Abstract
Identifying the determinants of health-related quality of life (HRQOL) improved assessment and decision-making in clinical practice. A few studies have focused on the determinants of HRQOL and their interrelationships in patients with hemorrhagic stroke. The aim of this study was to identify the factors contributing to HRQOL and exam their interrelationships.
A total of 202 patients with hemorrhagic stroke who were discharged from the neurological unit participated in this study. Stroke-specific quality of life was used to assess HRQOL. The Hamilton Rating Scale for Anxiety, the Hamilton Rating Scale for Depression, the Scandinavian Stroke Scale and the Barthel Index were collected as potential predictors as well as social-demographic data. A path analysis was used to explore the potential interrelationships between various factors based on the International Classification of Functioning model.
The final model reasonably fitted the data. The activities of daily living, neurological function and anxiety had direct effects on quality of life. Age, comorbidities, hemorrhage type, financial status, anxiety, and neurological function also had indirect influences on quality of life. All these factors explained 82.0% of all variance in quality of life.
HRQOL in patients with stroke can be predicted by anxiety, neurological function, activities of daily living and other personal and environmental factors. These identified predictors and their interrelationships may assist clinical professions focusing their assessments and developing strategies for modifiable factors to improve HRQOL.
Keywords: determinants, path analysis, quality of life, stroke
1. Introduction
Stroke is the second leading cause of death and is also the main reason for long-term disability worldwide.[1–3] Furthermore, stroke, especially hemorrhagic stroke, is a major health concern in China and is the first leading cause of death and disability.[4] According to a report from The Stroke Control Project Committee of National Health and Family Planning Commission of China (2015), the prevalence rate of hemorrhagic stroke was 125.78/100,000 in urban areas and 159.91/100,000 in rural areas. The morbidity of hemorrhagic stroke in China was nearly 3 times that of the world level (60–80/100,000 vs 24.6/100,000) and increased approximately 8.7% per year.[5,6] The burden of hemorrhagic stroke is expected to worsen in the coming years. Much progress has been made in treatments and rehabilitations for stroke survivors to increase survival rates, and quality of life has been an important outcome of medical care.[7] Health-related quality of life (HRQOL) reflects the impact of the health of an individual on functional and perceived wellness in different life domains.[8] Identifying the predictors for HRQOL can provide deep insights into the treatment and rehabilitation intervention strategies in clinical practice.[9]
Several studies have examined the predictors of HRQOL in patients with stroke. According to a literature review, age,[10–12] sex,[10,11] education level,[9] financial status,[13] physical function,[9,13,14] cognitive function,[12,15] comorbidities,[11,16] anxiety, and depression[17,18] have been reported to be predictors of HRQOL. Despite the large number of studies examining the predictors of HRQOL, little is known about the factors that predict quality of life in hemorrhagic stroke patients. Additionally, most studies used multiple regression analysis to identify predictors of HRQOL, which could not take the potential interrelationships between various variables into account. Thus, path analysis is an alternative method to identify the direct and indirect pathways through which potential interrelationships affect HRQOL.[19] To account for the interrelationships between predictor variables using path analysis, a theoretical framework is needed.
The International Classification of Functioning, Disability and Health (ICF) framework[20] was selected to examine the relationships between the predictor variables of HRQOL. The ICF is a universal framework, that covers the spectrum of problems involved in patients with acute and chronic health conditions.[21,22] The 3 key components of ICF are body function and structure, activity and participation.[20] Loss or deviations from normal body functions and structures are defined as body function or structure impairments; difficulties in daily activities are referred to as activity limitation; problems involving life situations are participation restrictions.[20] Moreover, the ICF framework takes personal and environmental factors into consideration. The multi-perspective domains of the ICF were adopted to describe the health conditions of hemorrhagic stroke (Fig. 1). According to the domains of the ICF framework, the potential predictor variables of HRQOL were divided into clearly defined groups. Thus, a theoretical model can be established based on the hypothesis that the HRQOL of patients with hemorrhagic stroke depends on personal and environmental factors, body function or structure impairments and activity limitations (Fig. 2).
Figure 1.
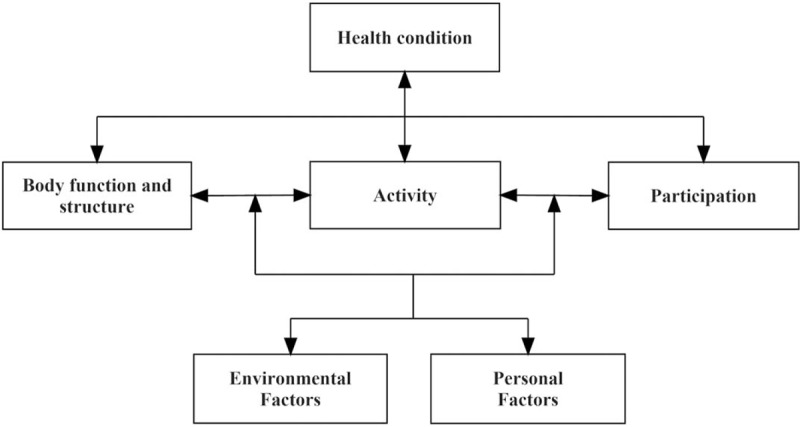
Examples of the interactions between the ICF components for people with stroke. ICF = International Classification of Functioning, Disability and Health.
