Abstract
Background:
Emerging published studies have indicated that adiponectin is involved in tumorigenesis of breast cancer. However, the results of available studies were inconsistent. The aim of this updated meta-analysis was to assess the association of adiponectin with breast cancer.
Materials and methods:
PubMed, EMBASE, Wanfang databases, and the China National Knowledge Infrastructure (CNKI) were systematically searched from inception to June 2018. The mean difference (MD) with 95% confidence interval (CI) were estimated and pooled to investigate the effect sizes.
Results:
Twenty-seven eligible articles that met the study criteria were included in the current meta-analysis. Overall, there was an evident inverse association between serum adiponectin levels and breast cancer (MD = −0.29, 95%CI = (−0.38, −0.21), P < .001). Asian subgroup showed a significant negative association between serum adiponectin concentrations and breast cancer in subgroup analysis by ethnicity (MD = −2.19, 95%CI = (−3.45, −0.94), P < .001). However, no statistical significance was found in Caucasian subgroup (MD = −0.65, 95%CI = (−1.47, 0.17), P = 0.12). Additionally, a further subgroup analysis of Asian stratified by menopausal status showed higher concentrations of adiponectin in healthy control group, whether they were premenopausal (MD = −0.85, 95%CI = (−1.50, −0.19), P = .01) or postmenopausal (MD = −2.17, 95%CI = (−4.17, −0.18), P = .03). No significant difference was observed concerning the association between serum adiponectin and breast cancer metastasis (MD = −1.56, 95%CI = (−4.90, 1.78), P = .36).
Conclusion:
The current meta-analysis suggests that the serum adiponectin may be inversely associated with breast cancer. Decreased serum adiponectin levels in premenopausal women may also be inversely associated with breast cancer risk other than postmenopausal status. In addition, low serum adiponectin levels in Asian women were more likely to be associated with breast cancer risk than Caucasian women.
Keywords: adiponectin, biomarker, breast cancer, meta-analysis
1. Introduction
Breast cancer, the most commonly diagnosed malignancy, is one of the dominating cause of cancer-related mortality among women worldwide.[1] As a result of enhanced lifespan, widely available screening techniques as well as higher prevalence of well-established risk factors, breast cancer incidence rates have been rising dramatically in recent years.[2–4] Many risk factors have been identified to contribute to the development of breast cancer, among which obesity was proved to be associated with breast cancer in postmenopausal population and shortened survival based on numerous strong evidence.[5–9] Recently, accumulating evidence has suggested that adipokines function as potential mediators linking obesity and breast cancer,[5,9] whereas underlying mechanisms have not been well elucidated.
Adiponectin, an insulin-sensitizing adipokine, which is secreted by adipocytes, along with other adipokines, maintain the metabolic homeostasis. In contrast to most adipokines, such as leptin and TNF-α (tumor necrosis factor-α), increased serum adiponectin levels were demonstrated to function as a protective factor with anti-vascular, anti-inflammation, antidiabetic, and insulin-sensitizing effects.[10] Moreover, several epidemiologic studies have demonstrated a significant inverse association of serum adiponectin with breast cancer risk.[11–14] Among these studies, Miyoshi et al found phenotype of patients with low serum adiponectin levels tended to be biologically aggressive[12] and Mantzoros et al found no statistically significant association in premenopausal women.[13] Furthermore, few studies have reported that increased adiponectin level was negatively correlated with lymph node metastasis and recurrence in patients already diagnosed with breast cancer.[15,16]
However, controversial results obtained in individual studies were insufficient to detect the exact effect of the adiponectin levels on breast cancer. Up to now, the association between adiponectin and breast cancer risk have already been investigated in several previously published meta-analyses with relatively small samples included.[17–19] Besides, adiponectin was the research hotspot in the breast cancer field, and relevant studies were continuously published. Henceforth, we performed an updated meta-analysis to further evaluate the association of the serum adiponectin levels with breast cancer.
2. Materials and methods
2.1. Literature search
PubMed, EMBASE, Wanfang databases, and CNKI were systematically searched to identify relevant studies involving the role of adiponectin in breast cancer up to June 2018. The key search terms were as follows: (“breast neoplasms” or “breast neoplasm” or “breast tumors” or “breast tumor” or “breast cancer” or “breast carcinoma” or “Human Mammary” or “human mammary neoplasms” or “human mammary carcinoma” or “ Mammary Neoplasm, Human”) and (“adiponectin”). The meta-analysis was limited to studies published in English or Chinese. Further, we also reviewed the reference lists of the obtained articles to identify more eligible studies. All analyses were based on previous published studies, thus no ethical approval and patient consent are required in this study.
2.2. Study selection
The inclusive criteria comprised of the following details:
-
(1)
a case-control design;
-
(2)
investigating the association between serum adiponectin levels and breast cancer;
-
(3)
the data provided in the study should be available for calculating mean difference (MD) with 95% confidence interval (CI);
-
(4)
the study subjects are human.
The exclusion criteria were as follows:
-
(1)
duplicative or overlapping publications as well as review;
-
(2)
no control cohort; and
-
(3)
study with incomplete data.
2.3. Quality score assessment
The quality of each eligible case-control study involving the role of serum adiponectin levels in breast cancer was evaluated on the basis of the Newcastle-Ottawa Scale (NOS). The quality was assessed by three aspects, including the selection, comparability, and the ascertainment of the exposure in each case-control study. The total scores ranged 0–9. A study with scores of more than 7 points was regarded as a high-quality study.
2.4. Data extraction
To avoid errors in the pooled analysis, two independent investigators (Zeping Yu and Shenli Tang) collected the information of each included study. To resolve any disagreements, a third investigator (Hongbing Ma) would assess the studies. The information was collected as: first author, publication date, country, ethnicity, age, sample size, pathological classification, cancer stage, assay methods, and serum adiponectin levels. Discrepancies were resolved by consensus.
2.5. Statistical method
All data analysis was performed with Review Manager (version 5.3). All of the data were calculated as MD with 95%CI to evaluate the effectiveness of the association between adiponectin and breast cancer. Heterogeneity was examined by Chi-squared-based Q-test and I2 statistics. The pooled effect size (MD and OR) was computed by the fixed-effects model (FEM) if no or low heterogeneity existed (I2 > 50% and P < .10). Otherwise, the random-effects model (REM) is used. The data collected was stratified on the basis of ethnicity (Asian and Caucasian), menopausal status (premenopausal or postmenopausal), study quality (high quality and low quality), and assay methods (enzyme-linked immunosorbent assay (ELISA) and radio-immunity assay (RIA)) and were then employed in subgroup analyses to investigate the potential source of heterogeneity. Furthermore, sensitivity analyses were also performed by omitting individual studies in sequence to evaluate the stability of the results. Potential publication bias was tested by Begg's and Egger's tests.[20,21] Visual inspection of asymmetry in funnel plots was carried out to further detect publication bias.
