Abstract
Background
Adequate nutrients early in life promote cognitive development and are critical for proper growth and functioning. The effect of individual nutrients consumed through food is often not the same as consuming the same nutrients in supplementary form due to 'food synergy', the biological and chemical interrelations that occur between nutrients. Animal‐source foods, such as eggs, meat, fish, and dairy, are energy dense and contain multiple micronutrients and essential fatty acids with high bioavailability. The benefits of animal‐source foods may include higher food synergy relative to fortified foods as well as decreasing dependence on external suppliers of fortified foods.
Objectives
To assess the effectiveness of animal‐source foods compared to any other feeding interventions or no intervention in improving growth and developmental outcomes in children aged 6 to 59 months.
Search methods
We searched CENTRAL, MEDLINE, Embase, CINAHL, 18 other databases, and three trials registers up to August 2018. We also contacted authors and known experts in the field for assistance in identifying ongoing or unpublished data, and searched the reference lists of included studies and reviews, and websites of relevant organizations, for other studies that may not have been captured by our electronic searches.
Selection criteria
We included randomized controlled trials and quasi‐randomized controlled trials of any duration, where children between 5 months and 59 months (6 years) of age were provided with an animal‐source food (e.g. consumption of milk, meat, or eggs), prepared with any cooking method, compared with any intervention or no intervention.
Data collection and analysis
Two review authors independently assessed trial eligibility using prespecified criteria, extracted data, assessed risk of bias, and graded the quality of the evidence using the GRADE approach.
Main results
Study characteristics
We included 6 studies that analyzed data from 3036 children aged 5 to 50 months. The studies were conducted in China, the Democratic Republic of Congo, Ecuador, Guatemala, Pakistan, the USA, and Zambia, and lasted between 5 and 12 months. Three studies were funded, in part, by government entities; one study was supported by a nonprofit organization. Two studies did not report a funding source.
Three studies compared the effects of feeding an animal‐source food with a fortified (iron or iron and zinc), or unfortified cereal; two used a control group with no intervention; one compared a meat‐based diet to a dairy‐based diet. The types of animal‐source foods tested included yogurt, eggs, cheese, lyophilized (freeze‐dried) beef product, ground and frozen pork, puréed and jarred beef with gravy or pork, and powdered whey protein.
We judged four studies to be at unclear risk of bias overall; three studies because they were funded by an industry with a plausible interest in the outcome of the intervention; and one study because there was insufficient information to assess five of the seven bias 'Risk of bias' domains. We judged two of the six studies to be at high risk of bias overall; one study because there was significant baseline imbalance in length‐for‐age z scores (LAZ) between groups and evidence of selective reporting; the other study because there there was both a significant baseline imbalance in LAZ and weight‐for‐age z scores (WAZ) between groups, and a large‐scale social media campaign that may have influenced care received at home in the control group.
Key results
Animal‐source foods versus cereal‐based foods or no intervention
Five studies (2972 children) measured change in linear growth with either height‐for‐age z scores (HAZ) or LAZ. Three studies (592 children) reported a significant increase in HAZ and LAZ in the intervention group compared to the control group. Two studies (2380 children) reported a decline in LAZ in both groups. In one study (1062 children) there was no difference between the groups in the rate of decline; in the other (1318 children) the decrease in LAZ was significantly smaller in the intervention group.
Five studies (2972 children) measured weight gain using WAZ. Three studies (592 children) reported a significant increase in WAZ in the intervention group compared to the control group. In two studies (2380 children), WAZ decreased in both groups. In one of these studies (1318 children), the decrease in the intervention group was significantly smaller than in the control group. In the other study (1062 children), there was no difference between the groups.
Three studies (1612 children) reported impacts on all‐cause morbidity, but metrics were inconsistent between studies. One study with yogurt (402 children) reported a significant reduction in duration and incidence of diarrhea and upper respiratory infections in the intervention group. One study with eggs (148 children) reported a significant increase in the incidence of diarrhea in the intervention group, but this may have been due to cultural associations with eggs and gastrointestional problems. There were no other significant differences in fever, respiratory infections, or skin conditions between groups. The third study (1062 children) found no differences between intervention and control groups across morbidity measures.
No studies reported data on anemia.
Meat‐based diet versus dairy‐based diet
One study (64 children) measured change in LAZ and WAZ in infants fed either a meat‐based diet or dairy‐based diet. There was a significant increase in LAZ among infants consuming the meat‐based diet and a significant decrease in LAZ among infants consuming a dairy‐based diet. WAZ increased in both groups, with no significant difference between groups.
The study did not assess all‐cause morbidity or anemia.
Quality of the evidence
We rated the quality of the evidence as very low overall due to baseline imbalances between intervention and control groups, high heterogeneity in meta‐analysis, and imprecision due to wide confidence intervals and inconsistent direction of effects. We have little confidence in the results; further research is likely to change the estimate of magnitude and direction of treatment effect.
Authors' conclusions
Given the limited quality of the evidence, we are uncertain of the effects of the provision of animal‐source food versus cereal products or no intervention on the growth or development of children. More adequately powered trials with deliberately selected animal‐source foods are needed.
Plain language summary
Animal‐source foods for growth and development in children 6 to 59 months of age
What is the aim of this review?
We reviewed the evidence about the effect of animal‐source foods on the growth and development of children between 6 and 59 months of age.
What is the rationale for studying this?
The nutrition a child receives during the first five years of life is important for his or her growth and development. Animal‐source foods such as meat, fish, eggs, or dairy provide critical nutrients. Compared to foods such as iron‐fortified cereal products, the nutrients in animal‐source foods may be better absorbed by, and used in, the body.
What studies were included?
We included 6 studies with a total of 3036 children aged between 5 months and 50 months of age at enrollment. The interventions were conducted in China, Democratic Republic of Congo, Ecuador, Guatemala, Pakistan, USA, and Zambia, and lasted between 5 and 12 months.
Three studies compared animal‐source foods to a fortified (iron‐fortified or iron and zinc‐fortified) or unfortified cereal product. Two studies compared animal‐source foods to no intervention. One study compared meat to dairy. The types of animal‐source foods provided included beef, pork, eggs, yogurt, cheese, and powdered whey protein.
Three studies were funded in part by government entities and in part by an agency with a commercial interest in the results of the studies; we rated these studies as at unclear risk of other bias. One study was supported by a nonprofit organization. Two studies did not report a funding source.
What were the main results?
Animal‐source foods versus cereal‐based foods or no intervention
Five studies (2972 children) reported data on growth (measured as height‐for‐age or length‐for‐age) and weight gain (measured as weight‐for‐age). Three studies (592 children) reported increases in weight‐for‐age as well as height‐for‐age or length‐for‐age in the intervention group, compared to the control group. Of the two remaining studies, one study (1062 children) found both groups decreased in both weight‐for‐age and length‐for‐age, with no differences between the groups. In the other study (1318 children), both groups also decreased for these outcomes, but the decrease was smaller in the intervention group compared to the control group.
Three studies (1612 children) reported data on disease. One study with yogurt (402 children) found that children who received yogurt were less likely to experience diarrhea and respiratory infection and recovered faster when they did. One study with eggs (148 children) showed an increase in the incidence of diarrhea in children fed eggs, but this may have been due to cultural associations between eggs and gastrointestional problems. There were no differences in fever, respiratory infections, or skin conditions between the groups. The third study (1062 children) found no differences between the intervention and control groups for any measures of disease.
No studies reported data on anemia.
Meat‐based diet versus dairy‐based diet
One study (64 children) reported data on growth (measured as length‐for age) and weight gain (measured as weight‐for‐age). Infants consuming a meat‐based diet showed a significant increase in length‐for‐age compared to infants consuming a dairy‐based diet who experienced a decrease in length for age. Both groups experienced an increase in weight‐for‐age but there was no difference between them.
The study did not measure disease or anemia.
Overall results
Given the limited and very low‐quality evidence overall, we are uncertain of the effects of giving children animal‐source food versus cereal products or no intervention on children's growth and development.
What was the quality of evidence?
We rated the quality of the evidence as very low overall. We found some evidence to suggest that animal‐source foods increase growth and weight gain, and other evidence that suggests they do not. The amount of growth and weight gain also varied widely between studies. In addition, we had serious concerns about bias, including the unclear role of industry sponsors. Future findings are very likely to change our confidence in our estimate of the effects of animal‐source foods on growth and weight gain.
How up‐to‐date is this review?
The review authors searched the scientific literature up to August 2018.
Summary of findings
Background
Exclusive breastfeeding is recommended during the first six months of life followed by continued breastfeeding with appropriate complementary foods for up to two years or beyond (Kramer 2002; WHO 2003). Complementary foods provide calories and nutrients beyond that which is provided in breast milk (PAHO/WHO 2003). Adequate nutrients early in life promote cognitive development and are critical for proper growth and functioning. Growth faltering is seen across global contexts and usually occurs between the ages of three months and two years (Victora 2010). Nearly half of all deaths in children under the age of five in low‐ and middle‐income countries (LMIC) are attributable to malnutrition (Black 2013). Diets in LMICs are often nutritionally poor, based on staple foods like rice, wheat, maize (corn), millet, sorghum, roots, and tubers (FAO 1995). Animal products, such as eggs, meat, fish, and dairy, are energy dense and contain multiple micronutrients (particularly iron, zinc, vitamin A, vitamin B12, and choline) and essential fatty acids in a highly bioavailable form (Leroy 2007). Their consumption is associated with improved growth and developmental outcomes in observational studies, however they may not be practical for the lowest‐income consumers due to availability, access, or sociocultural norms (Leroy 2007).
The World Health Organization (WHO) Global Strategy on Diet, Physical Activity and Health, endorsed by the 57th World Health Assembly, recognizes the need to draft, update, and implement national food‐based dietary and physical activity guidelines (WHO 2004). The Brazilian Dietary Guidelines of 2014 (Brazilian MoH 2015), for example, emphasize the importance of understanding nutrition in terms of food and meals rather than individual nutrients. As countries develop economically, animal‐source foods, vegetable oils, and sugars begin to replace a larger portion of calories (Popkin 2001). In high‐income contexts, meat consumption is associated with obesity and its sequelae in adults, but not children (Bradlee 2010; Wang 2009). For this reason, it is important to understand the impact of animal‐source food consumption on growth and development outcomes in children across global contexts.
Description of the condition
Malnutrition in children encompasses both undernutrition and overweight and obesity. Undernutrition includes stunting (low height‐for‐age), wasting (low weight‐for‐height), and micronutrient deficiencies. In 2011, undernutrition contributed to 45% of all deaths in children under five years of age (Black 2013). Stunting affects 156 million children, while a further 50 million children are wasted and 42 million are overweight (WHO 2016). Of the major micronutrient deficiencies, vitamin A, zinc, iron, and iodine are responsible for the largest proportion of years of life lost (YLLs) and disability‐adjusted life years (DALYs) (Black 2008). Deficiencies of vitamin A and zinc result in increases in all‐cause morbidity and mortality; deficiencies in iron and iodine, in addition to omega‐3 fatty acids, impair children’s ability to reach their development potential (Nyaradi 2013).
Global estimates report that in 2015, 42 million children under 5 years of age, or 6.2%, were classified as overweight (weight‐for‐height score greater than 2 z scores above the median WHO standard) (WHO 2006; WHO 2016). Overweight in children under five years of age may result in type 2 diabetes and high blood pressure, and is a risk for adult obesity and its sequelae. Although stunting is less prevalent among overweight or obese children, deficiencies in micronutrients and essential fatty acids—'hidden hunger'—may persist, with negative impacts on neurocognitive development (Black 2013).