Figure 2.
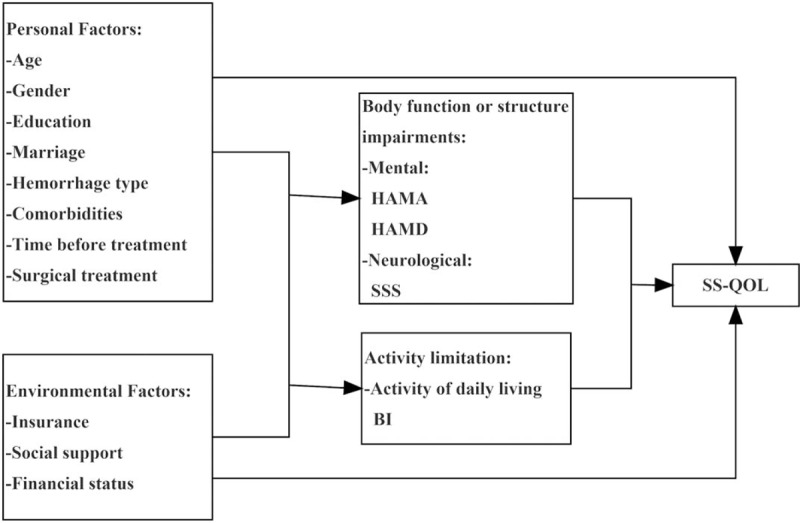
Measurement model based on the ICF framework. BI = Barthel Index, HAMA = Hamilton Rating Scale for Anxiety, HAMD = Hamilton Rating Scale for Depression, ICF = International Classification of Functioning, Disability and Health, SS-QOL = stroke specific quality of life, SSS = Scandinavian Stroke Scale.
The aim of this study was to identify the predictors of HRQOL in patients with hemorrhagic stroke and explore the direct and indirect relationships between the identified determinants under the framework of ICF.
2. Methods
2.1. Study design and participants
This study was a cross-sectional study performed in the neurosurgery unit of a teaching hospital in Chengdu, China from January 2016 to September 2017. A total of 2437 consecutive patients who were discharged from the ward were screened, and 202 agreed to take part in and complete this study. The inclusion criteria of the stroke survivors were:
-
(1)
a diagnosis of first-ever hemorrhagic stroke by CT/MRI;
-
(2)
a Glasgow Coma Scale score of 9 or above; and
-
(3)
age older than 18 years.
The exclusion criteria were:
-
(1)
patients in the acute stroke phase (less than 1 month after onset); and
-
(2)
unable to read or understand the questionnaire.
The study was approved by the Ethnics Committee of West China Hospital, Sichuan University. Written informed consent was obtained from the participants before the questionnaires were distributed.
2.2. Data collection
2.2.1. Health-related quality of life
The stroke-specific quality of life (SS-QOL) questionnaire is specifically designed for patients with stroke, which is significantly more valid and sensitive compared to traditional instruments.[23,24] The questionnaire consists of 12 domains encompassing 49 items, including the social role, mobility, energy, language, self-care, mood, personality, thinking, upper extremity function, family role, vision, and work/productivity. Each item is scored on a 5-point Likert scale in which 1 means complete agreement and 5 means complete disagreement. The total score ranges from 49 to 245, with higher scores indicating a better quality of life. Wang, Jian-Guo, Jun-Tao[25] translated the SS-QOL questionnaire into Chinese version and investigated the reliability and validity in patients with stroke in China. All 12 domains showed excellent reliability in test-retest, inter-test (kappa coefficient ranged from 0.82–1.00) and internal consistent (Cronbach’α coefficient > 0.76).[25]
2.2.2. Personal and environmental factors
Personal factors, including age, gender, education level, and marriage status (Fig. 2), were examined. Characteristics of disease including hemorrhage type, comorbidities, time before treatment and whether to receive surgical treatment were also collected. Environmental factors included social support, insurance, and finical status. Participants were categorized as having adequate social support if they lived with caregivers, such as family members and formal caregivers.