3. Results
3.1. Description of the studies
Study search and selection process were summarized as a flow diagram in Figure 1. We identified 502 articles according to the previously described search strategy. Four hundred studies were excluded because they were duplicated studies, and 64 items were excluded due to reviews, conference abstracts, or no relevance, leaving 38 articles. After further, full-view screening, 11 articles were excluded with reasons. Four articles were eliminated because they focused on the association between breast cancer and tissue adiponectin expression levels.[22–25] Another four studies were not qualified for the reasons as follows: Al-Delaimy et al[26] designed their study using breast cancer recurrence among breast cancer patients as endpoint; Lee et al[27] designed breast cancer mortality as endpoint; and the studies reported by George et al[28] and Beg et al[29] were not case-control design. Two articles were excluded for the reason that the serum adiponectin levels cannot be calculated due to lacking of essential information.[30,31] Sonmez et al investigated the adiponectin from multi-source on the same patient, which was excluded.[32] Overall, 27 eligible articles were included in the current meta-analysis.[8,12–16,33–52] All articles included were published in English. Among the studies, twelve articles were conducted in Asians[8,12,14,16,33–35,42,44,46,50,53] while twelve studies were conducted in Caucasians,[13,15,37–40,43,45,48,51,52,54] and the remainder studies were composed of multi-ethnicity participants.[41,47,52] The characteristics of the included studies were summarized in Table 1 and Table 2.
Figure 1.
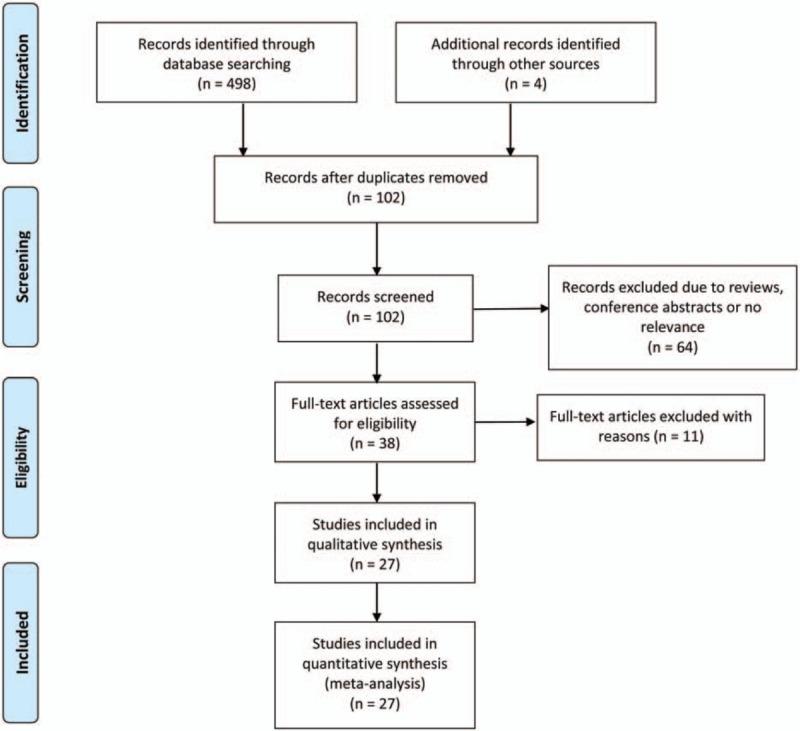
The flow diagram of the included and excluded studies.
Table 1.
Characteristics of studies involving association between the serum adiponectin and breast cancer.
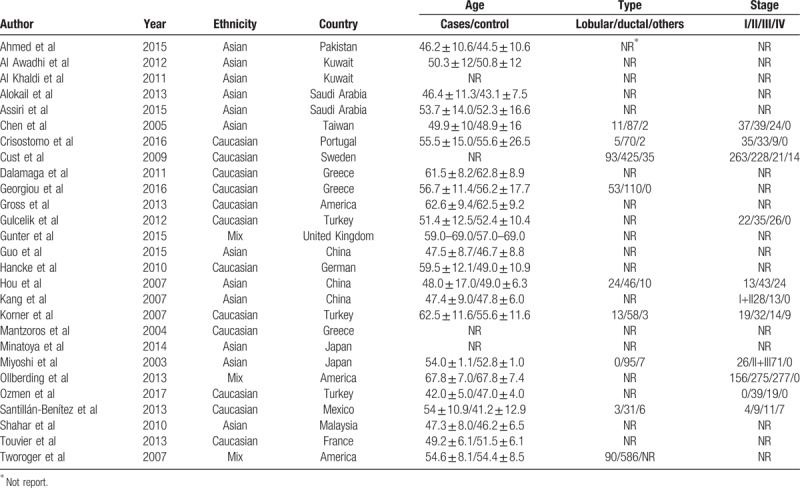
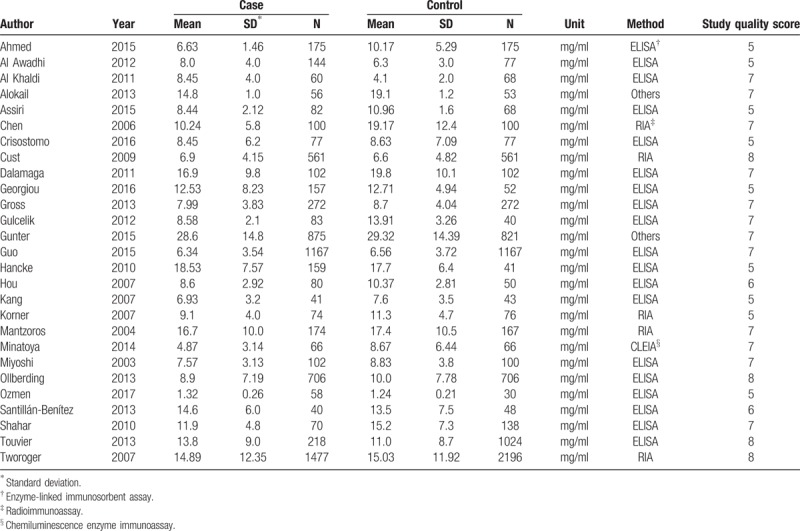
Table 2.
The serum adiponectin levels of studies included in the meta-analysis.
3.2. Overall meta-analysis
A total of 27 eligible case-control studies (7176 cases and 8318 controls) were included in the meta-analysis on the association between serum adiponectin concentrations and breast cancer. As showed in Table 3, the overall meta-analysis results of the random-effect model suggested that patients diagnosed with breast cancer had lower adiponectin values compared with control group (MD = −0.29, 95%CI = (−0.38, −0.21), P < .001) (Fig. 2). However, a significant heterogeneity among studies was observed (I2 = 97%). Whence we conducted subgroup analyses of different specific effects to investigate the source of heterogeneity.
Table 3.
The pooled results of the serum adiponectin levels in breast cancer patients compared with healthy controls.