Historically, the majority of nutrition interventions in LMIC have used micronutrient powders or fortified complementary or supplementary foods, which were usually cereal based. Evidence for these point‐of‐use multiple micronutrient powder supplementation or supplementary feeding interventions on growth and development outcomes are unclear. A Cochrane Review of eight trials found that a micronutrient powder containing at least iron, zinc, and vitamin A provided for home fortification was associated with a reduced risk of anemia and iron deficiency in children under two years of age, but had no impact on growth (De‐Regil 2011). A Cochrane Review of community‐based supplementary feeding for promoting growth in children under 5 years of age in LMIC found a small but statistically significant effect on length in children under 12 months of age but, due to the variance in outcomes between studies, reached no firm conclusions (Sguassero 2012).
Strategically developed and implemented food‐based strategies that take into account relevant ecological, cultural, and socioeconomic factors could be acceptable and sustainable forms of intervention (FAO/WHO 1998). Animal‐source foods in particular contain multiple micronutrients (particularly iron, zinc, vitamin A, vitamin B12, and choline) and essential fatty acids in a highly bioavailable form (Leroy 2007).
Description of the intervention
The effect of individual nutrients consumed through food is often not the same as consuming the same nutrients in supplementary form. This may be due to 'food synergy', the biological and chemical interrelations that occur between nutrients when consumed in foods rather than in supplement form (Jacobs 2009). When consumed in food form, nutrients may work in concert with each other to improve absorption, and likely have a different impact than their technologically produced counterparts.
This review incorporates interventions that include provision of animal‐source foods or foods containing an animal‐source food component. Animal‐source foods include eggs, meat, fish, and dairy, prepared with any cooking method. We considered foods containing animal‐source components if they accounted for 75% of the energy density in the food provided. We only considered interventions in which the food was given to infants and children or their caretakers, or where it was produced within the home and provision was verified, and not interventions that only promoted animal‐source food consumption through education or behavior change.
There is also growing concern, particularly in high‐income countries, of allergies associated with some animal‐source foods, especially eggs and shellfish, although there is currently no evidence to suggest that restrictive diets after six months of age have an allergy‐preventing effect (PAHO/WHO 2003). Exposure to livestock‐borne pathogens in areas of high human‐to‐animal contact are also a concern (Headey 2016). We included adverse effects, such as allergies and zoonotic illness associated with livestock proximity, in our outcome measures.
How the intervention might work
To date, most complementary feeding interventions that have used animal‐source foods, including milk and meat, have been shown to improve both growth and cognitive outcomes in intervention trials across a range of international contexts, mostly in school‐aged children (Dror 2011). The role that animal‐source foods play during the complementary‐feeding window, however, is less well researched.
Animal‐source foods are calorie dense and are high sources of protein and fatty acids, vitamins, and minerals. Milk, for example, is intended to support the growth and development of nursing mammals, and thus may have a positive impact on linear growth (Dror 2011). This may be due to energy or protein content, a combination of micronutrients, or other factors present in milk. Eggs are considered a perfect protein source and a good source of essential fatty acids, choline, vitamins A and B12, and selenium (Iannotti 2014).
Importantly, animal‐source foods have the benefit of food synergy (Jacobs 2009). The vitamins and minerals found in animal‐source foods are more highly bioavailable than when consumed in plant‐based foods, particularly when consumed in concert with other ingredients. For example, animal‐source foods are typically good sources of fat, critical to absorption of fat‐soluble vitamins like vitamin A. Moreover, consuming critical nutrients in naturally found forms minimizes risk of excess consumption. In addition, although fortified staple foods may be cheaper than animal‐source foods, they are often consumed in conjunction with antinutrients that inhibit absorption. In particular, phytic acid, found in fortified staples like wheat and corn, and in alternative protein sources such as pulses and legumes, binds to nutrients such as zinc and calcium, decreasing their bioavailability (Michaelsen 1998).
Processed foods, specifically fortified products, have the advantage of the ability to address site‐specific nutrient deficiencies and can include many of the key limiting nutrients found in commonly consumed complementary foods such as staple grains. Additionally, they may present a lower risk for food contamination. However, there are also numerous disadvantages. The impact of the level of food processing in children has not been well studied. A 2015 study from Brazil showed that consumption of ultra‐processed products is associated with an increase in total cholesterol and low‐density lipoprotein cholesterol from preschool to school age (Rauber 2015). Most epidemiological studies have not taken level of food processing into account (Fardet 2015). Particularly in rural areas, access to processed foods also requires an external supply chain and source of funding that locally raised animal‐source foods do not.
Although the benefits of animal‐source foods for children in LMIC have been reported, the role that animal‐source foods play in the development of overweight and obesity in older children has not been well studied. Animal‐source foods are calorie‐dense, which has been implicated in the development of obesity across contexts. However, unlike processed foods, animal‐source foods provide a wide range of nutrients and may also promote feelings of satiety, which can help prevent obesity (Jacobs 2009; Speakman 2013). Separating the role that animal‐source foods play in proper growth and cognitive development versus non‐communicable, diet‐related disease is critical in moving nutritional policy and programming forward.
Why it is important to do this review
To date, the literature on randomized controlled trials on the impact of animal‐source foods on growth and development in infants and children has not been systematically reviewed. Dror and Allen conducted a narrative review in 2011 that included both observational studies and interventions (Dror 2011). That review found evidence that animal‐source foods improved child growth and cognition, but it did not involve a meta‐analysis and was less strict in study eligibility. Previous systematic reviews of complementary feeding have included studies of animal‐source foods (Dewey 2008), but none have conducted an exclusive analysis.
A growing body of research has examined the impact of increasing the intake of energy, protein, vitamins, and minerals through fortified infant and child foods, oral micronutrient supplements, or lipid‐based nutrient supplements on growth and development in the case of moderate or severe malnutrition. While these interventions provide key nutrients, they usually rely on external suppliers, may be highly processed, and contain other ingredients that may be detrimental in the diet if consumed in excess, such as sugar (Popkin 2014). In addition, many interventions incorporate an animal‐based ingredient in a processed form, such as skimmed‐milk powder.
Barriers related to local availability, affordability, and accessibility, in addition to cultural preferences against animal‐source feeding in some contexts, have meant that, to date, animal‐source food‐based approaches to nutrition have received little research and programming attention (Demment 2003). However, as animal‐source food consumption increases worldwide due to the Westernization of diets and rising incomes, it is likely that animal‐source foods will grow increasingly more accessible and accepted across country contexts (Pingali 2007; Popkin 2014). This review will help inform future policy and programming related to animal‐source foods.
Objectives
To assess the effectiveness of animal‐source foods compared to any other feeding interventions or no intervention in improving growth and developmental outcomes in children aged 6 to 59 months.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs), both individually and cluster randomized, as well as quasi‐RCTs.
Types of participants
Infants and children of any sex, aged between 5 and 59 months (i.e. less than 5 years of age), independently of their breastfeeding history, living in any country, and not more than 3 standard deviations (SD) above or below the WHO growth standards for length/height‐for‐age, weight‐for‐age, and weight‐for‐length/height. See Differences between protocol and review.
We excluded interventions for children with severe malnutrition (children below 3 SD of WHO growth standards for weight‐for‐length/height) and obesity (children above 3 SD of WHO growth standards for weight‐for‐length/height) (WHO 2006). We excluded children with severe malnutrition because they are at heightened risk of death; the appropriate nutritional regimen is different than for other forms of malnutrition; and rigorous guidelines already exist for community‐based management of severe malnutrition (Prudhon 2006).
Types of interventions
We included studies that directly provided animal‐source foods or foods containing an animal‐source food component of any duration.
Animal‐source foods include eggs, meat, fish, and dairy, prepared with any cooking method. We considered foods containing animal‐source components if they accounted for 75% of the energy density in the food provided. The reasons for selecting a 75% energy threshold were two‐fold. First, animal‐source foods are commonly added in small amounts to other complementary foods (i.e. small fish added to porridge or milk powder in a biscuit), decreasing the ability to isolate the impact of the animal‐source food in particular. Second, because infants and young children are only able to digest small amounts of food in a given feeding, we sought to include studies in which animal‐source foods were the predominant ingredient provided. Where this was unclear from the abstracts, we defined this threshold by calculating the energy provided by the animal‐source food if the food was adequately described in the report, or by extrapolating the density by comparing nutritional profiles of the food provided with the nutritional profile of the animal‐source food.
We did not consider interventions where only counseling or nutrition education promoting consumption of animal‐source foods was provided.
Comparator
Any comparison group or no intervention.
Types of outcome measures
Primary outcomes
Linear growth (measured by height‐for‐age z (HAZ) scores or length‐for‐age z scores (LAZ))
Weight gain (measured by weight‐for‐age z scores (WAZ))
All‐cause morbidity (number of children with at least one episode of any disease during the trial)
Secondary outcomes
Anemia (defined as hemoglobin lower than 110 g/L for children aged 6 to 59 months, adjusted by altitude where appropriate)
Iron deficiency (measured by serum/plasma ferritin below WHO cut‐off, adjusted for inflammation of 12 μg/L, for both boys and girls under five years of age)
Developmental outcomes (e.g. motor skills (measured by, for example, Movement Assessment of Infants (Chandler 1980) or Peabody Developmental Gross Motor Scale (Folio 1983)), visual and cognitive ability (measured by Forced Preferential Looking), and others as assessed by trialists)
Allergic reaction (e.g. rash, angioedema, diarrhea)
Search methods for identification of studies
Electronic searches
We first searched the databases and trials registers listed below between August and September 2017. We did not restrict the search by date, publication status, or language. We updated the searches in August 2018 using the same search strategies, limiting the search to the years 2017 to 2018. The search strategies are provided in Appendix 1.
International databases and trial registers
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 7) in the Cochrane Library, which includes the Cochrane Developmental, Psychosocial and Learning Problems Specialized Register (searched 15 August 2018).
MEDLINE Ovid (1946 to August, 2018 week 2).
MEDLINE In Process and Other Non‐Indexed Citations Ovid (searched 13 August 2018).
MEDLINE Epub Ahead of Print Ovid (searched 13 August 2018).
Embase Ovid (1974 to 2018 week 33).
CINAHL EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1981 to 13 August 2018).
Science Citation Index Web of Science (SCI; 1980 to 12 August 2018).
Social Science Citation Index Web of Science (SSCI; 1980 to 12 August 2018).
Conference Proceedings Citation Index ‐ Science Web of Science (CPCI‐S; 1990 to 12 August 2018).
Conference Proceedings Citation Index ‐ Social Science & Humanities Web of Science (CPCI‐SS&H; 1990 to 13 August 2018).
Cochrane Database of Systematic Reviews (CDSR; 2018, Issue 8), part of the Cochrane Library (searched 13 August 2018).
Epistemonikos (www.epistemonikos.org/en/advanced_search; searched 12 August 2018).
POPLINE (www.popline.org; searched 12 August 2018).
ClinicalTrials.gov (clinicaltrials.gov; searched 14 August 2018).
WHO International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch; searched 12 August 2018).
UK Clinical Trials Gateway (www.ukctg.nihr.ac.uk; searched 14 August 2018).
Regional databases
IBECS (ibecs.isciii.es; searched 12 August 2018).
SciELO (Scientific Electronic Library Online; www.scielo.br; searched 12 August 2018).
LILACS (Latin American and Caribbean Health Sciences Literature; lilacs.bvsalud.org/en; searched 12 August 2018).
PAHO (Pan American Health Library; www1.paho.org/english/DD/IKM/LI/library.htm; searched 12 August 2018).
WHOLIS (WHO Library; dosei.who.int; searched 12 August 2018).
WPRO (Western Pacific Region Index Medicus; www.wprim.org; searched 12 August 2018).
IMSEAR (Index Medicus for the South‐East Asia Region; imsear.searo.who.int; searched 12 August 2018).
IndMED (Indian medical journals; indmed.nic.in; 1985 onwards; searched 12 August 2018).
Native Health Research Database (hscssl.unm.edu/nhd; searched 12 August 2018).
Searching other resources
We contacted authors and known experts for assistance in identifying any ongoing or unpublished data. We searched the reference lists of all included studies for other trials that may not have been captured by the electronic searches. We also searched websites of nutrition‐focused entities (as reported in Appendix 1).
