase in the BMD of the femur was significantly greater in the non-switched-IBN subgroup than in the non-switched-ALN subgroup at 6 months (Fig. 5).
Figure 4.
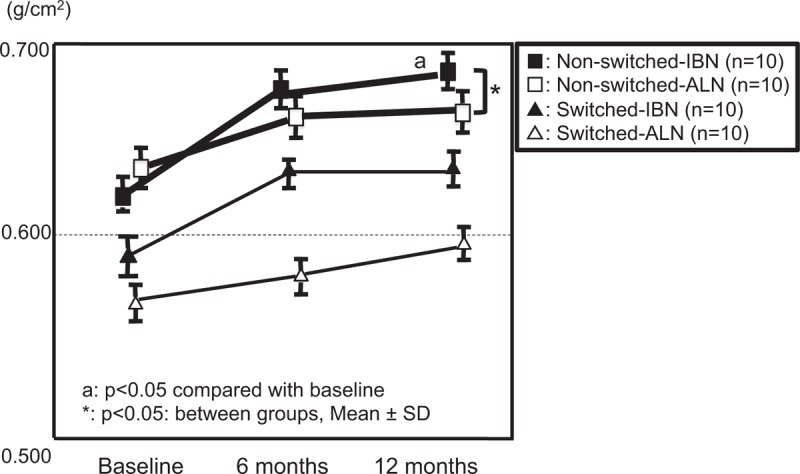
Comparison of changes in BMD of the lumbar spine between groups. BMD = bone mineral density.
Figure 5.
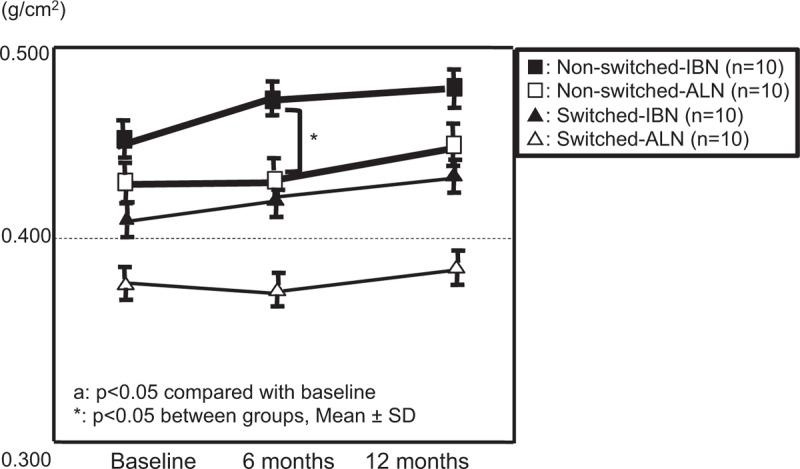
Comparison of changes in BMD of the femur between groups. BMD = bone mineral density.
4. Discussion
Our previous study, which was performed to attempt to confirm the efficacy and safety of intravenous IBN, suggested that it was non-inferior to oral ALN.[15] However, to the best of our knowledge, there have been no previous reports that compared the efficacy and safety of ALN and IBN given intravenously once a month in patients with osteoporosis. Therefore, the present study was performed to attempt to confirm the effects on BMD and bone metabolic markers of intravenous ALN and IBN. Although this was a limited developmental study with a short duration (52 weeks) and a relatively small number of patients, there were significant differences between the groups in bone turnover markers and adverse drug reactions. This study also showed the effectiveness of IBN compared to ALN in the percentage changes of BMD from baseline in the lumbar spine and femur, and with respect to the safety of ALN, no clinically significant differences in the incidences of fractures and non-responders were found.