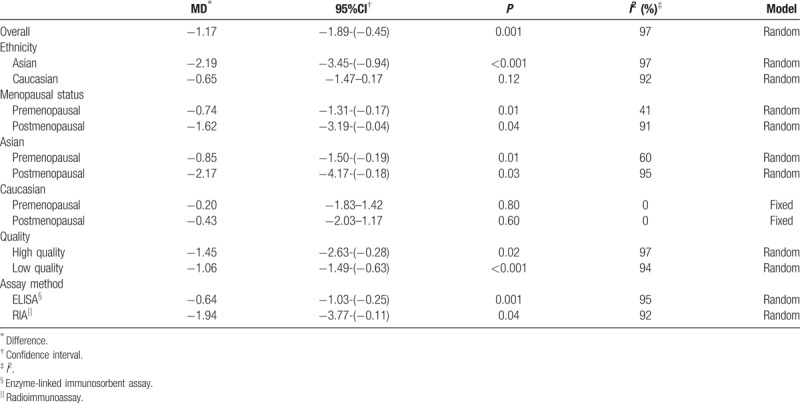
Figure 2.
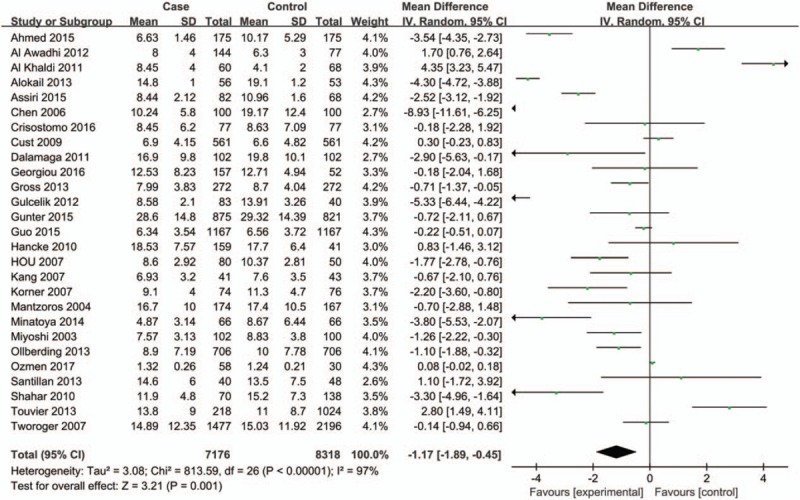
Forest plots of serum adiponectin levels and breast cancer risk in random-effects model for overall population.
3.3. Subgroup meta-analysis
A subgroup analysis of ethnicity was firstly carried out (Caucasian or Asian), and we found higher serum adiponectin concentrations of Asian in the control group with significant difference while no statistical significance was observed in Caucasian (MD = −2.19, 95%CI = (−3.45, −0.94), P < .001; MD = −0.65, 95%CI = (−1.47, 0.17), P = 0.12, respectively) (Fig. 3). The overall studies were then stratified by menopausal status to perform subgroup analysis, which showed higher adiponectin values in healthy control groups in both menopausal statuses (premenopausal status: MD = −0.74, 95%CI = (−1.31, −0.17), P = .01; postmenopausal status: MD = −1.62, 95%CI = (−3.194, −0.04), P = .04) (Fig. 4). A further subgroup analysis was performed to investigate the results in Asian by menopausal status which showed higher concentrations of adiponectin in healthy control group regardless of whether they were premenopausal (MD = −0.85, 95%CI = (−1.50, −0.19), P = .01) or postmenopausal (MD = −2.17, 95%CI = (−4.17, −0.18), P = .03) (Fig. 5). In contrast, the further stratification by menopausal status in Caucasian subgroup showed no statistical results (premenopausal: MD = −0.20, 95%CI = (−1.83, 1.42), P = .80; postmenopausal: MD = −0.43, 95%CI = (−2.03, 1.17), P = .60) (Fig. 6).
Figure 3.
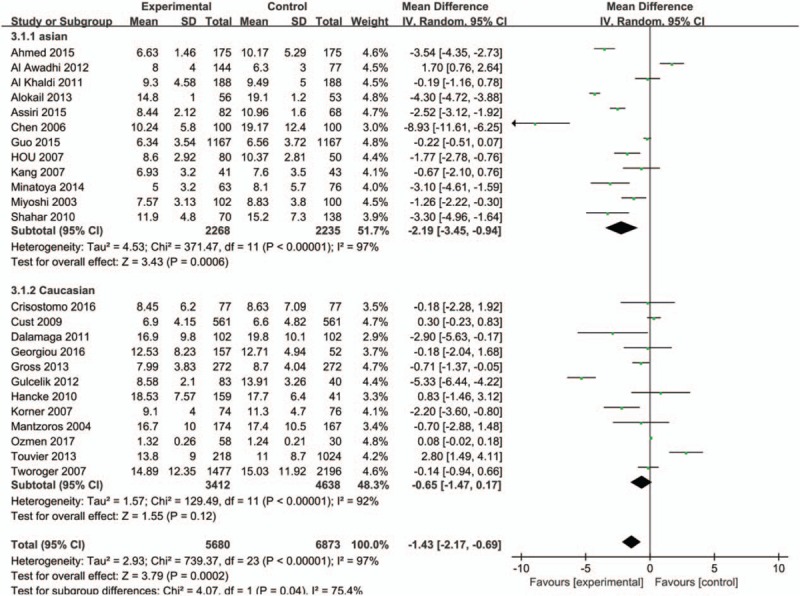
Forest plots of serum adiponectin levels and breast cancer risk in random-effects model for subgroup analysis by ethnicity (Asian and Caucasian).
Figure 4.
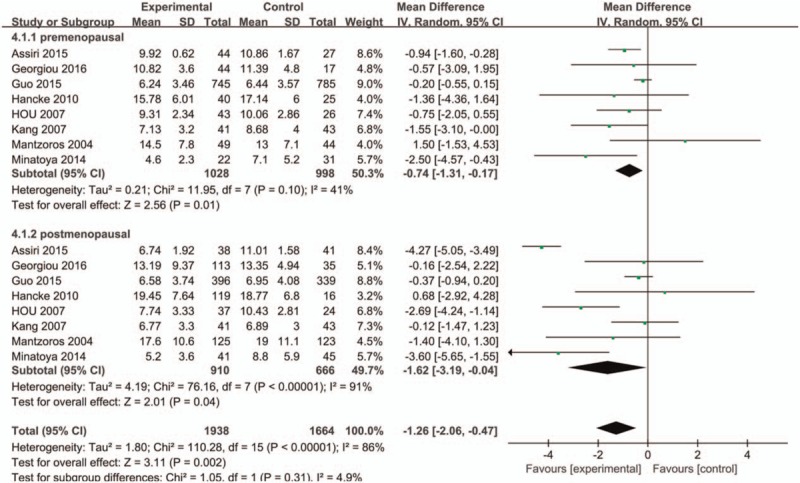
Forest plots of serum adiponectin levels and breast cancer risk in random-effects model for subgroup analysis by menopausal status (premenopausal and postmenopausal).