Data collection and analysis
We have reported only the methods used in this review in successive sections. All unused methods are reported in Table 3.
1. Unused methods.
| Measures of treatment effect |
Dichotomous data We will present dichotomous data as OR with 95% CI (Deeks 2011). |
|
Continuous data We will use the SMD with 95% CI to combine trials that measure the same outcome using different measurement methods. | |
| Unit of analysis issues |
Studies with more than two treatment groups If a control group is shared by two or more study arms, we will divide the control group over the number of relevant categories using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions so as to avoid double counting study participants (Higgins 2011). |
| Dealing with missing data | We will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by conducting a sensitivity analysis. The denominator for each outcome in each trial will be the number randomized minus any participants whose outcomes are known to be missing. For missing summary data, we will first contact the lead study authors for clarification. If this information is not available, and we judge that missing data may not be missing at random, we will aim to impute missing summary data using other statistical information (e.g. CI, standard errors) provided in the primary paper and impute the SD from other studies in the review. |
| Assessment of reporting biases | If more than 10 studies reporting the same outcome of interest are available, we will generate funnel plots in Review Manager 5 and visually examine them for asymmetry (Review Manager 2014). |
| Data synthesis | If continuous measures are not available for primary outcomes (such as LAZ scores), and we are unable to obtain the data from the study authors, we will use dichotomous outcomes and re‐express ORs as SMD (or vice versa) and combine the results using the generic inverse variance method, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). |
| Subgroup analysis and investigation of heterogeneity | We will conduct subgroup analyses by:
We will use the primary outcomes for our subgroup analyses (see Primary outcomes). We will not conduct subgroup analyses for those outcomes with 10 or fewer trials. We will visually explore the forest plots and identify where CIs do not overlap to identify differences between subgroup categories. We will also formally investigate differences between two or more subgroups by conducting t‐tests or F‐tests to calculate the significance of the ratio of MD to standard error. Using Review Manager 2014 (Review Manager 2014), we will compute an I2 statistic to describe variability in effect estimates from different subgroups that is due to genuine subgroup differences. The main focus of the analysis will be comparing magnitudes of effects across the different subgroups. |
| Sensitivity analysis | We will consider the impact of removing studies at high risk of bias (due to allocation concealment or baseline imbalances in outcomes between groups). We will also carry out a sensitivity analysis for quasi‐RCTs using a range of ICC values. |
CI: confidence interval LAZ: length‐for‐age z score MD: mean difference OR: odds ratio SD: standard deviation SMD: standardized mean difference
Selection of studies
Two review authors (JE, PRP) independently scanned the titles and abstracts of all records retrieved by the searches for relevance. The same two review authors then retrieved the full‐text reports of all potentially eligible studies and assessed these against the selection criteria (Criteria for considering studies for this review). Any disagreements were resolved through discussion or in consultation with a third review author (PRS) when necessary.
If records were only available as abstracts or as clinical trial registries, we attempted to locate the full‐text reports or trial registry pages in order to assess eligibility.
We recorded the selection process in a PRISMA diagram (Moher 2009).
Data extraction and management
Except for data on outcomes, one review author (JE) extracted data from each included study onto a data extraction form designed by the Cochrane Effective Practice and Organisation of Care Group (EPOC) and modified for this review (EPOC 2013). Two review authors (JE, PRP) extracted data on primary and secondary outcomes onto a pre‐designed spreadsheet in duplicate, resolving any disagreements through discussion.
We extracted the following information from each included study: source (e.g. contact details and citation); location of intervention; method of random allocation to treatment and control groups; details about participants (including age, baseline nutritional status, and standard diet (if available)); description and length of the intervention (including nutritional characteristics of the food provided); description of co‐interventions; data on outcomes related to child growth and development; rates of withdrawals; and compliance with diet (if available).
Where information regarding methods or results was unclear, we contacted the authors of the original studies for further details (see Dealing with missing data).
Assessment of risk of bias in included studies
Two review authors (JE, PRP) independently assessed the risk of bias in each included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions and set out in Appendix 2 (Higgins 2017). For each study, we rated the risk of bias as low, high, or unclear (uncertain), across the following seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential sources of bias. Where information related to risk of bias was not provided, we reached out to study authors for clarification. Any disagreements were resolved by discussion or in consultation with a third review author (PRS). The review authors were not blinded to the study authors, institution, or journal.
We considered the following to be key domains in our assessment of overall risk of bias in a study: random sequence generation, incomplete outcome data, selective reporting, and other risk (specifically, baseline imbalances in primary outcomes between intervention and control groups or the presence of funding from industries with an interest in the results). Where we rated a study at unclear risk of bias on one of these domains, we considered that study to be at unclear risk of bias overall. Where we rated a study at high risk of bias on one of these domains, we considered that study to be at high risk of bias overall. If a study appeared at both unclear and high risk of bias on two or more of the domains, we considered it to be at high risk of bias overall.
Measures of treatment effect
Dichotomous data
Trials reported dichotomous data differently, so we provided a narrative description of these outcomes.
Continuous data
Trials measured continuous outcomes in the same way, so we reported these using the mean difference (MD) with 95% confidence interval (CI).
Unit of analysis issues
Cluster‐randomized trials
We labeled cluster‐randomized trails with a (C). Where study authors had not appropriately accounted for the cluster design in the analysis, we used an intracluster correlation coefficient (ICC) from another source to calculate the trial's effective samples sizes.
Studies with more than two treatment groups
We did not include studies with more than two intervention arms.
Dealing with missing data
We noted levels of attrition in all included studies on the data extraction form and reported this information in the 'Risk of bias' tables in the Characteristics of included studies tables.
Assessment of heterogeneity
We assessed studies for clinical heterogeneity by comparing the distribution of study participants, study setting, dose and duration of the intervention. We evaluated methodological heterogeneity on the basis of trial factors such as the method of sequence generation, allocation concealment, blinding of outcome assessment, and losses to follow‐up.
To assess statistical heterogeneity, we used the Chi2 statistic to quantify the level of heterogeneity of intervention effects, considering a P value less than 0.10 as significant heterogeneity (Deeks 2011). We used the I2 statistic to assess the impact that heterogeneity had on the meta‐analysis. Where heterogeneity could not be explained, we used Tau2 to quantify between‐study variance in a random‐effects meta‐analysis. We considered substantial or considerable heterogeneity as Tau2 greater than 0.
Assessment of reporting biases
Statistical methods for identifying within‐study selective reporting are not yet well developed (Sterne 2011). We conducted a matrix of reported outcomes to examine patterns in reporting between studies, as well as examining protocols if these were available.
Data synthesis
We conducted statistical analysis using Review Manager 5 (Review Manager 2014). As we expected variation between trials in both population and intervention, we used a random‐effects model to combine the data. Because of the variation in time points at which outcomes were measured, we used mean changes from baseline. Due to high heterogeneity, we have provided a narrative synthesis of growth outcomes. Data were insufficient to pool in meta‐analysis for all other outcomes, therefore we have presented the results in a narrative synthesis.
'Summary of findings' table
We have presented our findings for linear growth, weight gain, all‐cause morbidity, and anemia for the comparison 'animal‐source foods versus a cereal‐based food or no intervention' in Table 1, and 'meat versus dairy' in Table 2, which we prepared using GRADEpro GDT (GRADEpro GDT 2015). The timing of outcome assessment ranged from 4 to 12 months. We have also reported the quality of the evidence for each outcome in these tables. Two review author (JE, PRP) assessed the quality of the evidence for each outcome as high, moderate, low, or very low using the GRADE approach (Balshem 2011), which takes into consideration the following five factors: study limitations, imprecision, inconsistency, indirectness, and publication bias. Any disagreements were resolved by discussion.
Summary of findings for the main comparison. Animal‐source foods compared to a cereal‐based food or no intervention for supporting optimal growth and development in children aged 6 to 59 months.
| Animal‐source foods compared to a cereal‐based food or no intervention for supporting optimal growth and development in children aged 6 to 59 months | ||||
| Patient or population: children aged 5 to 59 months Setting: China, the Democratic Republic of Congo, Ecuador, Guatemala, Pakistan, the USA, Zambia Intervention: animal‐source food Comparison: a cereal‐based food or no intervention | ||||
| Outcomes | Impacts | № of participants (studies) | Quality of the evidence (GRADE) | Comments |
|
Linear growth Assessed with: HAZ or LAZ scores Follow‐up: 5 to 12 months |
3 studies found a significant increase in HAZ and LAZ scores in the intervention group compared to the no intervention (2 studies) or cereal‐based (1 study) control groups. | 2972 (5 RCTs) |
⊕⊝⊝⊝ Very lowa,b,c | |
| 1 study found no significant difference between the intervention group and the control group receiving a fortified cereal; LAZ scores declined in both groups. | ||||
| 1 study found a significant, smaller decrease in LAZ scores in the intervention group compared to the control group receiving a fortified or an unfortified cereal. | ||||
|
Weight gain Assessed with: WAZ scores Follow‐up: 5 to 12 months |
3 studies found a small but significant increase in WAZ scores in the intervention group compared to the no intervention or cereal‐based control groups | 2972 (5 RCTs) |
⊕⊝⊝⊝ Very lowa,b,c | |
| 1 study found no significant difference between the groups; WAZ scores decline in both groups. | ||||
| 1 study found a significant, smaller decrease in WAZ scores in the intervention group compared to the control group receiving a fortified cereal; both groups declined. | ||||
|
All‐cause morbidity Assessed with: number of participants with at least 1 episode of any disease during the study Follow‐up: 6 to 12 months |
1 study found significant reductions in incidence and duration of respiratory infections and diarrhea in the intervention group compared to the control group. | 1612 (3 RCTs) |
⊕⊝⊝⊝ Very lowa,d,e | |
| 1 study found a significant increase of 5.5% in acute diarrhea in the intervention group compared to the control group, but no differences in fever, respiratory infections, or skin conditions between the groups. | ||||
| 1 study found no significant differences between the groups for morbidities, including pneumonia, malaria, and diarrhea. | ||||
| Anemia (not measured) | ‐ | ‐ | ‐ | Not measured |
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
| HAZ: height‐for‐age z score; LAZ: length‐for‐age z score; RCT: randomized controlled trial; WAZ: weight‐for‐age z score. | ||||
aDowngraded one level due to high risk of bias: baseline imbalances between groups or study funding. bDowngraded two levels for inconsistency: substantial heterogeneity (I2 > 90%) and varying directions of intervention effects. cDowngraded one level for imprecision: wide magnitude of effects. dDowngraded one level for imprecision in measures used to assess morbidities. eDowngraded one level for inconsistency between reported differences.
Summary of findings 2. Meat‐based diet compared to a dairy‐based diet for supporting optimal growth and development in children aged 6 to 59 months.
| Meat‐based diet compared to a dairy‐based diet for supporting optimal growth and development in children aged 6 to 59 months | ||||
|
Patient or population: children aged 5 to 59 months Settings: USA Intervention: meat‐based diet (puréed and jarred infants' foods) Comparison: dairy‐based diet (yogurt, cheese, and whey) | ||||
| Outcomes | Impacts | № of participants (studies) | Quality of the evidence (GRADE) | Comments |
|
Linear growth Assessed with: LAZ scores Follow‐up: 7 months |
1 RCT of formula‐fed infants found that LAZ scores increased in those children given a meat‐based diet and decreased in those children given a dairy‐based diet. | 64 (1 RCT) |
Moderatea | |
|
Weight gain Assessed with: WAZ scores Follow‐up: 7 months |
1 RCT of formula‐fed infants found no significant difference in WAZ scores between children given a meat‐based diet and those given a dairy‐based diet. | 64 (1 RCT) |
Moderatea | |
| All‐cause morbidity (not measured) | ‐ | ‐ | ‐ | Not measured |
| Anemia (not measured) | ‐ | ‐ | ‐ | Not measured |
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
| LAZ: length‐for‐age z score; RCT: randomized controlled trial; WAZ: weight‐for‐age z score. | ||||
aDowngraded one level due to indirectness.