2.2.3. Impairments in body structure or function
The Hamilton Rating Scale for Anxiety (HAMA) and the Hamilton Rating Scale for Depression (HAMD) were used to measure the impairments in mental function of patients.[26,27] The HAMA consists of 14 items measured on a 5-point scale. A score of 0 represents no and 4 represents extremely. The total score was classified as no (<7), potential (7–13), assured (14–29) and severe anxiety (>29). The HAMD contains 24 items, of which 9 items are defined from 1 to 2, 1 item is defined from 0 to 2, and 14 items are defined from 0 to 4. The total score was classified as no (<8), potential (8–19), assured (20–35), and severe depression (>35).
Impairments in neurological function were assessed using the Scandinavian Stroke Scale (SSS).[28] The SSS contains 9 items measured with 1 item scoring 0 to 2, 1 item scoring 0 to 4, 5 items scoring 0 to 6, 1 item scoring 0 to 10 and 1 item scoring 0 to 12. The total score was classified as good (>50), intermediate (30–49) or poor (<29).
2.2.4. Activity limitations
Limitations in performing self-care activities and mobility were examined in relation to HRQOL (Fig. 2). Self-care activities referring to the performance of daily tasks such as dressing and washing were evaluated via the ability of daily living. The Barthel Index (BI) served to measure the activities of daily living of the patients.[29] The scale describes 10 tasks in daily life and is scored according to the time or assistance required by patients. Two items range from 0 to 5, 6 range from 0 to 10 and 2 range from 0 to 15. The total score ranges from 0 to 100, with a higher score representing greater independence.
2.3. Procedure
Participants were screened from the medical records and interviewed over the telephone or face-to-face to obtain informed consent. Then, social-demographic data and clinic characteristics were collected from the medical records. The HRQOL, body function or structure impairment and activity limitation were assessed using SS-QOL, HAMA, HAMD, SSS, and BI scales by a trained research assistant.
2.4. Data analysis
Descriptive statistics of the predictor variables based on the ICF framework (Fig. 2) were presented as the mean, standard deviation, median, interquartile range and percentages as appropriate. Path analysis was used to examine the hypothesized causal relationships between social-demographic factors, mental impairments, neurological function impairments, activity limitations and quality of life. As illustrated in Fig. 2, these relationships were assumed to be unidirectional. Structural equational modeling (SEM) was conducted to assess the fitness of the final model. The χ2 static was used to assess the magnitude of the discrepancy between the sample and fitted covariance matrices, where the P >.05 indicated that the model and data were consistent.[30] The root mean square error of approximation (RMSEA), the goodness-of-fit index (GFI), the adjusted goodness-of-fit index (AGFI), the standardized root mean square residual (SRMR), and the Akaike Information Criterion (AIC) was used to evaluate the optimum model, with RMSEA <0.08, GFI >0.90, AGFI >0.90, and SRMR < 0.05 indicating good model fitness. The path analysis was performed using Amos 24 (SPSS Inc., Chicago, IL).
3. Results
3.1. Social-demographic and clinical characteristics
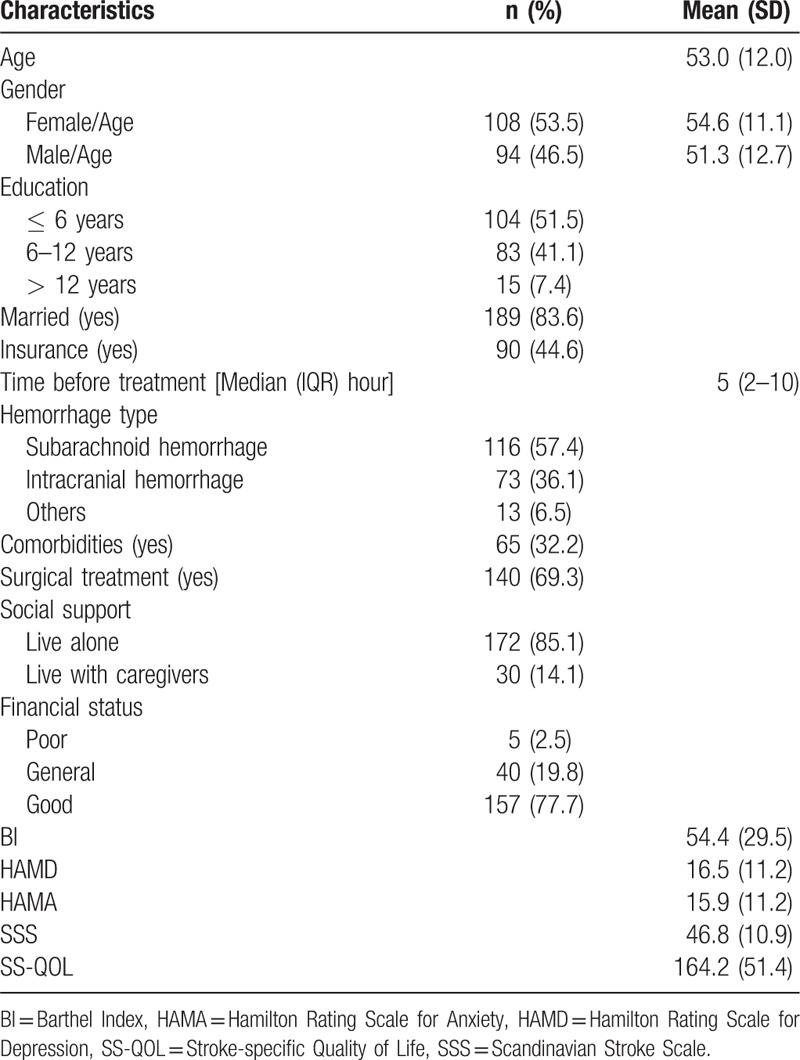
Table 1 shows the social-demographic and clinical characteristics of patients. The participants consisted predominantly of females (53.5%) with a mean age of 54.6 years old (SD = 11.1). Of all participants, 92.6% had a low to moderate education level, and only 44.6% had medical insurance. Subarachnoid hemorrhage was the main cause of hemorrhagic stroke in this sample (57.4%). Most of the participants underwent surgical treatment (69.3%). The participants had moderate activity limitations and neurological function impairments. The participants experienced assured anxiety and potentially borne depression.
