Abstract
Rationale:
Detection of aquaporin-4 (AQP4) antibody in cerebrospinal fluid (CSF) was not suggested for the diagnosis of neuromyelitis opica spectrum disorders (NMOSD). However, some patients with NMOSD have only AQP4 antibody positive in CSF but not in serum with unknown cause. Besides, it is rarely reported that NMOSD complicated with renal clear cell carcinoma. So, the relationship between AQP4-Ab, NMOSD and malignant tumors warrants an investigation.
Patients concerns:
A 31-year-old female presented in our hospital with chief complaints of urinary retention and weakness in bilateral lower extremities for more than 10 days.
Diagnoses:
The patient was diagnosed as NMOSD by neuroimaging and laboratory examination, with AQP4 antibody positive only in CSF. Besides, asymptomatic clear cell carcinoma was also found in left kidney.
Interventions:
The patient underwent 2-month immunosuppressive therapy for NMOSD at first, including intravenous administration of immunoglobulin (IVIG) and methylprednisone, with oral drugs of predisone and tacrolimus. After that, Partial nephrectomy of left kidney was performed.
Outcomes:
The patient demonstrated almost complete remission for NMOSD after immunosuppressive therapy, and the renal tumor was cured by partial nephrectomy.
Lesson:
This case indicates that neuromyelitis optica (NMO)-IgG positive only in CSF could have potential association with the etiology of NMOSD, and renal clear cell carcinoma could be found complicated with NMOSD coincidently. Besides, it is necessary to examine NMO-IgG in CSF for patients suspicious with NMOSD, even when the serum test is negative, especially for those with complicated malignant tumors.
Keywords: aquaporin 4 antibody, cerebral spinal fluid, neuromyelitis optica spectrum disorders, renal carcinoma
1. Introduction
Neuromyelitis optica (NMO) is a severe relapsing autoimmune inflammatory demyelinating disease that preferentially affects the optic nerves and spinal cord, thus mimicking multiple sclerosis, from which it is distinguished by a serum autoantibody specific for the astrocytic water channel aquaporin-4 (AQP4).[1,2] Related forms of NMO, such as optic neuritis and transverse myelitis, are also often positive for the anti-AQP4 antibody and are diagnosed as NMO spectrum disorder (NMOSD).[3] AQP4 is a protein expressed in foot-processes of astrocytes throughout the central nervous system (CNS), as well as in skeletal muscle and epithelial cells in kidney, lung, and gastrointestinal organs.[4] The origins of the anti-AQP4 antibody, as well as the pathogenesis of NMOSD, remain to be elucidated. NMOSD occurring in the course of renal carcinoma have not yet been reported in the literature. Here we describe a patient with renal carcinoma who presented with NMOSD.
2. Case report
A 31-year-old female, otherwise healthy, complained of fever and urinary retention lasting 15 days and weakness in bilateral lower extremities lasting 10 days. The maximum body temperature observed was 40.3°C and she was treated with oral cefixime for 2 days, intravenous penicillin/levofloxacin for 4 days, and moxifloxacin for 3 days. However, in the days following, body temperature remained fluctuating 38.0 °C to 38.5°C and urinary retention and weakness of bilateral lower extremities worsened. Physical examination revealed rough breath sounds and no rash. Neurological examination showed somnolence. Coarse vision, other cranial nerves, and upper limb strength (grade V) all appeared normal. Bilateral lower extremities were graded II. There were no sensory disturbances or meningeal signs. Algesthesis was somewhat reduced on the left side but deep sensation was normal bilaterally. Her reflexes were normal with bilateral negative Babinski's sign.