Subgroup analysis and investigation of heterogeneity
We did not conduct subgroup analyses because we did not include more than 10 studies.
Sensitivity analysis
We conducted sensitivity analyses on the pooled effect estimates of a cluster‐randomized trial, to consider the impact of an ICC of 0.02 and 0.05 on linear growth and weight gain.
Results
Description of studies
Results of the search
Our searches generated 7033 records. After removal of duplicates, we screened 5806 records, of which 28 were deemed potentially eligible for inclusion. Six studies met our inclusion criteria (Criteria for considering studies for this review). See Figure 1.
1.
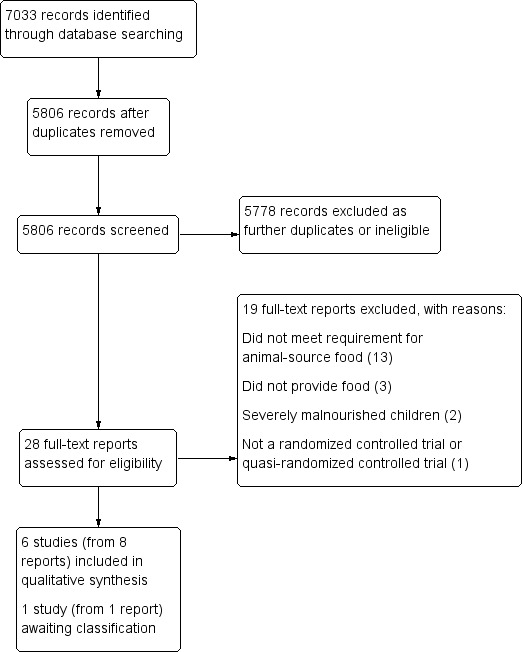
Study flow diagram.
Included studies
We included six studies (from eight reports) that analyzed data from 3036 children (He 2005; Iannotti 2017; Krebs 2012a (C); Tang 2014 (C); Tang 2018a; Tang and Krebs 2014).
See Characteristics of included studies tables for further detail.
Study design
All six included studies were RCTs. Two studies were cluster randomized by village (Krebs 2012a (C); Tang 2014 (C)).
Location
Four studies were conducted in LMICs (He 2005; Iannotti 2017; Krebs 2012a (C); Tang 2014 (C)). Two studies were conducted in the USA (Tang 2018a; Tang and Krebs 2014). One study, Krebs 2012a (C), was a multisited study conducted in four countries: the Democratic Republic of Congo, Guatemala, Pakistan, and Zambia. Two studies were conducted in China (He 2005; Tang 2014 (C)), and one study was conducted in Ecuador (Iannotti 2017).
Participants
Children in the included studies ranged in age from 5 months to 50 months at enrollment. Children began the intervention at five months of age in two studies (Tang 2018a; Tang and Krebs 2014), and at six months of age in two studies (Krebs 2012a (C); Tang 2014 (C)). The mean age at enrollment was approximately eight months in Iannotti 2017 and approximately 50 months in He 2005.
Description of intervention
Three studies compared the effects of feeding an animal‐source food versus a micronutrient‐fortified (iron‐fortified or iron and zinc‐fortified) or unfortified cereal (Krebs 2012a (C); Tang 2014 (C); Tang and Krebs 2014), while in two studies the control group received no intervention (He 2005; Iannotti 2017). The types of animal‐source foods included: yogurt (He 2005), eggs (Iannotti 2017), lyophilized (freeze‐dried) beef product (Krebs 2012a (C)), ground and frozen pork (Tang 2014 (C)), and puréed and jarred beef with gravy or pork (Tang and Krebs 2014).
Tang 2018a compared a meat‐based diet (consisting of commercially available puréed meats) to a dairy‐based diet (consisting of yogurt, cheese, and powdered whey protein).
Foods were provided to families on an every‐other‐day or weekly basis in four studies (Iannotti 2017; Krebs 2012a (C); Tang 2014 (C); Tang and Krebs 2014), with recommendations to provide an allotted amount every day. In one study, He 2005, the intervention was delivered Monday to Friday while children were in preschool. In another study, Tang 2018a, parents were provided with food and given detailed guidelines on how much to feed by responding to infant hunger cues. Detailed characteristics are provided in the Characteristics of included studies tables.
Duration of the intervention
In one study apiece the duration of the interventions was: five months (Tang and Krebs 2014), six months (Iannotti 2017), seven months (Tang 2018a), and nine months (He 2005). In two studies the intervention lasted 12 months (Krebs 2012a (C); Tang 2014 (C)).
Outcomes
Linear growth
All six studies reported on linear growth using change in HAZ (He 2005) or LAZ scores (Iannotti 2017; Krebs 2012a (C); Tang 2014 (C); Tang 2018a; Tang and Krebs 2014).
Weight gain
All six studies reported on weight gain using change in WAZ scores (He 2005; Iannotti 2017; Krebs 2012a (C); Tang 2014 (C); Tang 2018a; Tang and Krebs 2014).
All‐cause morbidity
Three studies reported on morbidity but without consistency as to the specific conditions (He 2005; Iannotti 2017; Krebs 2012a (C)).
Anemia
No studies reported data on anemia status.
Iron deficiency
Two studies reported biomarkers of iron status at endline (Krebs 2012a (C); Tang and Krebs 2014 (results reported in a separate article: Krebs 2013)). We were able to obtain biomarkers of iron status for a third study after personal communication with the study author (Tang 2014 (C)).
Developmental outcomes
One study, Krebs 2012a (C), measured psychomotor and mental development using the Bayley Scales of Infant Development II, delivered once at endline at 18 months.
Allergic reaction
One study, Iannotti 2017, monitored for allergic reaction to eggs at weekly visits made to households and via observations and self‐reports at baseline and endline. No immediate allergic reactions were observed or reported.
Excluded studies
We formally excluded 19 studies. The most common reason for exclusion was failure to meet the 75% threshold for animal‐source food (13 studies: Batra 2016; Bauserman 2015; Bhandari 2001; Dube 2010; Engelmann 1998; Jalil 2013; Lartey 1999; Lin 2008; Long 2012; NCT02272543; Rosado 2011; Schlossman 2015; Skau 2015). Other reasons included the following: studies did not provide food (three studies: NCT02516852; NCT02791100; Tang 2016); interventions treated severely malnourished children (two studies: Baker 1978; de Oliveira 1966); and studies were not an RCT or quasi‐RCT (one study: Tavill 1969).
See Characteristics of excluded studies for further details.
Studies awaiting classification
We assessed one registered clinical trial as potentially eligible for inclusion (NCT02496247), but were unable to find published results and were not able to access unpublished data after contacting the study authors (Eaton 2017 [pers comm]). See Characteristics of studies awaiting classification.
Risk of bias in included studies
We have presented our 'Risk of bias' ratings for each included study in the 'Risk of bias' tables in the Characteristics of included studies tables and summarised them below and in Figure 2 and Figure 3.
2.
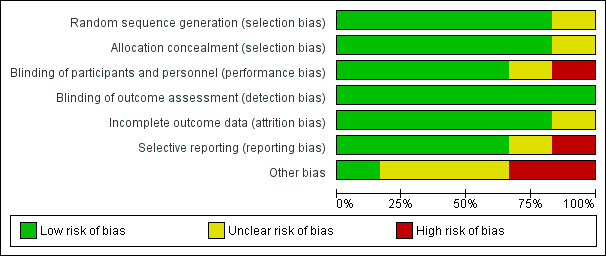
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
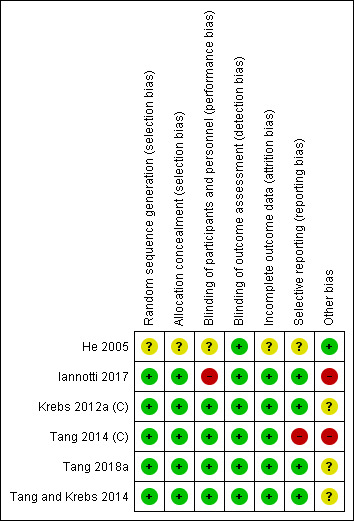
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We were unable to locate contact information for He 2005 and thus assessed several domains in that study as unclear.
Allocation
We considered five studies to be at low risk of selection bias, as they either described randomization and allocation in sufficient detail or provided procedures to the review authors via personal communication (Iannotti 2017; Krebs 2012a (C); Tang 2014 (C); Tang 2018a; Tang and Krebs 2014). We judged one study, He 2005, to be at unclear risk of bias, as methods were not described and we were unable to locate contact details for the study authors.
Blinding
Performance bias
Although it was impossible to blind caregivers to group assignment due to the nature of the interventions, we rated four studies as at low risk of performance bias because the intervention was unlikely to influence the care received as children were randomized to either an animal‐source food or cereal group (Krebs 2012a (C); Tang 2014 (C); Tang 2018a; Tang and Krebs 2014). We rated one study, He 2005, as at unclear risk of performance bias as the study authors did not provide sufficient information to assess whether non‐blinding was likely to influence care received at home. We judged another study, Iannotti 2017, to be at high risk of performance bias as non‐blinding was likely to influence care received in the control group. In that study, a large‐scale social media campaign promoting the intervention was carried out in the areas in which the trial was conducted, and 24‐hour dietary recalls indicated that the control group also increased their consumption of eggs between baseline and endline, although this was likely to bias results towards the null.
Detection bias
We judged all six studies to be at low risk of detection bias (He 2005; Iannotti 2017; Krebs 2012a (C); Tang 2014 (C); Tang 2018a; Tang and Krebs 2014). Aside from one study, Krebs 2012a (C), which administered the Bayley Scales of Infant Development II, all studies used objective outcomes; in Krebs 2012a (C) individuals administering the test were randomly assigned to both meat and cereal groups in order to improve inter‐rater reliability, so this study was also rated as at low risk of detection bias.
Incomplete outcome data
We judged five studies to be at low risk of attrition bias, as they had either no or minimal loss to follow‐up, or attrition was balanced between control and intervention groups (Iannotti 2017; Krebs 2012a (C); Tang 2014 (C); Tang 2018a; Tang and Krebs 2014). We judged one study, He 2005, to be at unclear risk of attrition bias as attrition was not reported.
Selective reporting
We judged four studies to be at low risk of reporting bias, as either protocols were available, or all expected outcomes of interest to the review were reported (Iannotti 2017; Krebs 2012a (C); Tang 2018a; Tang and Krebs 2014). We judged one study, Tang 2014 (C), to be at high risk of reporting bias, as micronutrient status was described as an outcome of interest but was not reported in the study, although we were able to obtain this information after communication with the author (Tang 2018a). He 2005 reported insufficient detail and we were unable to contact the author for further information, therefore we judged this study as at unclear risk of reporting bias.
Other potential sources of bias
We assessed all studies as having a low risk of attrition bias. Loss to follow‐up was low for all studies (< 15%), and where it was present it was balanced between groups with detailed reporting of reasons for the missing data, thus we did not employ methods to adjust for missing data.
We judged three studies as at unclear risk and two studies at high risk of other potential sources of bias. In two studies (Krebs 2012a (C); Tang and Krebs 2014), unclear risk was due to partial funding from the National Cattlemen's Beef Association, a trade and lobbying organization for beef producers in the USA. Tang 2018a was funded by the same organization, in addition to the National Pork Board and a food manufacturer that supplied foods to the trial. Although all three studies stated that this funding had no impact on study design or analysis, evaluations of research in other areas have concluded that industry sponsors may bias the results of research (Bes‐Rastrollo 2013), therefore we judged the risk of bias for these studies as unclear. We judged Tang 2014 (C) as at high risk of bias due to baseline imbalances in LAZ, and Iannotti 2017 as at high risk of bias due to baseline imbalances in LAZ and WAZ. We judged He 2005 to be at low risk of other potential sources of bias.