Interestingly, the present data indicated that the changes in BMD and bone turnover markers were different between the 2 drugs in the non-switched groups for osteoporosis, while no difference was found in the switched groups. Hashimoto et al also reported that there were significant differences in BMD of the lumbar spine between the non-switched groups (8%) and switched groups (1%) with intravenous IBN for the treatment of primary osteoporosis, and it was attributed to the past treatment history.[16] These facts suggest that there might be some differences between these intravenously administered drugs in their effects in osteoporotic patients with a past treatment history, although there have been few reports comparing the changes in BMD of osteoporotic patients based on their history of osteoporosis treatment. As for bone turnover markers, Duan et al mentioned that IBN had a relatively low affinity for bone compared to other bisphosphonates.[17] IBN has been reported to show a lower affinity to bone, which results in wide infiltration of bone, not staying only at the surface of bone, but spreading throughout all bone tissues.[18] Therefore, it was considered that IBN showed relatively restricted bone turnover in patients with no previous treatment for osteoporosis due to reduced restriction of bone turnover, as shown by a significant decrease in NTX.
On the other hand, intravenous infusion of bisphosphonates is known to induce a transient acute phase reaction with bone and muscle pain, pyrexia, myalgia, fatigue, lymphopenia, and so on.[10,19] Sieber et al explained that only half a dose was given at the first IBN injection for safety.[20] This implies that most symptoms that occurred after IBN injections might be due to the high dose. The present study showed that IBN had higher rates of adverse drug reactions than ALN, which might be attributed to the small numbers for statistical analysis.
Finally, we believe that this is the first trial to compare the efficacy and safety between these 2 intravenously administered drugs,
5. Limitations
There were several limitations in this study, including the short duration of follow-up, the small number of patients, and the non-randomized nature of the study.
6. Future directions
A further randomized, controlled trial with a larger number of patients and longer follow-up period would be needed to evaluate the effectiveness of the intravenous bisphosphonates. However, this study may provide useful information for selecting treatment for patients with osteoporosis from the many options in our daily clinical practice.
In conclusion, while intravenous IBN showed significant differences in the changes of BMD and NTX, it had a higher incidence of adverse drug events than ALN.
Acknowledgments
The authors would like to thank Dr Yusuke Sugimura who assisted and supported our research.
Author contributions
AH and HK designed this study. AH and MH analyzed the data. AH wrote the manuscript in consultation with NM and MH. All authors have read and approved the final manuscript.
Conceptualization: Michio Hongo.
Data curation: Hiroyuki Kodama.
Formal analysis: Yuji Kasukawa.
Supervision: Naohisa Miyakoshi.
Validation: Yoichi Shimada.
Writing – original draft: Akira Horikawa.
Footnotes
Abbreviations: ALN = alendronate, BAP = bone-specific alkaline phosphatase, BMD = bone mineral density, GERD = gastroesophageal reflux disease, IBN = ibandronate, NTX = serum collagen type I cross-linked telopeptide, SERMs = selective estrogen receptor modulators.
The authors have no conflicts of interest to disclose.