Laboratory analysis showed elevated white blood cell count (13,250/mm3 with 84.5% neutrophil, 10.3% lymphocyte), low sodium (112.1 mmol/L), low chloride (85.3 mmol/L) and slightly elevated liver enzymes (aspartate transaminase, 58.5 U/L). Antinuclear antibody, SS-A antibody, and anti-cytoplasmic neutrophil antibody levels were within normal limits. Quantitative immunoglobulins, complement C3/4, and tumor markers were also within normal limits. Cerebrospinal fluid (CSF) examination showed the initial pressure to be 280mmH2O, with pleocytosis (135/mm3), protein concentration of 1346.7 mg/dL, glucose 3.1mmol/L, and chloride 94mmol/L. Oligoclonal band was negative in serum and positive in CSF, with an IgG index of 1.30 (well above the cutoff of 0.85). IgG synthesis in CSF within 24 hours was 28.56 mg (highly above the cutoff of 7.0 mg/24 h). Both serum and CSF anti-myelin oligodendrocyte glycoprotein antibodies were within normal limits. Neither serum nor CSF was positive for paraneoplastic-associated antibodies such as anti-Hu, anti-Yo, anti-Ri anti-Ma2, anti-CV2, or anti-Amphiphysin, which rules out a diagnosis of paraneoplastic neurologic syndromes. AQP4 antibodies were detected with a cell-based assay using a commercially available kit (Euroimmun, Luebeck, Germany) or by transfection of HEK293T cells with a construct containing human AQP4-M1 and AQP4-M23 genes. The precise titer of AQP4-Abs was 1:100 for CSF as per the kit instructions. According to the instructions of commercial kits provided by Euroimmun, 2 operators (HH and FG) independently ran the test to assess intralaboratory and interrater reproducibility of the assay. Tissue-based immunoassays revealed that CSF was strongly positive for anti-NMO IgG, while the serum sample was negative. What is more, we tested CSF for AQP4-Abs at different time points of 24 hours and 48 hours after lumber puncture to confirm that the positive findings of AQP4-Abs were not transitional.
Chest computed tomography (CT) showed inflammation of bilateral lower lung and minimal bilateral pleural effusion. These findings suggested a diagnosis of pneumonia and she was treated with ceftriaxone intravenously for 7 days. After antibiotic therapy, body temperature and white blood cell count returned to normal range. Enhanced abdominal CT showed a mass on the left kidney (Fig. 1A), indicative of renal clear cell carcinoma. Nerve conduction examinations of the left upper and lower extremities showed normal motor and sensory function. Cranial magnetic resonance imaging (MRI) images were fairly typical, showing only mild maxillary sinusitis. Enhanced cervical MRI revealed swelling of the spinal cord from C2-T4 with gadolinium enhancement (Fig. 2). Examination of bilateral visual evoked potential (VEP) showed a prolonged latency of the P100 response. These findings supported a diagnosis of NMOSD, according to the criteria of Wingerchuk et al published in 2015.[5] Therefore, the patient was started on immunosuppressive therapy with intravenous immunoglobulin 0.4 g/kg for 5 days and methylprednisolone 1 g for 3 days, followed by 500 mg for 3days, 240 mg for 3 days, 120 mg for 3 days, as well as prednisone (1 mg/kg) and tacrolimus (3 mg per day). Shortly after the course of immunosuppressive therapy, the patient's motor symptoms resolved, with the strength of the legs improved to grade V. She was discharged without urine tube or wheelchair. Two months later, she underwent partial nephrectomy with laparoscope under general anesthesia. The pathology of the renal mass was determined as renal clear cell carcinoma (Fig. 1B). The tumor tissues stained positive for AQP4 (Fig. 3).
Figure 1.
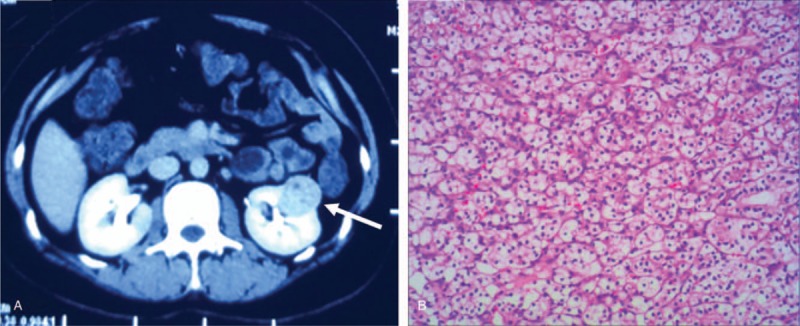
Abdominal CT shows a mass on the left kidney with slight iodine contrast enhancement (A, arrow head), and pathological tissue under 10 × 10 magnification is characteristic of clear cell carcinoma (B). CT = computed tomography.
Figure 2.