Table 1.
Descriptive summary of patient characteristics (n = 202).
3.2. Relationships between SS-QOL, personal and environmental factors
A path analysis model based on the ICF framework (Fig. 1) was used to examine the relationships between personal and environmental factors, mental function impairments, neurological impairments, and activity limitations with quality of life. This model explained 82% of all variance in SS-QOL, and no personal and environmental factors directly contributed to SS-QOL (Fig. 3a). However, age, hemorrhage type, comorbidities, and financial status contributed to SS-QOL indirectly via neurological function impairments (β = −0.20, P <.001; β = 0.24, P <.001; β = 0.18, P = .003; and β = 0.30, P <.001). Additionally, comorbidities were also indirectly related to SS-QOL through HAMA (β = −0.28, P <.001).
Figure 3.
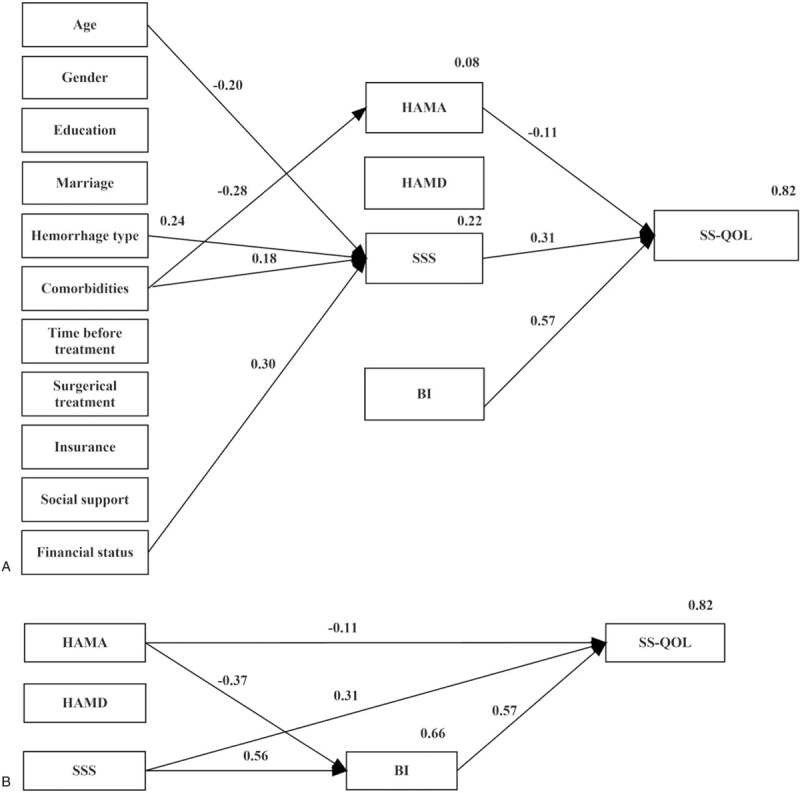
Model of HRQOL illustrating the relationship between HRQOL and a personal and environmental factors; b stroke impairments and activity limitations only. BI = Barthel Index, HAMA = Hamilton Rating Scale for Anxiety, HAMD = Hamilton Rating Scale for Depression, HRQOL = health-related quality of life, SS-QOL = stroke specific quality of life, SSS = Scandinavian Stroke Scale.