Figure 5.
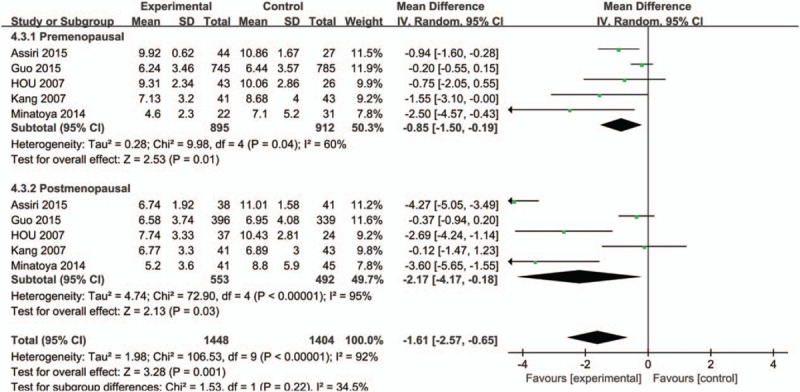
Forest plots of serum adiponectin levels and breast cancer risk in random-effects model for subgroup analysis by menopausal status (premenopausal and postmenopausal) in Asians.
Figure 6.
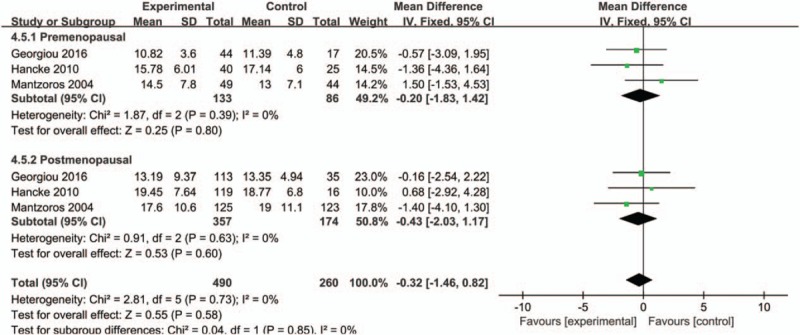
Forest plots of serum adiponectin levels and breast cancer risk in fixed-effects model for subgroup analysis by menopausal status (premenopausal and postmenopausal) in Caucasians.
Study quality was then characterized for subgroup analysis, and we found that adiponectin values in healthy control group were higher than patients with breast cancer both in high-quality studies (NOS score ≥7, 6086 cases and 7591 controls) (MD = −1.45, 95%CI = (−2.63, −0.28), P = .02) and in low-quality studies (NOS score <7, 970 cases and 693 controls) (MD = −1.06, 95%CI = (−1.49, −0.63), P < .001) (Fig. 7). The subgroup analysis of detection method (ELISA or RIA) showed that statistical significances were observed both in ELISA group (MD = −0.64, 95%CI = (−1.03, −0.25), P = .001), and RIA group (MD = −1.94, 95%CI = (−3.77, −0.11), P = 0.04) (Fig. 8). The meta-analysis results of overall studies stratified were presented in Table 3.
Figure 7.
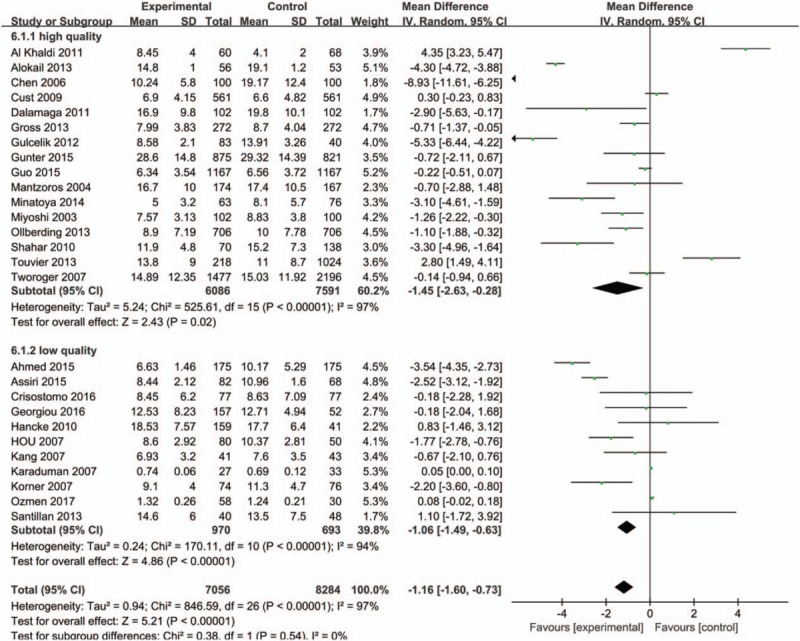
Forest plots of serum adiponectin levels and breast cancer risk in random-effects model for subgroup analysis by quality scores (high quality and low quality).
Figure 8.
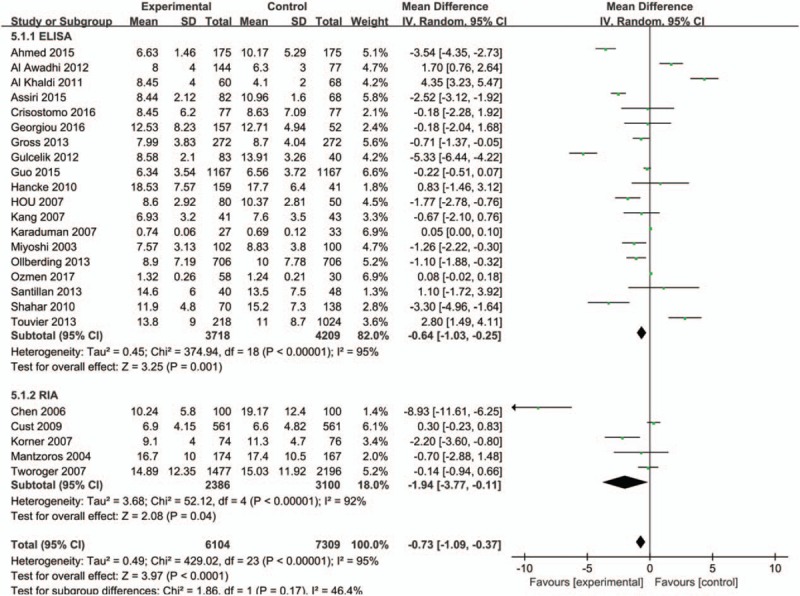
Forest plots of serum adiponectin levels and breast cancer risk in random-effects model for subgroup analysis by detection methods (ELISA and RIA).