References
- [1].Miyakoshi N, Kasukawa Y, Sasaki H, et al. Impact of spinal kyphosis on gastroesophageal reflux disease symptoms in patients with osteoporosis. Osteoporosis Int 2009;20:1193–8. [DOI] [PubMed] [Google Scholar]
- [2].Gertz BJ, Holland SD, Kline WF, et al. Studies of the oral bioavailability of alendronate. Clin Pharmacol Ther 1995;58:288–98. [DOI] [PubMed] [Google Scholar]
- [3].Brookhart MA, Avorn J, Katz JN, et al. Gaps in treatment among users of osteoporosis medications: the dynamics of noncompliance. Am J Med 2007;120:251–6. [DOI] [PubMed] [Google Scholar]
- [4].Siris ES, Harris ST, Rosen CJ, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporosis women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clinic Proc 2006;81:1013–22. [DOI] [PubMed] [Google Scholar]
- [5].Shiraki M, Nakamura T, Fukunaga M, et al. A multicenter randomized double-masked comparative study of different preparations of alendronate in osteoporosis- monthly (four weeks) intravenous versus once weekly oral administrations. Curr Med Res Opin 2012;28:1357–67. [DOI] [PubMed] [Google Scholar]
- [6].Horikawa A, Miyakoshi N, Shimada Y, et al. A comparative study between intravenous and oral alendronate administration for the treatment of osteoporosis. SpringerPlus 2015;4:675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hagino H, Yoshida S, Hashimoto J, et al. Increased bone mineral density with monthly intravenous ibandronate contributes to fracture risk reduction in patients with primary osteoporosis: three-year analysis of the MOVER Study. Calcif Tissue Int 2014;95:557–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ito M, Tobinai M, Yoshida S, et al. Effect of monthly intravenous injections on vertebral or non-vertebral fracture risk in Japanese patients with high-risk osteoporosis in the MOVER study. J Bone Mineral Metab 2015;35:58–64. [DOI] [PubMed] [Google Scholar]
- [9].Nakamura T, Nakano T, Ito M, et al. MOVER Study Group. Clinical efficacy on fracture risk and safety of 0.5 mg or 1 mg/month intravenous ibandronate versus 2.5 mg/day oral risedronate in patients with primary osteoporosis. Calcif Tissue Int 2013;93:137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nakamura T, Ito M, Hashimoto J, et al. MOVER Study Group. Clinical efficacy and safety of monthly oral ibandronate 100 mg versus monthly intravenous ibandronate 1 mg in Japanese patients with primary osteoporosis. Osteoporosis Int 2015;26:2685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].WHO: Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser 1994;843:1–29. [PubMed] [Google Scholar]
- [12].Hadji P, Felsenberg D, Amling M, et al. The non-interventional BonViva Intravenous Alendronate (VIVA) study: real-world adherence and persistence to medication, efficacy, and safety, in patients with postmenopausal osteoporosis. Osteoporosis Int 2014;25:339–47. [DOI] [PubMed] [Google Scholar]
- [13].Nakano T, Yamamoto M, Hashimoto J, et al. Higher response with bone mineral density increase with monthly injectable ibandronate 1 mg compared with oral risedronate in the MOVER study. J Bone Miner Metab 2016;34:678–84. [DOI] [PubMed] [Google Scholar]
- [14].Genant HK, Jergas M, Palermo L, et al. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res 1996;11:984–96. [DOI] [PubMed] [Google Scholar]
- [15].Eisman JA, Civitelli R, Adami S, et al. Efficacy and tolerability of intravenous ibandronate injections in postmenopausal osteoporosis: 2-year results from the DIVA study. J Rheumatol 2008;35:488–97. [PubMed] [Google Scholar]
- [16].Hashimoto S, Hara S, Gouhara T, et al. Clinical efficacy and safety of intravenous ibandronate for primary osteoporosis. Therap Res 2015;36:267–72. [Google Scholar]
- [17].Duan X, Xia Z, Zhang H, et al. The effects of pH on the relative bone mineral-binding affinities of bisphosphonates determined by hydroxyapatite-column chromatography. J Bone Miner Res 2010;25:S347. [Google Scholar]
- [18].Leu CT, Luegmayr E, Freedman LP, et al. Relative binding affinities of bisphosphonates for human bone and relationship to antiresorptive efficacy. Bone 2006;38:628–36. [DOI] [PubMed] [Google Scholar]
- [19].Chesnut CH, III, Skag A, Christiansen C, et al. Oral ibandronate osteoporosis vertebral fracture trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 2004;19:1241–9. [DOI] [PubMed] [Google Scholar]
- [20].Sieber P, Lardelli P, Kraenzlin CA, et al. Intravenous bisphosphonates for postmenopausal osteoporosis: safety profiles of zoledronic acid and ibandronate in clinical practice. Clin Drug Invest 2013;33:117–22. [DOI] [PubMed] [Google Scholar]