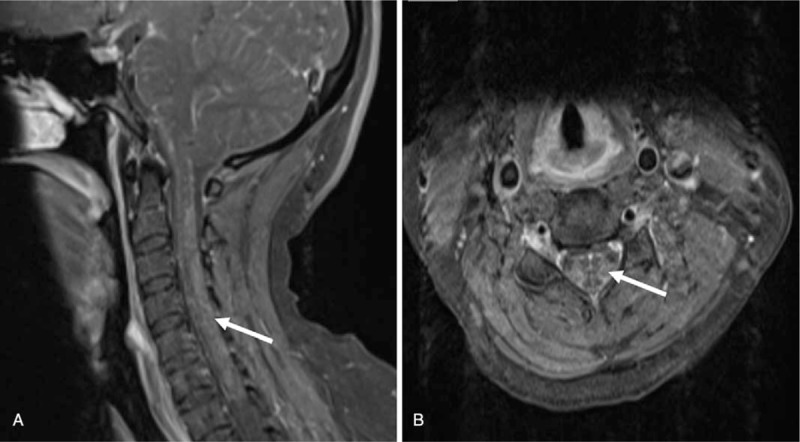
Cervical MRI shows a hyperintense swelling lesion with moderate gadolinium enhancement extending from C2 to T2 on sagittal view (A, arrow head), which is prominent in the central gray matter at C4 on the axial view (B, arrow head). MRI = magnetic resonance imaging.
Figure 3.
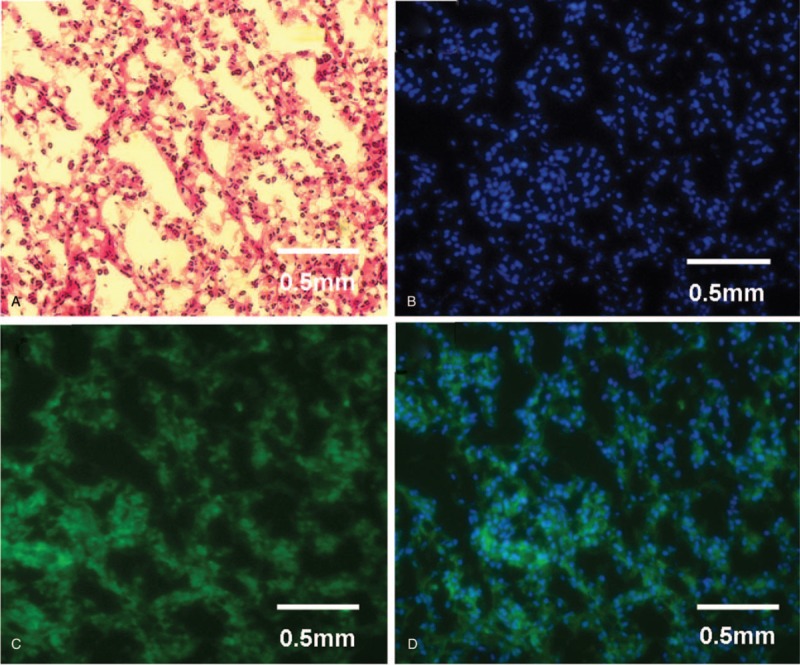
Renal tumor tissues stained with H&E (A), DAPI (B), and aquaporin-4 (AQP4) (C); DAPI and AQP4 are also shown in a merged image (D). Scale bar indicates 0.5 mm. DAPI = 4’,6-diamidino-2-phenylindole dihydrochloride.
3. Discussion
Currently, there is no published literature of a case of NMOSD coincident with NMO-IgG-positive CSF and renal clear cell carcinoma. NMO/AQP4-IgG is normally produced and expressed in the serum of patients with NMOSD, but rarely in the CSF. There have also been rare cases of unknown etiology encountered in clinical work with AQP4-IgG positive only in CSF but not in serum. In the present case, we thought it unlikely that NMO/AQP4-IgG synthesis had been induced by AQP4 ectopically expressed in the renal tissue, because NMO/AQP4-IgG were not detected in the serum. These results suggest that it is still necessary to test for the presence of NMO-IgG in the CSF when the serum test was negative if a diagnosis of NMOSD is likely.
Recently, Majed M et al[6] reported that fluorescence-activated cell sorting (FACS) and commercial cell-based assay (CBA) detection of AQP4-IgG are less sensitive in CSF than in serum, and suggest that most AQP4-IgG is produced in peripheral lymphoid tissues and penetrates the CNS via serum/CSF gradient. However, not all NMOSD patients were positive for serum NMO-IgG. It remains unknown why NMO lesions localize mainly in spinal cord and optic nerve without affecting peripheral, AQP4-expressing organs, and what role AQP4 antibodies play in NMOSD. In our patient, the elevated levels of intrathecal Ig suggest that the intrathecal humoral immune response against AQP4 may be involved in the pathogenesis of the disease. With CSF positive for AQP4-IgG, and serum negative, plus positive expression of AQP4 in the renal carcinoma sample, our case supports the hypothesis that AQP4-IgG is initially produced intrathecally, and may be involved in the pathogenesis of NMOSD. Jeffrey et al[7] similarly demonstrated that AQP4-specific Ig is synthesized intrathecally at disease onset and directly contributes to CNS pathology. In addition, there is also the possibility of a secondary origin of AQP4-Abs resulting from massive astrocytic damage due to myelitis of other origin, as suggested by the presence of glial fibrillary acidic protein (GFAP) antibodies in CSF.[8] In that case, the presence of AQP4-Abs would be transient.