3.4. Serum adiponectin levels in breast cancer patients
A total of two studies reported calculable data to obtain serum adiponectin levels in breast cancer patients. For the meta-analysis, the random effect model was applied due to high heterogeneity (I2 = 97%). The results indicated no significant association between serum adiponectin levels and lymph node metastasis in breast cancer patients (MD = −1.56, 95%CI = (−4.90, 1.78), P = 0.36) (Fig. 9).
Figure 9.
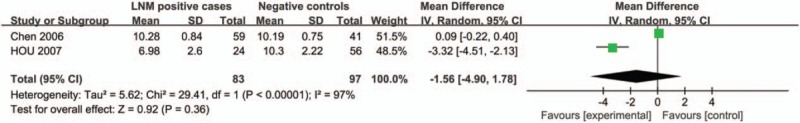
Comparison of differences of the adiponectin levels in breast cancer patients with metastasis and without metastasis.
3.5. Sensitivity analysis
Further, we performed a sensitivity analysis by excluding studies in sequence and evaluating the pooled results to investigate the influence of the corresponding study. The variations regarding heterogeneity and direction of the effect were too minor among residual studies to change our results, which, therefore, indicated that the results of our meta-analysis were stable and robust.
Publication bias in this meta-analysis was evaluated by using Begg's funnel plot and Egger's regression intercept tests. The results of Begg's (P = 0.106) and Egger's tests (P = 0.069) indicated that no publication bias was found in this meta-analysis (Fig. 10).
Figure 10.
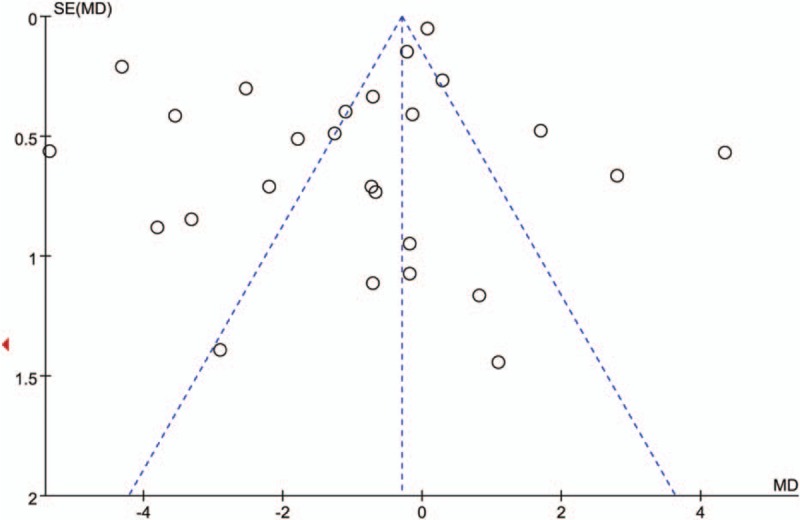
Funnel plot for evaluating publication bias on association between serum adiponectin levels and breast cancer. Circles in the funnel plot implied no asymmetrical distribution, which indicates no publication biases were observed.
4. Discussion
Breast cancer is the most prevalent malignancy in women globally, whose prevalence tends to be younger, with the incidence sharply rising in multiple countries.[1,3,4,55] Obesity, a well-established risk factor associated with breast cancer, indicates final-stage cancer with poor prognosis, especially in postmenopausal population.[39,56,57] Moreover, emerging studies revealed that obese patients diagnosed with breast cancer tended to present a worse prognosis and also suffer an increased risk of cancer progression and recurrence regardless of menopausal status.[58,59] Therefore, adipocytokines were studied to identify as potential biomarkers for possible preventive screening capable of early diagnosis. Many studies have indicated that adiponectin may serve as a protective effect on breast cancer progression. Adiponectin, a polypeptide secreted exclusively by adipose tissue,[36] has been proven as the most abundant adipose tissue sourced protein possessing insulin-sensitizing, anti-inflammatory, and antiatherogenic properties.[10] Further investigations have also revealed three configurations of adiponectin with diverse functions.[60] However, different forms of circulating adiponectin levels might play different roles in breast cancer risk, among which increased circulating high molecular weight (HMW) adiponectin level was reported as a risk factor in females with breast cancer family history.[42] However, the underlying mechanisms all remained unclear. Hence, we undertook a meta-analysis to investigate the association between the concentrations of adiponectin and breast cancer.
Our meta-analysis including 27 primary case-control studies (7176 cases and 8318 controls) was to investigate the association between serum adiponectin values and breast cancer. The overall results suggested that the serum adiponectin values were statistically higher in healthy control groups compared with breast cancer patients. However, we observed a noticeable level of heterogeneity across the studies included in the current meta-analysis (I2 = 97%). The overall result may be affected by multiple factors. Hence we identified several factors which may contribute to this significant heterogeneity:
-
(1)
the discrepancies of demographic characteristics and inheritance backgrounds in Asian and Caucasian populations;
-
(2)
different menopausal status of individuals in the included studies lead to various proportions of postmenopausal women;
-
(3)
different assay methods used in individual study;
-
(4)
included studies were of diverse quality;
-
(5)
the cases diagnosed with breast cancer were at different tumor stages in each study.
In order to investigate the source of heterogeneity, firstly we performed a sensitivity analysis by omitting each study in sequence to investigate the underlying causes. The results showed a minor variation in the test effect, and the excluded studies did not affect the statistical result, indicating the stability of our meta-analysis. Therefore, we identified several characteristics such as ethnicity, menopausal status, study quality, assay methods, ethnicity, and metastasis status to carry out subgroup meta-analysis.
An inverse association between serum adiponectin values and breast cancer was observed in Asian subgroup. However, no statistical result was found in Caucasian subgroup. In these findings, we intended to investigate the effect of ethnicities on adiponectin values variations between breast cancer patients and healthy individuals. Therefore, a further subgroup analysis was then performed to investigate the ethnicity results by menopausal status. Interestingly, similar results were obtained in both menopausal statuses of Asian group, showing higher adiponectin values in healthy controls, which were more significant in postmenopausal group. Coincidentally, no statistical significance was observed in Caucasian subgroup regardless of menopausal status. We continued to perform the subgroup analysis stratified by menopausal status and an inverse association between serum adiponectin and breast cancer was found whether in premenopausal group or postmenopausal group. Only two previously published meta-analysis, which analyzed the association between serum adiponectin levels and breast cancer risk across ethnicities, showed no significant difference.[17,18] However, it was inconsistent with our results showing a significant inverse association in Asian group. Unfortunately, they did not perform a further stratification analysis by ethnicity in their menopausal subgroup to identify the possible impacts that ethnics may possess. The possible reasons contributing to this finding may be as follows. The sample of Asian group (cases and controls: 4503) is relatively minor compared with Caucasian (cases and controls: 8050) in the overall population. The larger group in Caucasian may be confronted with more confounding factors because the direction of association in Caucasian group is the same as Asian group, though insignificant. In another aspect, the dietary habits, life backgrounds, and the obesity populations between the two groups differ substantially. Circulating adiponectin levels have been identified to be inversely correlated with obesity and type 2 diabetes mellitus.[61] Serum adiponectin level increased in obesity population and generally associates negatively with visceral (intra-abdominal) fat, which is independent from menopausal status.[62–64] Since high-calorie diets prevail among European and American areas, which is distinguished from Asian areas, our results may also be partly explained. Our findings could possibly be a potential direction for the current research on breast cancer risk.