Overall risk of bias
We judged four of the six studies to be at unclear risk of bias overall; three studies because of the role of industry with a plausible interest in the outcome of the intervention (Krebs 2012a (C); Tang 2018a; Tang and Krebs 2014); and one study because there was insufficient information to assess five of the seven bias 'Risk of bias' domains (He 2005). We judged two of the six studies to be at high risk of bias overall; one study because there was significant baseline imbalance in LAZ between groups and evidence of selective reporting (Tang 2014 (C)); the other study because there there was both a significant baseline imbalance in length‐for‐age z‐scores (LAZ )and weight‐for‐age z‐scores (WAZ) between groups, and a large‐scale social media campaign that may have influenced care received at home in the control group (Iannotti 2017).
Effects of interventions
We have presented the results of our analysis below.
We obtained mean changes in LAZ from two studies (Krebs 2012a (C); Tang and Krebs 2014), and mean change in WAZ from three studies (Iannotti 2017; Krebs 2012a (C); Tang and Krebs 2014).
We did not adjust the results from Krebs 2012a (C), as clustering effects were adjusted for in the data analysis. We used an assumed ICC value from Krebs 2011 to calculate effective sample size in Tang 2014 (C).
Two studies used two control groups each (Tang 2014 (C); Tang and Krebs 2014). For both of these studies, the trial authors collapsed the control groups to enable a single pairwise comparison.
Animal‐source foods versus no intervention or a cereal‐based food
Primary outcomes
Linear growth
Five studies with a total of 2972 children evaluated the effects of animal‐source food compared to a cereal‐based food or no intervention on linear growth assessed using either HAZ (He 2005) or LAZ (Iannotti 2017; Krebs 2012a (C); Tang 2014 (C); Tang and Krebs 2014).
We pooled these studies in a meta‐analysis and found substantial heterogeneity (I2 = 99%; Analysis 1.1). Removing Iannotti 2017 from the analysis resulted in the most significant reduction in heterogeneity (I2 = 93%; Analysis 1.2), but because of the small number of included studies, it was not possible to investigate this by subgroup analysis. Given the degree of heterogeneity, we have presented a narrative synthesis of the results below.
1.1. Analysis.
Comparison 1 Animal‐source foods versus a cereal‐based food or no intervention, Outcome 1 Linear growth.
1.2. Analysis.
Comparison 1 Animal‐source foods versus a cereal‐based food or no intervention, Outcome 2 Linear growth (without Iannotti 2017).
Three studies with a total of 592 children found a statistically significant increase in HAZ and LAZ in the intervention group compared to the control group (He 2005; Iannotti 2017; Tang and Krebs 2014). In He 2005 (402 children), the mean difference (MD) change in HAZ in children receiving yogurt compared to those receiving no intervention was 0.05 (95% confidence interval (CI) 0.01 to 0.08). In Iannotti 2017 (148 children), the MD change in LAZ between children receiving eggs and those receiving no intervention was 0.64 (95% CI 0.61 to 0.67). In Tang and Krebs 2014 (42 children), the MD between infants receiving puréed and jarred beef with gravy or pork and controls receiving a fortified cereal snack was 0.41 (95% CI 0.27 to 0.55).
The two remaining studies with a total of 2380 children reported conflicting results (Krebs 2012a (C); Tang 2014 (C). One study, Krebs 2012a (C) (1062 children), found no significant difference between those receiving lyophilized beef product and those receiving fortified cereal (MD 0.03, 95% CI ‐0.08 to 0.14); LAZ declined in both groups. In Tang 2014 (C) (1318 children), both groups declined, but children receiving pork experienced a significantly slower decline in LAZ (MD 0.11, 95% CI 0.03 to 0.19) compared to those receiving fortified or unfortified cereal.
We rated the quality of this evidence as very low for the following reasons (Table 1).
Inconsistency. There was substantial heterogeneity (I2 = 99%) between studies in a pooled analysis, which could not be explained by age, type of control, or intervention length. When examining studies individually, the change in LAZ was inconsistent in direction between the studies.
Imprecision. The CI straddled the null finding in a pooled analysis. When examining studies individually, the magnitude of change varied widely.
Risk of bias. We assessed the overall risk of bias as serious, due to high risk of other bias in Iannotti 2017 and Tang 2014 (C) from baseline imbalances and unclear risk of other bias from industry funding in Krebs 2012a (C) and Tang and Krebs 2014.
Weight gain
You‐for‐age z scores
Five studies with a total of 2972 children evaluated the effects of animal‐source food compared to a cereal‐based food or no intervention on weight gain assessed using WAZ (He 2005; Iannotti 2017; Krebs 2012a (C); Tang 2014 (C); Tang and Krebs 2014).
We pooled these studies in a meta‐analysis and found substantial heterogeneity (I2 = 93%; Analysis 1.3). Removing Iannotti 2017 from the analysis resulted in the most significant reduction in heterogeneity (I2 = 83%; Analysis 1.4), but because of the small number of included studies, it was not possible to investigate this by subgroup analysis. Given the degree of heterogeneity, we have presented a narrative synthesis of the results below.
1.3. Analysis.
Comparison 1 Animal‐source foods versus a cereal‐based food or no intervention, Outcome 3 Weight gain.
1.4. Analysis.
Comparison 1 Animal‐source foods versus a cereal‐based food or no intervention, Outcome 4 Weight gain (without Iannotti 2017).
Three studies with a total of 592 children found a significant increase in WAZ in the intervention group compared to the control group (He 2005; Iannotti 2017; Tang and Krebs 2014). In He 2005 (402 children), the MD between children receiving yoghurt and children receiving no intervention was 0.12 (95% CI 0.06 to 0.19). In Iannotti 2017 (148 children), the MD between children receiving eggs and no intervention was 0.72 (95% CI 0.54 to 0.90). In Tang and Krebs 2014 (42 children), the MD in infants receiving puréed and jarred beef with gravy or pork compared to controls receiving a fortified cereal snack was 0.31 (95% CI 0.19 to 0.43).
Two studies (2380 children) found a decrease in WAZ in both groups, with conflicting results on whether animal‐source foods had a protective effect on growth faltering (Krebs 2012a (C); Tang 2014 (C). In one study, Krebs 2012a (C) (1062 children), both groups declined at roughly the same rate; the MD between children receiving lyophilized beef product compared to the control group was 0.04 (95% CI −0.08 to 0.16), with no significant difference between groups. In another study, Tang 2014 (C) (1318 children), both groups declined, but the WAZ scores of children receiving pork decreased marginally but significantly more slowly (MD 0.08, 95% CI 0.01 to 0.15) than the WAZ scores of children receiving cereal.
We rated the quality of this evidence as very low for the following reasons (Table 1).
Inconsistency. There was substantial heterogeneity (I2 = 93%) between studies in a pooled analysis, which could not be explained by age, control, or intervention length.
Imprecision. When examining studies individually, the magnitude of change varied widely.
Risk of bias. We assessed the overall risk of bias as serious, due to high risk of other bias in Iannotti 2017 from baseline imbalances and unclear risk of other bias from industry funding in Krebs 2012a (C) and Tang and Krebs 2014.
All‐cause morbidity
Three studies with a total of 1612 children reported on all‐cause morbidity (He 2005; Iannotti 2017; Krebs 2012a (C)). Two studies (1360 children) monitored all‐cause morbidity but did not report any data (Tang 2014 (C); Tang and Krebs 2014).
He 2005 (402 children) reported the incidence and duration of upper respiratory infections and diarrhea. At endline, children receiving the yogurt supplement had a significantly lower total incidence of upper respiratory infection (7.51% versus 13.21%, P < 0.001) and diarrhea (1.23% versus 2.43%, P = 0.02) compared to controls. The duration of these symptoms was also significantly lower in the yogurt group for both upper respiratory infection (3.4 days versus 4.8 days, P = 0.01) and diarrhea (2.0 days versus 2.8 days, P = 0.01).
In Iannotti 2017 (148 children), children receiving the egg intervention had a higher prevalence of acute diarrhea at baseline (26%) than controls (15%). This increased 5.5% at endline in the intervention group compared to no change in the control group (P = 0.05). However, this may have been due to the non‐blinding of care givers and cultural associations between eggs and gastrointestinal disorders in children. There were no differences in fever, respiratory infections, or skin conditions between the groups.
In Krebs 2012a (C) (1062 children), overall morbidity and morbidity related to specific conditions (diarrhea, respiratory illness, pneumonia, severe pneumonia, and malaria) did not differ between the meat and cereal groups. No specific results were reported.
We rated the quality of this evidence as very low due to concerns about bias related to baseline imbalances between groups, inconsistency between studies, and an inability to assess the precision of the morbidity measures used (Table 1).
Secondary outcomes
Anemia
No studies reported data on anemia.
Iron deficiency
Two studies with a total of 1104 children reported on biomarkers of iron status (Krebs 2012a (C); Tang and Krebs 2014). A third study, Tang 2014 (C) (1318 children), provided biomarkers via personal communication with the review authors.
Krebs 2012a (C) (1062 children) reported hemoglobin status at 18 months of age from a subsample of the total study at three of the four country sites. Following 12 months of supplementation, there was no significant difference (P = 0.19) in hemoglobin levels between the groups: beef (11.5 g/dL (± 1.5), 95% CI 11.3 to 11.7; 287 children) and cereal (11.7 g/dL (± 1.3), 95% CI 11.5 to 11.8; 267 children).
Tang 2014 (C) reported hemoglobin levels for a subsample of participants (410 children) after 12 months (endline) of intervention and found no significant difference between groups: pork (122.3 g/dL (± 11.4); 137 children); fortified cereal (121.6 g/dL (± 11.7); 140 children); and local cereal (119.5 g/dL (± 12.1); 133 children).
Tang and Krebs 2014 reported hemoglobin levels for 41 children reported in a separate analysis, Krebs 2013, of the same trial and found no significant difference between groups: puréed and jarred beef with gravy or pork (12.4 g/dL (± 0.3); 12 children); iron‐fortified cereal (12.1 g/dL (± 0.2); 13 children); and iron‐ and zinc‐fortified cereal (11.8 g/dL (± 0.2); 14 children).
We rated the quality of this evidence as low due to concerns about selective reporting bias, Tang 2014 (C), and indirectness, as we were unable to assess change in hemoglobin levels over time.
Developmental outcomes
One trial with 1236 children, Krebs 2012a (C), reported results for both the Psychomotor Developmental Index and Mental Developmental Index of the Bayley Scales of Infant Development II (which reports standardized scores with a mean of 100 and standard deviation of 15), delivered at 18 months—the endline of a 12‐month intervention. The study authors found no significant difference in scores between the meat (99.1 points, 95% CI 97.9 to 100.3) and cereal groups (99.7 points, 95% CI 98.8 to 100.7) on the Psychomotor Developmental Index (P = 0.54), or between the meat (95.2 points, 95% CI 94.2 to 96.2) and cereal groups (95.3 points, 95% CI 94.5 to 96.2) on the Mental Developmental Index (P = 0.82).
Allergic reaction
One trial with 160 children, Iannotti 2017, reported on allergic reactions to the food provided, which was one egg per day. That study reported that no incidents were observed by field researchers or reported by caregivers during weekly home visits.
Meat‐based diet versus dairy‐based diet
One trial with 64 formula‐fed children assessed the effects of a meat‐based diet consisting of puréed jarred meats to a dairy‐based diet consisting of yogurt, cheese, and whey protein powder (Tang 2018a).
Primary outcomes
Linear growth
Tang 2018a measured infant growth using LAZ and found a significant increase in the meat‐based group (0.33, 95% CI 0.16 to 0.50) compared to a significant decrease in the dairy‐based group (−0.30, 95% CI −0.49 to −0.11).