3.3. Relationship between SS-QOL, function impairments and activity limitations
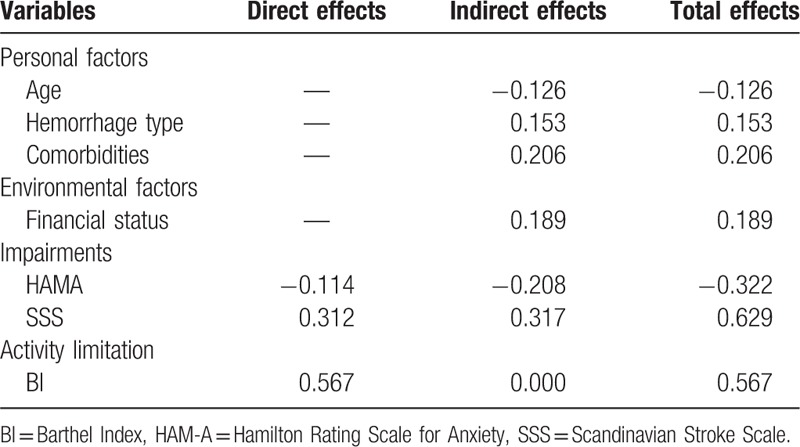
The relationships between SS-QOL function impairments and activity limitations are displayed in Fig. 3b. Activity limitation (BI) directly contributed to SS-QOL most strongly (β = 0.57, P <.001). Anxiety (HAMA) and neurological function impairments (SSS) were also significant contributing factors. Anxiety contributed to SS-QOL directly via a relationship with SS-QOL (β = −0.11, P = .004) and indirectly through activity limitation (β = −0.37, P <.001). Similarly, the contribution of neurological function impairments was established via a direct relationship with SS-QOL (β = 0.31, P <.001) and indirectly through activity limitation (β = 0.57, P <.001). However, depression had no contribution to SS-QOL through either direct or indirect pathway. Table 2 shows the standardized direct, indirect and total effects of personal and environmental factors, function impairments and activity limitation on SS-QOL.
Table 2.
Standardized direct, indirect and total effects of personal and environmental factors on SS-QOL.
3.4. The model of SS-QOL in patients with hemorrhagic stroke
The statistics to assess the fit of the model are shown in Table 3. The χ2 test was non-significant (P = .246) and the RMSEA was 0.032 (less than 0.08), indicating that the model and data were consistent. The discrepancy divided by degrees of freedom was 1.248 (less than 2), suggesting a good fit of this model. The GFI and AGFI were 0.973 and 0.941 (greater than 0.90). The SRMR was 0.048 (less than 0.05). All the statistics showed the good fitness of this model.
Table 3.
Summary of the final model fit statistics.

4. Discussion
The model examined in this study reasonably fitted with the data, indicating that it was effective to identify the key predictors of HRQOL in patients with hemorrhagic stroke using path analysis. Complex interrelationships between personal and environmental factors, body function or structure impairments and activity limitations were found and explained 82% of all variance in the HRQOL of patients with hemorrhagic stroke.
4.1. Direct effects
The model coefficients showed that the activity limitation, mental and neurological impairments had direct effects on HRQOL. The activity limitation had the largest effect and explained most of the variance in HRQOL.
Similar to previous studies,[13,14,31] the activity limitation in this study significantly predicted the HRQOL of patients with hemorrhagic stroke. The stroke survivors always lived with functional impairments, such as motor disorder, sensory disorder or language disorder.[31–33] The decrease of function level decided the lower ability to cope with self-care activities of daily living, which played an important role in HRQOL. In fact, the quality of life was the individual's self-perceived position within the social contexts.[34] The decrease of activities of daily living indicates the care needs from others, which may change the self-perceived position of patients, leading to a decrease in quality of life. This result indicated that professions should provide more interventions to improve the daily living activities of patients. Functional rehabilitations for patients were confirmed to be effective in improving the HRQOL.[12] Additionally, using supportive tools appropriately for the daily activities of stroke survivors may also be useful to obtain better quality of life.[35]
The neurological function impairments were also a strong predictor of HRQOL in this study. This result confirmed the significant relationship between neurological function and HRQOL in previous studies.[15,36] Cognitive impairment was the most common outcome caused by neurological function impairments. The relationship between HRQOL and cognitive function was well-known.[37] Carod-Artal, Trizotto, Coral, Moreira[36] found that better cognitive function predicted better communication and memory domains of HRQOL in patients with stroke. Cognitive function was also recognized as a predictor of the physical function domain of HRQOL.[38] The motor disorder also had side effects on the HRQOL of patients. Typically, the upper limb and lower extremity motor function recovery helped patients perceive HRQOL.[39,40] This result indicated that neurological function recovery was the key component for patients’ rehabilitation after discharge, including sensory, memory, motion, cognition, and communication exercise.