The impact of menopausal status on the association between adiponectin levels and breast cancer risk has been substantially investigated in individual studies and researchers were largely in agreement that postmenopausal women were at a higher risk of suffering breast cancer than premenopausal women. This conclusion was supported by the previously published meta-analyses.[17,18] However, in this updated meta-analysis, we also found an increased risk of breast cancer in premenopausal women with lower serum adiponectin levels, which was inconsistent with most studies. Macis et al conducted a meta-analysis and found the association between serum adiponectin levels and breast cancer risk in premenopausal women, which was in the same direction as postmenopausal women, though nonsignificant. Besides, Miyoshi et al reported a case-control study with a sample of 202 patients included and found an inverse association between serum adiponectin levels and breast cancer risk in premenopausal women. One possible reason may be the distinct difference of estrogen generation in different menopausal status. Aromatization impacts on ovarian and adrenal androgens induced by adipocytes of postmenopausal women turn androgens into androgens, which were proved to be associated with breast cancer risks.[65] Adipocytes in postmenopausal obese women generate a larger amount of biologically active estrogen stimulating MEC (mammary epithelial cell mitosis) and advance the tumor progression.[66] Furthermore, Tworoger et al[52] speculated that adiponectin might only make impacts on the proliferation of breast tumor cells in a low estrogen environment. On the other hand, serum leptin levels, negatively correlated with adiponectin levels, were reported to be positively correlated with breast cancer risks in premenopausal women.[67] These findings may partially explain that serum adiponectin levels in postmenopausal women were more likely to be associated with breast cancer risk.
The stronger association between adiponectin and postmenopausal status breast cancer were well-established in various studies, indicating that higher breast cancer risk is specifically associated with decreased levels of adiponectin.[6,7,11,68,69] Our subgroup analysis results suggested an inverse association between serum adiponectin and breast cancer, which was confirmed by many studies from different aspects.[6,7,68–70] Some cytological studies revealed that adiponectin had been proven to inhibit MCF-7 and MD-MB-231 breast cancer cell proliferation in vitro and to present increased expression of the proapoptotic genes Bax and p53.[71,72] Falk Libby et al claimed that specific isoforms of adiponectin might strengthen breast cancer invasiveness.[73] Globular adiponectin (gAd) substantially promotes the breast cancer cells migration and invasiveness, which was not observed on full-length adiponectin (fAd).[73] Several epidemiological studies of adiponectin also concluded that lower serum adiponectin levels were associated with higher breast cancer risk.[12–14]
When it comes to study quality, consistent results were observed showing that adiponectin values were significantly higher in healthy controls regardless of their study quality. The subgroup of assay methods presented the same result as study quality group did, which indicated that both detective methods were acceptable. However, ELISA group presented a more significant difference, which may be a more effective way as most studies adopted.
Previously published articles indicated that adiponectin was identified to present a negative correlation with metastasis,[16,44] tumor grade, and stage.[15,16] In the current subgroup meta-analysis of lymph node metastasis, only two studies[14,16] were analyzed due to insufficient information to calculate relevant adiponectin values in most published studies. Our results with no statistical significance were of low statistical efficiency, and then this made us unable to draw a sound conclusion in this aspect. In a case-control study conducted on 102 breast cancer patients, those who were identified with decreased serum adiponectin levels were more vulnerable to present a biologically aggressive phenotype breast cancer.[12] Conversely, in a prospective study including 1477 incident breast cancer cases, adiponectin was modestly correlated with ductal type of breast cancer instead of lobular tumors.[52] A larger sample based meta-analysis is essential to be carried out in the future for further investigation.
There were several limitations in this meta-analysis. First, our meta-analysis was totally based on observational studies, which is vulnerable to the potential biases and confounding factors not stratified in the current analysis. Second, although we endeavored to search eligible studies, it was possible that few existing or unpublished studies might be missed. Third, we failed to carry out further subgroup analyses to identify other factors due to insufficient information to calculate specific adiponectin values, such as cancer stage, estrogen receptor, or progesterone receptor as well as obesity (based on specific-classified Body Mass Index) which may add to confounding factors. Finally, the relatively minor Caucasian sample compared with Asian in the further subgroup analysis by menopausal status may affect the results obtained. Thus, a further meta-analysis might be essential in the future. Although these limitations exist, we eliminated the feasibility of bias throughout the whole study by executing an elaborate protocol and by study identification, data selection, statistical analysis, and publication bias control. Although controversial results were found in several studies, the accumulating evidence based on etiological, cellular, in vitro, and clinical studies reviewed above with the addition of our large sample included meta-analysis may suggest an inverse association between serum adiponectin values and breast cancer.
In summary, this meta-analysis suggested that serum adiponectin values were inversely associated with breast cancer. Decreased serum adiponectin levels in premenopausal women may also be inversely associated with breast cancer risk, while stronger in postmenopausal status. In addition, low serum adiponectin levels in Asian women were more likely to be associated with breast cancer risk than Caucasian women. These results seem to provide a potential direction into a better understanding of the association between adiponectin and breast cancer risk.
Author contributions
Data curation: Zeping Yu, Hongbing Ma.
Formal analysis: Zeping Yu, Shenli Tang.
Methodology: Zeping Yu, Shenli Tang, Hong Duan.
Validation: Hong Duan, Yong Zeng.
Visualization: Hong Duan, Yong Zeng.
Writing – original draft: Zeping Yu, Shenli Tang, Hongbing Ma.
Footnotes
Abbreviations: CI = confidence interval, CNKI = the China National Knowledge Infrastructure, ELISA = enzyme-linked immunosorbent assay, MD = mean difference, NOS = Newcastle-Ottawa Scale, RIA = radio-immunity assay.
ZY, ST, and HM have contributed equally to this work.
Funding: There is no funding source in this study.