We rated the quality of this evidence as moderate, downgrading one level due to indirectness, as we did not hypothesize about the role of different types of animal‐source foods in linear growth, making comparison between studies difficult. See Table 2.
Weight gain
Tang 2018a measured weight gain using WAZ, and found that WAZ increased in both the meat group (0.43, 95% CI 0.25 to 0.61) and the dairy group (0.53, 95% CI 0.32 to 0.74), with no significant differences between groups.
We rated the quality of this evidence as moderate, downgrading one level due to indirectness, as we did not hypothesize about the role of different types of animal‐source foods in linear growth, making comparison between studies difficult. See Table 2.
The study did not assess all‐cause morbidity, anemia, iron deficiency, developmental outcomes, or allergic reaction.
Sensitivity analyses
We conducted sensitivity analyses for linear growth and weight gain. We compared the impact on the pooled summary estimates using ICCs of 0.02 (Analysis 2.1; Analysis 2.2) and 0.05 (Analysis 3.1; Analysis 3.2), adjusting one cluster‐randomized study that had not already adjusted the effective sample size (Tang 2014 (C)). Increasing the ICC did not impact the results of either outcome.
2.1. Analysis.
Comparison 2 Animal‐source foods versus a cereal‐based food or no intervention: sensitivity analysis (ICC = 0.02), Outcome 1 Linear growth.
2.2. Analysis.
Comparison 2 Animal‐source foods versus a cereal‐based food or no intervention: sensitivity analysis (ICC = 0.02), Outcome 2 Weight gain.
3.1. Analysis.
Comparison 3 Animal‐source foods versus a cereal‐based food or no intervention: sensitivity analysis (ICC = 0.05), Outcome 1 Linear growth.
3.2. Analysis.
Comparison 3 Animal‐source foods versus a cereal‐based food or no intervention: sensitivity analysis (ICC = 0.05), Outcome 2 Weight gain.
Discussion
Summary of main results
Our review aimed to assess the effects of animal‐source foods on the growth, nutritional status, and development of children aged 6 to 59 months. We found six eligible studies, two of which were cluster‐randomized trials. Three studies compared the provision of an animal‐source food with a fortified (iron‐fortified or iron and zinc‐fortified) or non‐fortified cereal supplement; two compared the provision of an animal‐source food with no intervention; and one compared the provision of a meat‐based diet to a dairy‐based diet. Seven different types of animal‐source foods were provided: yogurt, eggs, whey, lyophilized beef product, ground and frozen pork, and puréed and jarred beef with gravy or pork. The duration of the interventions ranged from 5 to 12 months. The total effective sample size was 3036 children, ranging from 5 to 50 months of age at the time of enrollment.
We found very low‐quality evidence for the effect of animal‐source food provision compared to fortified cereals or no intervention on both linear growth and weight gain. There was high heterogeneity in random‐effects meta‐analysis. The magnitude and direction of effect sizes varied. See Table 1.
We found moderate‐quality evidence for the effect of a meat‐based intervention compared to a dairy‐based intervention on linear growth and weight gain. See Table 2.
Assessments of morbidity were inconsistently provided, making it difficult to assess the impact of animal‐source food provision on other important markers. There was not enough evidence to assess the impact of animal‐source food provision on anemia, iron deficiency, developmental outcomes, or allergic reaction.
Overall completeness and applicability of evidence
In this review, we sought to determine the effectiveness of providing animal‐source foods to support growth and development in children aged 6 to 59 months. Our goal in reviewing this literature was twofold: 1) to systematically review what evidence already exists for animal‐source foods as a broad category; and 2) to compare animal‐source food provision with the provision of fortified foods or no supplementation.
Although numerous studies have assessed the impact of an animal‐source food component in feeding interventions, we excluded any research in which the animal‐source food component did not meet a 75% threshold for energy density. Given the high heterogeneity in the six included studies, the evidence was highly inconsistent. Only three studies assessed the same type of animal‐source food, thereby limiting our ability to reach conclusions about differences in the types of animal‐source food. There was also insufficient evidence to assess the impact of the duration of the intervention. Overall, differences in effect sizes and directions suggest that interventions are likely to be influenced by the type of animal‐source food and the context in which it is delivered. More research is needed to understand not only the effects of types and duration of interventions, but the sustainability of providing or promoting animal‐source foods.
We did not find a sufficient number of studies to assess the impact of food processing on animal‐source food supplementation. Given the importance of adapting food‐based interventions to local contexts, including relevant ecological, cultural, and socioeconomic factors for food provision, future research would benefit from including greater information on costs and sustainability of interventions.
Overall, estimates of the effect of animal‐source food for supporting infant and young child growth is uncertain, and future research is likely to have a large impact on findings. None of the studies included in this review provided high‐quality evidence in support of animal‐source foods for the following outcomes: anemia, iron deficiency, developmental outcomes, or allergic reaction.
Quality of the evidence
We rated the quality of the evidence as very low overall. Neither the type of animal‐source food nor the age of participants explained the high levels of heterogeneity found in meta‐analyses. There was a high degree of inconsistency in results, as indicated by varying directions in growth, and high inconsistency in reported measures or morbidity that made comparison difficult. We also considered results to be imprecise: for growth markers, the magnitude of effect sizes was highly variable between studies, whereas for morbidity outcomes, small sample sizes made it difficult to assess precise effect estimates. We also downgraded the overall quality of the evidence due to indirectness around morbidity measures.
We did not downgrade the quality of evidence due to publication bias, as due to the small number of included studies we were unable to calculate publication bias through funnel plots.
We judged four of the six studies to be at unclear risk of bias overall; three studies because they were funded by an industry with a plausible interest in the outcome of the intervention; and one study because there was insufficient information to assess five of the seven bias 'Risk of bias' domains. We judged two of the six studies to be at high risk of bias overall; one study because there was significant baseline imbalance in LAZ between groups and evidence of selective reporting; the other study because there there was both a significant baseline imbalance in LAZ and WAZ between groups, and a large‐scale social media campaign that may have influenced care received at home in the control group.
Our rating of the overall quality of evidence as very low, as indicated by our 'Risk of bias' and GRADE assessments (see Table 1; Table 2), means that future research is very likely to change our findings.
Potential biases in the review process
The possibility of authors' bias was relevant at every stage of the review process. We attempted to minimize this bias through dual study selection, data extraction, assessment of risk of bias, and grading of evidence. However, this process does not preclude the possibility of human error involved in personal judgements. We did not find sufficient studies to adequately assess publication bias, which we considered to be unclear.
We were unable to obtain results from one potentially eligible registered trial (NCT02496247). Given the few included studies, the lack of this information is likely to further bias findings.
Two of the review authors (LI, CL) were authors of one of the included studies (Iannotti 2017). Neither of these review authors were involved in selecting studies for inclusion, extracting data, assessing risk of bias, or grading the quality of the evidence.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first study to systematically review the effect of the provision of animal‐source foods in children under five years of age. A previous systematic review of complementary‐feeding interventions in which education was the main strategy found that the most effective programs included key messages encouraging caregivers to provide animal‐source foods (Dewey 2008). A 2012 Cochrane Review of community‐based supplementary feeding for children under five years of age in LMIC found similarly high levels of clinical heterogeneity (Sguassero 2012). Although the conclusions of that review were presented with caution, that analysis found smaller effect sizes on growth than our analysis.
In addition, a 2017 analysis of Demographic and Health Survey data of 112,553 children aged 6 to 23 months from 46 LMIC found strong associations between the consumption of animal‐source food and child growth, which were consistent for fish and dairy products and, in some geographic areas, for eggs and meat (Headey 2017).
Authors' conclusions
Implications for practice.
Given the limited evidence base currently available, we are uncertain of the effects of the provision of animal‐source foods versus cereal products or no intervention on the growth or development of children.
Implications for research.
Given the lack of high‐quality evidence on animal‐source foods for supporting optimal growth and development in children 6 to 59 months of age, we conclude that further well‐designed research is needed across geographic contexts. Study authors should provide sufficient rationale for the selection of type of animal‐source food, taking into account nutritional considerations as well as cultural and economic factors.
Future research should endeavour to study the differences between processing levels (i.e. fresh versus freeze‐dried) and nutrient content of different animal‐source foods. Only one study included in this review compared two types of animal‐source foods, and no studies compared animal‐source protein sources to plant‐based sources such as pulses or legumes. Given the lower access to and availability of animal‐source food in low‐ and middle‐income countries, understanding the trade‐offs between these food sources is important for future policy and programming. Further research might also address how the provision of animal‐source food impacts overall dietary patterns in children and how the dosing of animal‐source food affects growth in contexts in which they may be overconsumed.
Feedback
Issue in PLS of 'Effectiveness of provision of animal‐source foods for supporting optimal growth and development in children 6 to 59 months of age', 27 February 2019
Summary
Comment: I was preparing to circulate your review to a paediatric interest group when I noticed that the "What were the main results?" in the plain language summary state "found both groups decreased in both height and weight". The rest of the review rightly reports that this is a decrease in length for age but in the plain language summary this has been lost. The summary that I receive to distribute is located on the Cochrane website not on the library and is based on the plain language summary and so throughout talks about a decrease in height and weight which should be corrected.
Name: Vanessa Jordan Email Address:v.jordan@auckland.ac.nz Affiliation: University of Auckland Declaration of interest: none declared
Reply
Response from Editorial Base: Thank you for your comment; we agree there is some ambiguity here. We have made alterations in the 'Main results' section of the Plain Language Summary at several points, expanding the concept of 'growth' into component parts of HAZ (height for age) and LAZ (length for age), as well as weight gain. The text is now explicit and we thank you and the review's first author for approving it.
Contributors
Jane Dennis, Feedback Editor, CDPLPG Joanne Duffield, Managing Editor, CDPLPG Jake Eaton, first author, Effectiveness of provision of animal‐source foods for supporting optimal growth and development in children 6 to 59 months of age Vanessa Jordan, New Zealand Cochrane Fellow/Senior Research Fellow
What's new
| Date | Event | Description |
|---|---|---|
| 14 May 2019 | Feedback has been incorporated | We amended the Plain Language Summary to address feedback. The term 'growth' was expanded as necessary, to deal with differences in meaning between 'height for age', 'length for age' and weight gain, where relevant. |
History
Protocol first published: Issue 10, 2017 Review first published: Issue 2, 2019
| Date | Event | Description |
|---|---|---|
| 27 August 2018 | New search has been performed | Updated for the top‐up search with the inclusion of one additional study. |
| 10 January 2017 | Feedback has been incorporated | Amended for JP's latest edits and proofread for content |
Acknowledgements
The protocol for this review began during the WHO/Cochrane/Nutrition International (formerly Micronutrient Initiative)/Cornell University Summer Institute for Systematic Reviews in Nutrition for Global Policy Making, hosted at the Division of Nutritional Sciences, Cornell University, Ithaca, USA, between 25 July and 5 August 2016. We are thankful to Juan Pablo Peña‐Rosas for his early guidance on the project and extensive input on the first draft of the protocol. We thank Joanne Duffield, Daniela Kuellenberg de Gaudry, and Nuala Livingstone of the Cochrane Development, Psychosocial and Learning Problems Group for their valuable advice and assistance on the review and Margaret Anderson for her assistance with the search strategy. We are also grateful to Zulfiqar A Bhutta and three anonymous reviewers for their comments on earlier drafts of the review.