It was not surprising to find that anxiety was a determinant of HRQOL. In fact, anxiety was a common psychological problem for stroke survivors with a prevalence of 22% to 25% in the first 6 months after stroke.[41] The health consequences, evolution, and fear of aggravation of disease, the change in financial status and care needs brought serious anxiety to patients.[33] High anxiety easily decreased energy levels and led to the social isolation of patients, thereby influencing HRQOL.[39,42] This finding revealed that mental health of patients with stroke was important. Detailed disease and cure information, available health care services after discharge and early interventions for anxiety symptoms may reduce anxiety. However, there was no evidence that depression had any impact on HRQOL in this study. This finding was inconsistent with previous studies, most of which suggested that depression or both anxiety and depression were related to HRQOL in patients with stroke.[17,18,39] Although patients with stroke had a higher prevalence of depression (24% to 30%) than anxiety,[41] depression showed less stability and persistence than anxiety, especially at the early stage after stroke.[39] This may explain why depression did not predict the HRQOL in this study. More studies are required to explore whether interaction between anxiety and depression exists and how this relationship influences HRQOL.
4.2. Indirect effects
Although all personal and environmental factors had no direct effects on HRQOL, age, hemorrhage type, and financial status had indirect effects on HRQOL via neurological impairments. This result may imply that patients with older age, subarachnoid hemorrhage, and poor financial status had lower HRQOL through the level of neurological function. Additionally, comorbidities had effects on HRQOL through anxiety and neurological impairments, confirming the effects of comorbidities on HRQOL in previous studies.[11,36]
Apart from the direct effect, anxiety had an indirect influence on HRQOL through activity limitations. Anxiety certainly influences the physical rehabilitations of patients by intensifying fear, nervousness and muscle tension.[43] This effect may result in a decrease of ability in daily living. As noted in a previous study, patients with post-stroke anxiety suffered more daily life activity limitations than social function limitations.[43] This may explain how anxiety influences HRQOL through activity limitation. Therefore, clinical professions should take into account the influence of anxiety on physical rehabilitation and HRQOL. Neurological impairments also had an indirect effect on HRQOL through activity limitation. The motor disorder, vision disorder, cognitive impairments and some other function impairments caused by neurological impairments made daily activities, such as bathing, washing and dressing much harder for patients. This result indicated that the rehabilitation of neurological function may improve HRQOL by increasing the activities of daily living of patients with stroke.
5. Limitations
There are several limitations in this study. First, the convenience sample with a Glasgow Coma Scale score of 9 or above reduces the representativeness of this study. However, it is necessary to exclude patients with Glasgow Coma Scale scores under 9 due to the anxiety and depression assessment. Second, the small sample size regarding the number of parameters that were examined in this study made it hard to draw definitive causal inferences. A larger sample size study is required to provide better insight into how patients with hemorrhagic stroke perceive HRQOL. Finally, the cross-sectional study cannot describe the change in HRQOL over time. Longitudinal studies are needed to examine the change of HRQOL over time and its determinants at different time points.
6. Conclusion
This study showed that anxiety, neurological impairments and activities of daily living of patients with hemorrhagic stroke directly contributed to HRQOL. The study also demonstrated a model based on the ICF framework including personal and environmental factors, body function or structure impairments and activity limitations. The determinants of HRQOL and the interrelationships between these factors may help clinical professions to develop specific interventions for some modifiable factors, such as anxiety, to improve the HRQOL of patients with hemorrhagic stroke.
Acknowledgments
The authors would like to thank the participants for their contribution to this study. And also, authors want to thank Professor Guanjian Liu for helping with data analysis in this project.
Author contributions
The manuscript has been read and approved by all authors. The authors are alone responsible for the content and writing of the paper. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Conceptualization: Wei Zhu, Yan Jiang.
Data curation: Wei Zhu.
Formal analysis: Wei Zhu.
Investigation: Wei Zhu.
Methodology: Yan Jiang.
Supervision: Yan Jiang.
Writing – original draft: Wei Zhu.
Writing – review & editing: Wei Zhu, Yan Jiang.
Wei Zhu orcid: 0000-0001-5032-6277.
Footnotes
Abbreviations: AGFI = adjusted goodness-of-fit index, BI = Barthel Index, GFI = goodness-of-fit index, HAMA = Hamilton Rating Scale for Anxiety, HAMD = Hamilton Rating Scale for Depression, HRQOL = health-related quality of life, ICF = International Classification of Functioning, Disability and Health, RMSEA = root mean square error of approximation, SRMR = standardized root mean square residual, SS-QOL = stroke-specific quality of life, SSS = Scandinavian Stroke Scale.
The authors declare no potential conflicts of interest involved in this study.