Conflicts of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, or publication of this article.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- [2].Althuis MD, Dozier JM, Anderson WF, et al. Global trends in breast cancer incidence and mortality 1973–1997. Int J Epidemiol 2005;34:405–12. [DOI] [PubMed] [Google Scholar]
- [3].Keramatinia A, Mousavi-Jarrahi SH, Hiteh M, et al. Trends in incidence of breast cancer among women under 40 in Asia. Asian Pac J Cancer Prev 2014;15:1387–90. [DOI] [PubMed] [Google Scholar]
- [4].Leclère B, Molinié F, Trétarre B, et al. Trends in incidence of breast cancer among women under 40 in seven European countries: a GRELL cooperative study. Cancer Epidemiol 2013;37:544–9. [DOI] [PubMed] [Google Scholar]
- [5].Engin A. Obesity-associated breast cancer: analysis of risk factors. Adv Exp Med Biol 2017;960:571–606. [DOI] [PubMed] [Google Scholar]
- [6].Grossmann ME, Ray A, Nkhata KJ, et al. Obesity and breast cancer: status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev 2010;29:641–53. [DOI] [PubMed] [Google Scholar]
- [7].Jardé T, Perrier S, Vasson MP, et al. Molecular mechanisms of leptin and adiponectin in breast cancer. Eur J Cancer 2011;47:33–43. [DOI] [PubMed] [Google Scholar]
- [8].Alokail MS, Al-Daghri N, Abdulkareem A, et al. Metabolic syndrome biomarkers and early breast cancer in Saudi women: evidence for the presence of a systemic stress response and/or a pre-existing metabolic syndrome-related neoplasia risk? BMC Cancer 2013;13:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol 2002;3:565–74. [DOI] [PubMed] [Google Scholar]
- [10].Housa D, Housová J, Vernerová Z, et al. Adipocytokines and cancer. Physiol Res 2006;55:233–44. [DOI] [PubMed] [Google Scholar]
- [11].Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br J Cancer 2006;94:1221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Miyoshi Y, Funahashi T, Kihara S, et al. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res 2003;9:5699–704. [PubMed] [Google Scholar]
- [13].Mantzoros C, Petridou E, Dessypris N, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab 2004;89:1102–7. [DOI] [PubMed] [Google Scholar]
- [14].Chen DC, Chung YF, Yeh YT, et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett 2006;237:109–14. [DOI] [PubMed] [Google Scholar]
- [15].Gulcelik MA, Colakoglu K, Dincer H, et al. Associations between adiponectin and two different cancers: breast and colon. Asian Pac J Cancer Prev 2012;13:395–8. [DOI] [PubMed] [Google Scholar]
- [16].Hou WK, Xu YX, Yu T, et al. Adipocytokines and breast cancer risk. Chin Med J (Engl) 2007;120:1592–6. [PubMed] [Google Scholar]
- [17].Liu LY, Wang M, Ma ZB, et al. The role of adiponectin in breast cancer: a meta-analysis. PLoS One 2013;8:e73183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ye J, Jia J, Dong S, et al. Circulating adiponectin levels and the risk of breast cancer: a meta-analysis. Eur J Cancer Prev 2014;23:158–65. [DOI] [PubMed] [Google Scholar]
- [19].Macis D, Guerrieri-Gonzaga A, Gandini S. Circulating adiponectin and breast cancer risk: a systematic review and meta-analysis. Int J Epidemiol 2014;43:1226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [21].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jeong YJ, Bong JG, Park SH, et al. Expression of leptin, leptin receptor, adiponectin, and adiponectin receptor in ductal carcinoma in situ and invasive breast cancer. J Breast Cancer 2011;14:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Karaduman M, Bilici A, Ozet A, et al. Tissue levels of adiponectin in breast cancer patients. Med Oncol 2007;24:361–6. [DOI] [PubMed] [Google Scholar]
- [24].Lim HY, Im KS, Kim NH, et al. Obesity, expression of adipocytokines, and macrophage infiltration in canine mammary tumors. Vet J 2015;203:326–31. [DOI] [PubMed] [Google Scholar]
- [25].Llanos AA, Dumitrescu RG, Marian C, et al. Adipokines in plasma and breast tissues: associations with breast cancer risk factors. Cancer Epidemiol Biomarkers Prev 2012;21:1745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Al-Delaimy WK, Flatt SW, Natarajan L, et al. IGF1 and risk of additional breast cancer in the WHEL study. Endocr Relat Cancer 2011;18:235–44. [DOI] [PubMed] [Google Scholar]
- [27].Lee SA, Sung H, Han W, et al. Serum adiponectin but not leptin at diagnosis as a predictor of breast cancer survival. Asian Pac J Cancer Prev 2014;15:6137–43. [DOI] [PubMed] [Google Scholar]
- [28].George SM, Neuhouser ML, Mayne ST, et al. Postdiagnosis diet quality is inversely related to a biomarker of inflammation among breast cancer survivors. Cancer Epidemiol Biomarkers Prev 2010;19:2220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Beg MS, Saleem S, Turer A, et al. A prospective analysis of plasma adiponectin and risk of incident cancer: the Dallas Heart Study. J Natl Compr Canc Netw 2015;13:873–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gaudet MM, Falk RT, Gierach GL, et al. Do adipokines underlie the association between known risk factors and breast cancer among a cohort of United States women? Cancer Epidemiol 2010;34:580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tian YF, Chu CH, Wu MH, et al. Anthropometric measures, plasma adiponectin, and breast cancer risk. Endocr Relat Cancer 2007;14:669–77. [DOI] [PubMed] [Google Scholar]
- [32].Sonmez B, Seker M, Bilici A, et al. Is there any correlation among adiponectin levels in serum, tumor tissue and normal tissue of the same patients with breast cancer? J BUON 2011;16:227–32. [PubMed] [Google Scholar]
- [33].Ahmed SD, Khanam A, Sultan N, et al. Serum adiponectin level association with breast cancer risk: evidence from a case-control study. Asian Pac J Cancer Prev 2015;16:4945–8. [DOI] [PubMed] [Google Scholar]
- [34].Al Awadhi SA, Al Khaldi RM, Al Rammah T, et al. Associations of adipokines & insulin resistance with sex steroids in patients with breast cancer. Indian J Med Res 2012;135:500–5. [PMC free article] [PubMed] [Google Scholar]
- [35].Al Khaldi RM, Al Mulla F, Al Awadhi S, et al. Associations of single nucleotide polymorphisms in the adiponectin gene with adiponectin levels and cardio-metabolic risk factors in patients with cancer. Dis Markers 2011;30:197–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Assiri AM, Kamel HF, Hassanien MF. Resistin, visfatin, adiponectin, and leptin: risk of breast cancer in pre- and postmenopausal Saudi females and their possible diagnostic and predictive implications as novel biomarkers. Dis Markers 2015;2015:253519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cust AE, Stocks T, Lukanova A, et al. The influence of overweight and insulin resistance on breast cancer risk and tumour stage at diagnosis: a prospective study. Breast Cancer Res Treat 2009;113:567–76. [DOI] [PubMed] [Google Scholar]
- [38].Dalamaga M, Karmaniolas K, Papadavid E, et al. Elevated serum visfatin/nicotinamide phosphoribosyl-transferase levels are associated with risk of postmenopausal breast cancer independently from adiponectin, leptin, and anthropometric and metabolic parameters. Menopause 2011;18:1198–204. [DOI] [PubMed] [Google Scholar]