Appendices
Appendix 1. Search strategies
International databases and trial registers
Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library
Searched 2 September 2017 (1283 records) and 15 August 2018 (19 records)
#1 (Beef or chicken? or Goat? or Pork or Poultry or Venison):ti,ab #2 ("fish" or "shellfish" or "seafood" or "sea‐food"):ti,ab #3 "eggs":ti,ab #4 "protein intake":ti,ab #5 "animal product?":ti,ab #6 ("butter" or "cheese$" or "dairy" or "milk" or "yo?urt") #7 MeSH descriptor: [Insects] explode all trees #8 MeSH descriptor: [Meat] explode all trees #9 MeSH descriptor: [Seafood] explode all trees #10 ("insect?" or "caterpillar?" or "spiders" or "beetle?" or "termite?" or "ant" or "ants"):ti,ab #11 MeSH descriptor: [Dairy Products] explode all trees #12 ("kefir" or "Kephir" or "bulgaros") #13 ("buffalo" or "camel?" or "cattle" or "cow?" or "deer" or "donkey?" or "goat?" or "Horse?" or "pig?" or "sheep" or "swine" or "reindeer?" or "Yak?") near/2 ("Protein?" or "product?") #14 "animal source" near/2 ("diet" or "feed*" or "food?" or "nutrition" or "protein?"):ti,ab #15 "animal source food?":ti,ab #16 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 #17 MeSH descriptor: [Infant] explode all trees #18 preschool child*:ti,ab #19 (baby or babies or infant* or preschool* or pre‐school* or child*):ti,ab #20 #17 or #18 or #19 #21 "child growth":ti,ab #22 "child development":ti,ab #23 "malnutrition" #24 "nutritional deficiency":ti,ab #25 "nutritional disorder?":ti,ab #26 "growth disorder?":ti,ab #27 MeSH descriptor: [Anthropometry] explode all trees #28 MeSH descriptor: [Body Composition] explode all trees #29 "body height":ti,ab #30 "body mass":ti,ab #31 "Muscle?":ti,ab #32 "Z score$":ti,ab #33 ("stunted" or "stunting"):ti,ab #34 "body fat":ti,ab #35 "length for age":ti,ab #36 "weight for age":ti,ab #37 "weight for length":ti,ab #38 "weight for height":ti,ab #39 "Lean mass":ti,ab #40 "growth":ti,ab #41 ("BMI" or "body mass index"):ti,ab #42 #21 or #22 #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 #43 #16 and #20 and #42
Ovid MEDLINE
Searched 21 August 2017 (1583 records) and 13 August 2018 (97 records)
1 Meat/ 2 Meat Products/ 3 Red Meat/ 4 (beef or chicken$ or goat$ or meat or pork or poultry or venison).tw,kf. 5 exp Seafood/ 6 (fish$ or shellfish$ or seafood$ or sea‐food$).tw,kf. 7 Insects/ 8 (insect$ or caterpillar$ or spider$ or beetle$ or termite$ or ant or ants).tw,kf. 9 exp Eggs/ 10 exp Egg Proteins, Dietary/ 11 exp Dairy Products/ 12 exp Milk Proteins/ 13 (butter or cheese$ or dairy or eggs or milk or yog?urt).tw,kf. 14 (kefir or kephir or bulgaros).tw,kf. 15 ((buffalo$ or camel$ or cattle or cow$ or deer$ or donkey$ or goat$ or horse$ or pig$ or sheep$ or swine or reindeer$ or yak$) adj3 (protein$ or product$)).tw,kf. 16 ((animal$ or livestock) adj2 source$ adj2 (diet$ or feed$ or food$ or nutrition$ or protein$)).tw,kf. 17 or/1‐16 18 infant/ 19 Child, Preschool/ 20 child/ 21 (infan$ or baby or babies or toddler$ or preschoo$ or pre‐school$ or child$ or schoolage$ or school‐age$).tw. 22 or/18‐21 23 exp Child Development/ 24 infant nutrition disorders/ 25 malnutrition/ 26 child nutrition disorders/ 27 growth disorders/ 28 nutrition disorders/ 29 ANTHROPOMETRY/ 30 exp Body Composition/ 31 exp body height/ 32 exp body weight/ 33 body mass index/ 34 muscle, skeletal/ 35 Z score$.tw,kf. 36 (stunted or stunting).tw,kf. 37 body fat$.tw,kf. 38 length for age.tw,kf. 39 weight for age.tw,kf. 40 weight for length.tw,kf. 41 weight for height.tw,kf. 42 lean mass.tw,kf. 43 growth.tw,kf. 44 (BMI or body mass index).tw,kf. 45 or/23‐44 46 17 and 22 and 45 47 randomized controlled trial.pt. 48 controlled clinical trial.pt. 49 randomi#ed.ab. 50 placebo.ab. 51 clinical trials as topic.sh. 52 randomly.ab. 53 trial.ti. 54 or/47‐53 55 exp animals/ not humans.sh. 56 54 not 55 57 46 and 56
Ovid MEDLINE In‐Process & Other Non‐Indexed Citations
Searched 21 August 2017 (71 records) and 13 August 2018 (1 record)
1 Meat.tw 2 Meat Products.tw 3 Red Meat.tw 4 (beef or chicken$ or goat$ or meat or pork or poultry or venison).tw. 5 Seafood.tw 6 (fish$ or shellfish$ or seafood$ or sea‐food$).tw. 7 Insects.tw 8 (insect$ or caterpillar$ or spider$ or beetle$ or termite$ or ant or ants).tw. 9 Eggs.tw 10 Egg Proteins.tw 11 Dairy Products.tw 12 Milk Proteins.tw 13 (butter or cheese$ or dairy or eggs or milk or yog?urt).tw. 14 (kefir or kephir or bulgaros).tw. 15 ((buffalo$ or camel$ or cattle or cow$ or deer$ or donkey$ or goat$ or horse$ or pig$ or sheep$ or swine or reindeer$ or yak$) adj3 (protein$ or product$)).tw. 16 ((animal$ or livestock) adj2 source$ adj2 (diet$ or feed$ or food$ or nutrition$ or protein$)).tw. 17 or/1‐16 18 infant.tw. 19 Preschool Child.tw. 20 child.tw. 21 (infan$ or baby or babies or toddler$ or preschoo$ or pre‐school$ or child$ or schoolage$ or school‐age$).tw. 22 or/18‐21 23 Child Development.tw 24 infant nutrition disorders.tw 25 malnutrition.tw 26 child nutrition disorders.tw 27 growth disorders.tw 28 nutrition disorders.tw 29 Anthropometry.tw 30 Body Composition.tw 31 body height.tw 32 body weight.tw 33 body mass index.tw 34 muscle, skeletal.tw 35 Z score$.tw. 36 (stunted or stunting).tw. 37 body fat$.tw. 38 length for age.tw. 39 weight for age.tw. 40 weight for length.tw. 41 weight for height.tw. 42 lean mass.tw. 43 growth.tw. 44 (BMI or body mass index).tw. 45 or/23‐44) 46 17 and 22 and 45 47 randomized controlled trial.pt. 48 controlled clinical trial.pt. 49 randomi#ed. 50 placebo.ab. 51 clinical trials as topic 52 randomly 53 trial.ti 54 or/47‐53 55 animals/ not humans 56 54 not 55 57 46 and 56
Ovid MEDLINE Epub Ahead of Print
Searched 21 August 2017 (16 records) and 13 August 2018 (96 records)
1 Meat.tw 2 Meat Products.tw 3 Red Meat.tw 4 (beef or chicken$ or goat$ or meat or pork or poultry or venison).tw. 5 Seafood.tw 6 (fish$ or shellfish$ or seafood$ or sea‐food$).tw. 7 Insects.tw 8 (insect$ or caterpillar$ or spider$ or beetle$ or termite$ or ant or ants).tw. 9 Eggs.tw 10 Egg Proteins.tw 11 Dairy Products.tw 12 Milk Proteins.tw 13 (butter or cheese$ or dairy or eggs or milk or yog?urt).tw. 14 (kefir or kephir or bulgaros).tw. 15 ((buffalo$ or camel$ or cattle or cow$ or deer$ or donkey$ or goat$ or horse$ or pig$ or sheep$ or swine or reindeer$ or yak$) adj3 (protein$ or product$)).tw. 16 ((animal$ or livestock) adj2 source$ adj2 (diet$ or feed$ or food$ or nutrition$ or protein$)).tw. 17 or/1‐16 18 infant.tw. 19 Preschool Child.tw. 20 child.tw. 21 (infan$ or baby or babies or toddler$ or preschoo$ or pre‐school$ or child$ or schoolage$ or school‐age$).tw. 22 or/18‐21 23 Child Development.tw 24 infant nutrition disorders.tw 25 malnutrition.tw 26 child nutrition disorders.tw 27 growth disorders.tw 28 nutrition disorders.tw 29 Anthropometry.tw 30 Body Composition.tw 31 body height.tw 32 body weight.tw 33 body mass index.tw 34 muscle, skeletal.tw 35 Z score$.tw. 36 (stunted or stunting).tw. 37 body fat$.tw. 38 length for age.tw. 39 weight for age.tw. 40 weight for length.tw. 41 weight for height.tw. 42 lean mass.tw. 43 growth.tw. 44 (BMI or body mass index).tw. 45 or/23‐44) 46 17 and 22 and 45 47 randomized controlled trial.pt. 48 controlled clinical trial.pt. 49 randomi#ed. 50 placebo.ab. 51 clinical trials as topic 52 randomly 53 trial.ti 54 or/47‐53 55 animals/ not humans 56 54 not 55 57 46 and 56
Embase Ovid
Searched 24 August 2017 (2347 records) and 15 August 2018 (222 records)
1 exp meat/ 2 (beef or chicken$ or goat$ or meat or pork or poultry or venison).tw,kw. 3 exp sea food/ 4 (fish$ or shellfish$ or seafood$ or sea‐food$).tw,kw. 5 insect/ 6 (insect$ or caterpillar$ or spider$ or beetle$ or termite$ or ant or ants).tw,kw. 7 egg/ 8 protein intake/ 9 animal product/ 10 exp dairy product/ 11 (butter or cheese$ or dairy or eggs or milk or yog?urt).tw,kw. 12 (kefir or kephir or bulgaros).tw,kw. 13 ((buffalo$ or camel$ or cattle or cow$ or deer$ or donkey$ or goat$ or horse$ or pig$ or sheep$ or swine or reindeer$ or yak$) adj3 (protein$ or product$)).tw,kw.14 ((animal$ or livestock) adj2 source$ adj2 (diet$ or feed$ or food$ or nutrition$ or protein$)).tw,kw. 15 or/1‐14 16 exp infant/ 17 toddler/ 18 preschool child/ 19 child/ 20 (infan$ or baby or babies or toddler$ or preschoo$ or pre‐ school$ or child$ or schoolage$ or school‐age$).tw. 21 or/16‐20 22 15 and 21 23 child growth/ 24 child development/ 25 malnutrition/ 26 nutritional deficiency/ 27 nutritional disorder/ 28 growth disorder/ 29 exp anthropometry/ 30 exp body composition/ 31 body height/ 32 body mass/ 33 muscle/ 34 Z score$.tw,kw. 35 (stunted or stunting).tw,kw. 36 body fat$.tw,kw. 37 length for age.tw,kw. 38 weight for age.tw,kw. 39 weight for length.tw,kw. 40 weight for height.tw,kw. 41 lean mass.tw,kw. 42 growth.tw,kw. 43 (BMI or body mass index).tw,kw. 44 or/23‐43 45 22 and 44 46 randomized controlled trial/ 47 controlled clinical trial/ 48 crossover procedure/ 49 double blind procedure/ 50 single blind procedure/ 51 random$.tw,kw. 52 (allocat$ or assign$).tw,kw. 53 (controlled adj7 (study or design or trial)).ti,ab. 54 trial$.ti. 55 crossover$.tw,kw. 56 cross over$.tw,kw. 57 placebo$.tw,kw. 58 (doubl$ adj blind$).tw,kw. 59 (single$ adj blind$).tw,kw. 60 placebo$.tw,kw. 61 or/46‐59 62 45 and 61 63 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 64 human/ or normal human/ or human cell/ 65 63 and 64 66 63 not 65 67 62 not 66
CINAHL EBSCOhost (Cumulative Index to Nursing and Allied Health Literature)
Searched 22 August 2017 (1721 records) and 13 August 2018 (90 records)