References
- [1].Geneva. Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016. 2018; Available at: http://www.who.int/healthinfo/global_burden_disease/estimates/en/ Accessed July 26, 2018. [Google Scholar]
- [2].Feigin VL, Mensah GA, Bo N, et al. Group GSPE. Atlas of the global burden of stroke (1990–2013): the GBD 2013 study. Neuroepidemiology 2015;45:230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Slomka A, Świtonska M, Sinkiewicz W, et al. Haemostatic factors do not account for worse outcomes from ischaemic stroke in patients with higher C-reactive protein concentrations. Ann Clin Biochem 2017;54:378–85. [DOI] [PubMed] [Google Scholar]
- [4].Zhijuan B. A summary of rehabilitation nursing of stroke patients. Pract J Card Cereb Pneumal Vasc Dis 2013;9:160–1. [Google Scholar]
- [5].Fu X, Wong KS, Wei JW, et al. Factors associated with severity on admission and in-hospital mortality after primary intracerebral hemorrhage in China. Int J Stroke 2013;8:73–9. [DOI] [PubMed] [Google Scholar]
- [6].Jia Q, Liu LP, Wang YJ. Stroke in China. Clin Exp Pharmacol Physiol 2010;37:259–64. [DOI] [PubMed] [Google Scholar]
- [7].Pain K. Quality of life: what does it mean in rehabilitaion. J Rehabil 1998;64:5–11. [Google Scholar]
- [8].Coons DSJ, Rao S, Keininger DL, et al. A comparative review of generic quality-of-life instruments. Pharmacoeconomics 2000;17:13–35. [DOI] [PubMed] [Google Scholar]
- [9].Chou CY. Determinants of the health-related quality of life for stroke survivors. J Stroke Cerebrovasc Dis Off J Nat Stroke Assoc 2015;24:655–62. [DOI] [PubMed] [Google Scholar]
- [10].Sturm JW, Donnan GA, Dewey HM, et al. Quality of life after stroke the north east Melbourne stroke incidence study (NEMESIS). Stroke 2004;35:2340–5. [DOI] [PubMed] [Google Scholar]
- [11].Pkb M, Gunathunga MW, Jayasinghe S, et al. Factors influencing pre-stroke and post-stroke quality of life among stroke survivors in a lower middle-income country. Neurol Sci 2018;39:287–95. [DOI] [PubMed] [Google Scholar]
- [12].Safaz İ, Kesikburun S, Adigüzel E, et al. Determinants of disease-specific health-related quality of life in Turkish stroke survivors. Int J Rehabil Res Int Zeitschrift Rehabilitationsforschung Revue Int Recherches Réadaptation 2016;39:130–3. [DOI] [PubMed] [Google Scholar]
- [13].Chang WH, Sohn MK, Lee J, et al. Predictors of functional level and quality of life at 6 months after a first-ever stroke: the KOSCO study. J Neurol 2016;263:1166–77. [DOI] [PubMed] [Google Scholar]
- [14].Gurcay E, Bal A, Cakci A. Health-related quality of life in first-ever stroke patients. Ann Saudi Med 2009;29:36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen CM, Tsai CC, Chung CY, et al. Potential predictors for health-related quality of life in stroke patients undergoing inpatient rehabilitation. Health Quality Life Outcomes 2015;13:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nichols-Larsen DS, Clark PC, Zeringue A, et al. Factors influencing stroke survivors’ quality of life during subacute recovery. Stroke 2005;36:1480–4. [DOI] [PubMed] [Google Scholar]
- [17].De WL, Theuns P, Dejaeger E, et al. Long-term impact of stroke on patients’ health-related quality of life. Disabil Rehabil 2016;39:1435–40. [DOI] [PubMed] [Google Scholar]
- [18].Fróes KS, Valdés MT, Lopes DP, et al. Factors associated with health-related quality of life for adults with stroke sequelae. Arq Neuropsiquiatr 2011;69:371–6. [DOI] [PubMed] [Google Scholar]
- [19].Soh S, Mcginley JL, Watts JJ, et al. Determinants of health-related quality of life in people with Parkinson's disease: a path analysis. Qual Life Res 2013;22:1543–53. [DOI] [PubMed] [Google Scholar]
- [20].Mandich M. International classification of functioning, disability and health. Phys Occup Ther Pediatr 2007;27:1–4. [PubMed] [Google Scholar]
- [21].Cieza A, Ewert T, Ustün TB, et al. Development of ICF Core Sets for patients with chronic conditions. J Rehabil Med 2004;36suppl 44:9–11. [DOI] [PubMed] [Google Scholar]
- [22].Grill E, Ewert T, Chatterji S, et al. ICF Core Sets development for the acute hospital and early post-acute rehabilitation facilities. Disabil Rehabil 2009;27:361–6. [DOI] [PubMed] [Google Scholar]