- [39].Georgiou GP, Provatopoulou X, Kalogera E, et al. Serum resistin is inversely related to breast cancer risk in premenopausal women. Breast 2016;29:163–9. [DOI] [PubMed] [Google Scholar]
- [40].Gross AL, Newschaffer CJ, Hoffman-Bolton J, et al. Adipocytokines, inflammation, and breast cancer risk in postmenopausal women: a prospective study. Cancer Epidemiol Biomarkers Prev 2013;22:1319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gunter MJ, Wang T, Cushman M, et al. Circulating adipokines and inflammatory markers and postmenopausal breast cancer risk. J Natl Cancer Inst 2015;107:djv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Guo MM, Duan XN, Cui SD, et al. Circulating high-molecular-weight (HMW) adiponectin level is related with breast cancer risk better than total adiponectin: a case-control study. PLoS One 2015;10:e0129246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hancke K, Grubeck D, Hauser N, et al. Adipocyte fatty acid-binding protein as a novel prognostic factor in obese breast cancer patients. Breast Cancer Res Treat 2010;119:367. [DOI] [PubMed] [Google Scholar]
- [44].Kang JH, Yu BY, Youn DS. Relationship of serum adiponectin and resistin levels with breast cancer risk. J Korean Med Sci 2007;22:117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Körner A, Pazaitou-Panayiotou K, Kelesidis T, et al. Total and high-molecular-weight adiponectin in breast cancer: in vitro and in vivo studies. J Clin Endocrinol Metab 2007;92:1041–8. [DOI] [PubMed] [Google Scholar]
- [46].Minatoya M, Kutomi G, Shima H, et al. Relation of serum adiponectin levels and obesity with breast cancer: a Japanese case-control study. Asian Pac J Cancer Prev 2014;15:8325–30. [DOI] [PubMed] [Google Scholar]
- [47].Ollberding NJ, Kim Y, Shvetsov YB, et al. Prediagnostic leptin, adiponectin, C-reactive protein, and the risk of postmenopausal breast cancer. Cancer Prev Res 2013;6:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ozmen HK, Erdemci B, Askin S, et al. Carnitine and adiponectin levels in breast cancer after radiotherapy. Open Med (Wars) 2017;12:189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Santillán-Benítez JG, Mendieta-Zerón H, Gómez-Oliván LM, et al. The tetrad BMI, leptin, leptin/adiponectin (L/A) ratio and CA 15-3 are reliable biomarkers of breast cancer. J Clin Lab Anal 2013;27:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Shahar S, Salleh RM, Ghazali AR, et al. Roles of adiposity, lifetime physical activity and serum adiponectin in occurrence of breast cancer among Malaysian women in Klang Valley. Asian Pac J Cancer Prev 2010;11:61–6. [PubMed] [Google Scholar]
- [51].Touvier M, Fezeu L, Ahluwalia N, et al. Association between prediagnostic biomarkers of inflammation and endothelial function and cancer risk: a nested case-control study. Am J Epidemiol 2013;177:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tworoger SS, Eliassen AH, Kelesidis T, et al. Plasma adiponectin concentrations and risk of incident breast cancer. J Clin Endocrinol Metab 2007;92:1510–6. [DOI] [PubMed] [Google Scholar]
- [53].Minatoya M, Kutomi G, Asakura S, et al. Relationship of serum isoflavone, insulin and adiponectin levels with breast cancer risk. Breast Cancer 2015;22:452–61. [DOI] [PubMed] [Google Scholar]
- [54].Crisóstomo J, Matafome P, Santos-Silva D, et al. Hyperresistinemia and metabolic dysregulation: a risky crosstalk in obese breast cancer. Endocrine 2016;53:433–42. [DOI] [PubMed] [Google Scholar]
- [55].Azim HA, Jr, Partridge AH. Biology of breast cancer in young women. Breast Cancer Res 2014;16:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Fletcher SJ, Sacca PA, Pistone-Creydt M, et al. Human breast adipose tissue: characterization of factors that change during tumor progression in human breast cancer. J Exp Clin Cancer Res 2017;36:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nagaraju GP, Rajitha B, Aliya S, et al. The role of adiponectin in obesity-associated female-specific carcinogenesis. Cytokine Growth Factor Rev 2016;31:37–48. [DOI] [PubMed] [Google Scholar]
- [58].Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–78. [DOI] [PubMed] [Google Scholar]
- [59].Loi S, Milne RL, Friedlander ML, et al. Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 2005;14:1686–91. [DOI] [PubMed] [Google Scholar]
- [60].Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev 2012;33:547–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Chandran M, Phillips SA, Ciaraldi T, et al. Adiponectin: more than just another fat cell hormone? Diabetes Care 2003;26:2442–50. [DOI] [PubMed] [Google Scholar]
- [62].Barb D, Williams CJ, Neuwirth AK, et al. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr 2007;86:s858–66. [DOI] [PubMed] [Google Scholar]
- [63].Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab 2002;13:84–9. [DOI] [PubMed] [Google Scholar]
- [64].Ziemke F, Mantzoros CS. Adiponectin in insulin resistance: lessons from translational research. Am J Clin Nutr 2010;91:258S–61S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].McTiernan A. Associations between energy balance and body mass index and risk of breast carcinoma in women from diverse racial and ethnic backgrounds in the U.S. Cancer 2000;88Suppl:1248–55. [DOI] [PubMed] [Google Scholar]
- [66].Han DF, Zhou X, Hu MB, et al. Polymorphisms of estrogen-metabolizing genes and breast cancer risk: a multigenic study. Chin Med J (Engl) 2005;118:1507–16. [PubMed] [Google Scholar]
- [67].Tessitore L, Vizio B, Pesola D, et al. Adipocyte expression and circulating levels of leptin increase in both gynaecological and breast cancer patients. Int J Oncol 2004;24:1529–35. [PubMed] [Google Scholar]
- [68].Wolf I, Sadetzki S, Kanety H, et al. Adiponectin, ghrelin, and leptin in cancer cachexia in breast and colon cancer patients. Cancer 2006;106:966–73. [DOI] [PubMed] [Google Scholar]
- [69].Jardé T, Caldefie-Chézet F, Goncalves-Mendes N, et al. Involvement of adiponectin and leptin in breast cancer: clinical and in vitro studies. Endocr Relat Cancer 2009;16:1197–210. [DOI] [PubMed] [Google Scholar]
- [70].Jardé T, Caldefie-Chézet F, Damez M, et al. Adiponectin and leptin expression in primary ductal breast cancer and in adjacent healthy epithelial and myoepithelial tissue. Histopathology 2008;53:484–7. [DOI] [PubMed] [Google Scholar]
- [71].Dieudonne MN, Bussiere M, Dos Santos E, et al. Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem Biophys Res Commun 2006;345:271–9. [DOI] [PubMed] [Google Scholar]
- [72].Dos Santos E, Benaitreau D, Dieudonne MN, et al. Adiponectin mediates an antiproliferative response in human MDA-MB 231 breast cancer cells. Oncol Rep 2008;20:971–7. [PubMed] [Google Scholar]
- [73].Falk Libby E, Liu J, Li YI, et al. Globular adiponectin enhances invasion in human breast cancer cells. Oncol Lett 2016;11:633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]