Aquaporin-4 is normally expressed in a variety of non-CNS tissues,[9,10] including kidney tissue. Aquaporin-4 has been observed in NMOSD patients presenting with breast carcinoma,[11] ovarian teratoma,[12] and hepatic metastasis from a small-bowel neuroendocrine tumor.[13] Together these reports suggest that in NMOSD patients, aquaporin-4 IgG in the serum may lead to neoplasia. However, whether aquaporin-4 IgG-mediated NMOSD is distinct from other paraneoplatic syndromes requires further investigation.
4. Conclusion
Our case demonstrates an interesting association between positive AQP4 in CSF of a patient with both NMOSD and renal clear cell carcinoma. It serves as a reminder to physicians that it is still necessary to check NMO-IgG in the CSF even when the serum test is negative in a suspected case of NMOSD. Further investigation into the relationship between AQP4-Ab, NMOSD, and renal clear cell carcinoma is needed.
Acknowledgments
The authors thank the patient for her contributions to this work.
Author contributions
Conceptualization: Hongjun Hao, Lei Wang, Yining Huang.
Data curation: Ding Nan, Jingjing Luo.
Funding acquisition: Haiqiang Jin.
Investigation: Hongjun Hao.
Methodology: Hongjun Hao, Ding Nan.
Supervision: Yining Huang.
Writing – original draft: Haiqiang Jin.
Writing – review & editing: Jingjing Luo, Feng Gao, Yining Huang.
Footnotes
Abbreviations: CNS = central nervous system, CSF = cerebrospinal fluid, CT = computed tomography, MRI = magnetic resonance imaging, NMO = neuromyelitis optica, NMOSD = neuromyelitis optica spectrum disorders.
This study was supported by a grant from the National Natural Science Foundation of China (81400941).
This study was approved by the Ethics Committee of Peking University First Hospital. The clinical and imaging data were obtained with the patient's consent for publication of this report.
The authors have no conflicts of interest.
References
- [1].Wingerchuk DM, Lennon VA, Pittock SJ, et al. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485–9. [DOI] [PubMed] [Google Scholar]
- [2].Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet Neurol 2007;6:805–15. [DOI] [PubMed] [Google Scholar]
- [3].Weinshenker BG, Wingerchuk DM. Neuromyelitis spectrum disorders. Mayo Clin Proc 2017;92:663–79. [DOI] [PubMed] [Google Scholar]
- [4].Verkman AS, Phuan PW, Asavapanumas N, et al. Biology of AQP4 and anti-AQP4 antibody: therapeutic implications for NMO. Brain Pathol 2013;23:684–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Majed M, Fryer JP, McKeon A, et al. Clinical utility of testing AQP4-IgG in CSF: guidance for physicians. Neurol Neuroimmunol Neuroinflamm 2016;3:e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bennett JL, Lam C, Kalluri SR, et al. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol 2009;66:617–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ikeshima-Kataoka H. Neuroimmunological implications of AQP4 in astrocytes. Int J Mol Sci 2016;17:1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Verkman AS, Shi LB, Frigeri A, et al. Structure and function of kidney water channels. Kidney Int 1995;48:1069–81. [DOI] [PubMed] [Google Scholar]
- [10].Van Hoek AN, Bouley R, Lu Y, et al. Vasopressin-induced differential stimulation of AQP4 splice variants regulates the in-membrane assembly of orthogonal arrays. Am J Physiol Renal Physiol 2009;296:F1396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Armagan H, Tuzun E, Icoz S, et al. Long extensive transverse myelitis associated with aquaporin-4 antibody and breast cancer: favorable response to cancer treatment. J Spinal Cord Med 2012;35:267–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Frasquet M, Bataller L, Torres-Vega E, et al. Longitudinally extensive transverse myelitis with AQP4 antibodies revealing ovarian teratoma. J Neuroimmunol 2013;263:145–7. [DOI] [PubMed] [Google Scholar]
- [13].Figueroa M, Guo Y, Tselis A, et al. Paraneoplastic neuromyelitis optica spectrum disorder associated with metastatic carcinoid expressing aquaporin-4. JAMA Neurol 2014;71:495–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