- [23].Mahmoodi M, Safari A, Vossoughi M, et al. Stroke specific quality of life questionnaire: test of reliability and validity of the Persian version. Iranian J Neurol 2015;14:94–100. [PMC free article] [PubMed] [Google Scholar]
- [24].Williams LS, Weinberger M, Harris LE, et al. Development of a stroke-specific quality of life scale. Stroke J Cereb Circ 1999;30:1362–9. [DOI] [PubMed] [Google Scholar]
- [25].Yi-Long W, Jian-Guo MA, Jun-Tao LI. The study on reliability,validity and responsiveness of the chinese version of stroke-specific quality of life. Chin J Geriatr Cardiovasc Cerebrovasc Dis 2003;5:391–4. [Google Scholar]
- [26].Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol 1959;32:50–5. [DOI] [PubMed] [Google Scholar]
- [27].Wang L, Zhong Z, Hu J, et al. Sertraline plus deanxit to treat patients with depression and anxiety in chronic somatic diseases: a randomized controlled trial. Bmc Psychiatry 2015;15:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Stückle D. Asia Pacific Stroke Conference 2016. Abstracts of the annual conference of the asia pacific stroke organization (APSO) Combined with stroke society of Australasia, Brisbane, Qld., Australia, July 14–17, 2016: abstracts. Cerebrovasc Dis 2016;42suppl 1:1. [DOI] [PubMed] [Google Scholar]
- [29].Granger CV, Albrecht GL, Hamilton BB. Outcome of comprehensive medical rehabilitation: measurement by PULSES profile and the Barthel Index. Arch Phys Med Rehabil 1979;60:145–54. [PubMed] [Google Scholar]
- [30].Keith T. Multiple Regression and Beyond. 2nd.New York: Routledge; 2015. [Google Scholar]
- [31].Rachpukdee S, Howteerakul N, Suwannapong N, et al. Quality of life of stroke survivors: a 3-month follow-up study. J Stroke Cerebrovasc Dis 2013;22:e70–8. [DOI] [PubMed] [Google Scholar]
- [32].Lopezespuela F, Zamorano JD, Portillacuenca JC, et al. Determinants of quality of life in stroke survivors after 6 months, from a comprehensive stroke unit: a longitudinal study. Biol Res Nurs 2015;17:461–8. [DOI] [PubMed] [Google Scholar]
- [33].Baumann M, Bihan EL, Chau K, et al. Associations between quality of life and socioeconomic factors, functional impairments and dissatisfaction with received information and home-care services among survivors living at home two years after stroke onset. BMC Neurol 2014;14:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pkb M, Gunathunga MW, Jayasinghe S, et al. Pre-event quality of life and its influence on the post-event quality of life among patients with ST elevation and non-ST elevation myocardial infarctions of a premier province of Sri Lanka. Health Qual Life Outcomes 2017;15:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jaracz K, Grabowska-Fudala B, Górna K, et al. Caregiving burden and its determinants in Polish caregivers of stroke survivors. Arch Med Sci AMS 2014;10:941–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Carod-Artal FJ, Trizotto DS, Coral LF, et al. Determinants of quality of life in Brazilian stroke survivors. J Neurol Sci 2009;284:63–8. [DOI] [PubMed] [Google Scholar]
- [37].Cumming TB, Brodtmann A, Darby D, et al. The importance of cognition to quality of life after stroke. J Psychosom Res 2014;77:374–9. [DOI] [PubMed] [Google Scholar]
- [38].Patel MD, Mckevitt C, Lawrence E, et al. Clinical determinants of long-term quality of life after stroke. Age Ageing 2007;36:316–22. [DOI] [PubMed] [Google Scholar]
- [39].Morris JH, Van WF, Joice S, et al. Predicting health related quality of life 6 months after stroke: the role of anxiety and upper limb dysfunction. Disabil Rehabil 2013;35:291–9. [DOI] [PubMed] [Google Scholar]
- [40].Franceschini M, La PF, Agosti M, et al. Is health-related-quality of life of stroke patients influenced by neurological impairments at one year after stroke. Eur J Phys Rehabil Med 2010;46:389–99. [PubMed] [Google Scholar]
- [41].De WL, Putman K, Baert I, et al. Anxiety and depression in the first six months after stroke. A longitudinal multicentre study. Disabil Rehabil 2008;30:1858–66. [DOI] [PubMed] [Google Scholar]
- [42].Aström M. Generalized anxiety disorder in stroke patients. A 3-year longitudinal study. Stroke J Cereb Circ 1996;27:270–5. [DOI] [PubMed] [Google Scholar]
- [43].D’Aniello GE, Scarpina F, Mauro A, et al. Characteristics of anxiety and psychological well-being in chronic post-stroke patients. J Neurol Sci 2014;338:191–6. [DOI] [PubMed] [Google Scholar]