S57 S46 AND S56 S56 S54 NOT S55 S55 animals NOT MW humans S54 S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 S53 TI trial S52 AB randomly S51 MH clinical trials S50 AB placebo S49 AB randomi#ed S48 "clinical controlled trial" S47 PT randomized controlled trial S46 S17 AND S22 AND S45 S45 S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 S44 (BMI or "body mass index") S43 growth Search modes S42 lean mass S41 ("weight for height" or weight for height) S40 ("weight for length" or weight for length) S39 ("weight for age" or weight for age) S38 ("length for age" or length for age) S37 body fat* S36 (stunted or stunting) S35 Z score* S34 (MH "Muscle, Skeletal") S33 body mass index S32 (MH "Body weight") S31 (MH "Body Height") S30 (MH "Body Composition+") S29 (MH "Anthropometry") S28 (MH "Nutrition Disorders") S27 (MH "Growth Disorders") S26 (MH "Child Nutrition Disorders") S25 (MH "Malnutrition") S24 (MH "Infant Nutrition Disorders") S23 (MH "Child Development") S22 S18 OR S19 OR S20 OR S21 S21 (infant* or baby or babies or toddler* or preschool* or pre‐school* or schoolage* or school‐age*) S20 (MH "Child") S19 (MH "Child, Preschool") S18 infant S17 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 S16 ((animal* or livestock) N2 source* N2 (diet* or feed* or food* or nutrition* or protein*)) S15 ((buffalo* or camel* or cattle or cow* or deer* or donkey* or goat* or horse* or pig* or sheep* or swine or reindeer* or yak*) N3 (protein* or product*)) S14 (kefir or kephir or bulgaros) S13 (butter or cheese* or daily or eggs or milk or youg#urt) S12 (MH "Milk Proteins+") or milk protein* S11 (MH "Dairy Products+") S10 (MH "Dietary Proteins+") OR "egg proteins" S9 (MH "Eggs") S8 insect* or caterpillar* or beetle* or termite* or ant or ant*) S7 (MH "Insects") S6 (fish* or shellfish* or seafood* or sea‐food*) S5 (MH "Seafood+") S4 (beef or Chicken* or goat* or meat or pork or poultry or venison) S3 red meat S2 meat products S1 meat
Science Citation Index (SCI); Social Science Citation Index (SSCI); Conference Proceedings Citation Index ‐ Science (CPCI‐S); Conference Proceedings Citation Index ‐ Social Sciences & Humanities (CPCI‐SS&H); all Web of Science
CPCI‐S and CPCI‐SS&H. Searched 2 September 2017 (83 records) and 13 August 2018 (0 records) SCI. Searched 2 September 2017 (1702 records) and 13 August 2018 (0 records) SSCI. Searched 2 Sept 2017 (199 records) and 13 August 2018 (0 records)
# 37 #36 NOT #34 # 36 #35 NOT #33 # 35 #32 AND #31 # 34 TS=("human" OR "normal human" OR "human cell") # 33 TS= ("animals" OR "invertebrate" OR "animal experiment" OR "animal model" OR "animal tissue" OR "animal cell" OR "nonhuman") # 32 TS=("clinical trial?") OR TS=(research design) OR TS=(comparative stud*) OR TS=(evaluation stud*) OR TS=(controlled trial$)OR TS=(follow‐up stud*) OR TS=(prospective stud*) OR TS=(random*) OR TS=(placebo$) OR TS=(single blind*) OR TS=(double blind*) # 31 #30 AND #16 AND #14 # 30 #29 OR #28 OR #27 OR #26 OR #25 OR #24 OR #23 OR #22 OR #21 OR #20 OR #19 OR #18 #29 #28 OR #27 OR #26 OR #25 OR #24 OR #23 OR #22 OR #21 OR #20 OR #19 OR #18 OR #17 # 28 TS=("BMI" OR "body mass index") # 27 TS="growth" # 26 TS= "lean mass" # 25 TS=("length for age" OR "weight for age" OR "weight for length" OR "weight for height") # 24 TS=("body fat") # 23 TS=("stunted" OR "stunting") # 22 TS=("muscle" OR "z score*") # 21 TS=("body height" OR "body mass") # 20 TS="body composition" # 19 TS=anthropometry # 18 TS=("malnutrition" OR "nutritional deficienc*" OR "nutritional disorder?" OR "growth disorder?") # 17 TS=( "child growth" OR "child development") # 16 #15 OR #14 # 15 TS=(Baby OR Babies OR preschool OR child* OR school‐age*) # 14 TS=("infant*" OR "toddler?") # 13 TS=((animal OR livestock) NEAR/1 (source OR sources) NEAR/1 (diet$ OR feed* OR food$ OR nutrition* OR protein?)) # 12 TS=((buffalo OR camel OR cattle OR cow OR deer OR donkey or horses OR pig OR sheep) NEAR/3 (protein$ OR Product$)) # 11 TS=("kefir" OR "Kephir" OR "bulgaros") # 10 TS=("butter" OR "cheese?" OR "dairy" OR "milk" OR "yog?urt") # 9 TS="dairy product" # 8 TS="animal product" # 7 TS="protein intake" # 6 TS=("egg" OR "eggs") # 5 TS=("insect") OR TS=("insect$") # 4 TS=("fish" OR "shellfish" OR "Seafood*" OR "sea‐food") # 3 TS=("sea food") # 2 TS=("beef" OR "chicken" OR "goat" OR "pork" OR "poultry" OR "venison") # 1 TS=("meat")
Cochrane Database of Systematic Reviews (CDSR), part of the Cochrane Library
Searched 2 September 2017 (121 records) and 13 August 2018 (26 records)
#1 (Beef or chicken? or Goat? or Pork or Poultry or Venison):ti,ab #2 ("fish" or "shellfish" or "seafood" or "sea‐food"):ti,ab #3 "eggs":ti,ab #4 "protein intake":ti,ab #5 "animal product?":ti,ab #6 ("butter" or "cheese$" or "dairy" or "milk" or "yo?urt") #7 MeSH descriptor: [Insects] explode all trees #8 MeSH descriptor: [Meat] explode all trees #9 MeSH descriptor: [Seafood] explode all trees #10 ("insect?" or "caterpillar?" or "spiders" or "beetle?" or "termite?" or "ant" or "ants"):ti,ab #11 MeSH descriptor: [Dairy Products] explode all trees #12 ("kefir" or "Kephir" or "bulgaros") #13 ("buffalo" or "camel?" or "cattle" or "cow?" or "deer" or "donkey?" or "goat?" or "Horse?" or "pig?" or "sheep" or "swine" or "reindeer?" or "Yak?") near/2 ("Protein?" or "product?") #14 "animal source" near/2 ("diet" or "feed*" or "food?" or "nutrition" or "protein?"):ti,ab #15 "animal source food?":ti,ab #16 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 #17 MeSH descriptor: [Infant] explode all trees #18 preschool child*:ti,ab #19 (baby or babies or infant* or preschool* or pre‐school* or child*):ti,ab #20 #17 or #18 or #19 #21 "child growth":ti,ab #22 "child development":ti,ab #23 "malnutrition" #24 "nutritional deficiency":ti,ab #25 "nutritional disorder?":ti,ab #26 "growth disorder?":ti,ab #27 MeSH descriptor: [Anthropometry] explode all trees #28 MeSH descriptor: [Body Composition] explode all trees #29 "body height":ti,ab #30 "body mass":ti,ab #31 "Muscle?":ti,ab #32 "Z score$":ti,ab #33 ("stunted" or "stunting"):ti,ab #34 "body fat":ti,ab #35 "length for age":ti,ab #36 "weight for age":ti,ab #37 "weight for length":ti,ab #38 "weight for height":ti,ab #39 "Lean mass":ti,ab #40 "growth":ti,ab #41 ("BMI" or "body mass index"):ti,ab #42 #21 or #22 #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 #43 #16 and #20 and #42
Epistemonikos (www.epistemonikos.org/en/advanced_search)
Searched 28 August 2017 (17 records) and 12 August 2018 (5 records)
Keywords: child growth; child development; ASF; animal source foods; supplementary foods; complementary foods
POPLINE (www.popline.org)
Searched 2 September 2017 (80 records) and 12 August 2018 (7 records)
TITLE/KEYWORDS: malnutrition/underweight/wasting/wasted/malnourished
KEYWORDS: NOT obesity/overweight
TITLE: AND Child*/ infant*/ baby/pediatric*/paediatrics*
ABSTRACT: AND food*/supplement*5 1 and 2 and 3 and 4
ClinicalTrials.gov (clinicaltrials.gov)
Searched 28 August 2017 (25 records) and 14 August 2018 (4 records)
Keywords: child growth; child development; ASF; animal source foods; supplementary foods; complementary foods
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialsearch)
Searched 2 September 2017 (34 records) and 12 August 2018 (0 records)
Keywords: child growth; child development; ASF; animal source foods; supplementary foods; complementary foods
UK Clinical Trials Gateway (www.ukctg.nihr.ac.uk)
Searched 2 September 2017 (0 records) and 14 August 2018 (0 records)
Keywords: child growth; child development; ASF; animal source foods; supplementary foods; complementary foods
Regional databases
IBECS (ibecs.isciii.es)
Searched 2 September 2017 (0 records) and 12 August 2018 (0 records)
Keywords: child growth; child development; ASF; animal source foods; supplementary foods; complementary foods
SciELO (Scientific Electronic Library Online; www.scielo.br)
Searched 2 September 2017 (5 records) and 12 August 2018 (5 records)
Keywords: child growth; child development; ASF; animal source foods; supplementary foods; complementary foods
LILACS (Latin American and Caribbean Health Sciences Literature; lilacs.bvsalud.org/en)
Searched 28 August 2017 (32 records) and 12 August 2018 (2 records)
(tw:(growth disorders)) AND (tw:(meat )) AND (tw:(dietary proteins))
(tw:(growth disorders)) AND (tw:(dietary proteins OR Livestock OR Meat OR animal‐source food)) AND (type_of_study:(Systematic Review))
(tw:(growth disorders)) AND (tw:(meat OR dairy OR fish OR pork OR cows OR insects)) AND (tw:(Child, preschool OR infant OR children)) AND (tw:(systematic review))
PAHO (Pan American Health Library; www1.paho.org/english/DD/IKM/LI/library.htm)
Searched 2 September 2017 (0 records) and 12 August 2018 (0 records)
Keywords: child growth; child development; ASF; animal source foods; supplementary foods; complementary foods
WHOLIS (WHO Library; dosei.who.int)
Searched 2 September 2017 (0 records) and 12 August 2018 (0 records)
Keywords: child growth; child development; ASF; animal source foods; supplementary foods; complementary foods
WPRO (Western Pacific Region Index Medicus; www.wprim.org)
Searched 02 September 2017 (20 records) and 12 August 2018 (4 records)
Keywords: Animal source food, Complementary, Supplemental, Child / infants/baby
IMSEAR (search.bvsalud.org/ghl/index.php)
Searched 2 September 2017 (1 record) and 12 August 2018 (0 records)
Keywords: child growth; child development; ASF; animal source foods; supplementary foods; complementary foods
IndMED (Indian medical journals; indmed.nic.in)
Searched 2 September 2017 (1 record) and 12 August 2018 (0 records)
Keywords: child growth; child development; ASF; animal source foods; supplementary foods; complementary foods
Native Health Research Database (hscssl.unm.edu/nhd/)
Searched 2 September 2017 (0 records) and 12 August 2018 (0 records)
Keywords: child growth; child development; ASF; animal source foods; supplementary foods; complementary foods
Other Sources
Searched 5 September to 8 September 2017 and 12 August 2018 to 15 August 2018.
The Department of Nutrition for Health and Development (www.who.int/nutrition/nhd/en). Searched 05 September 2017 (0 records) and 12 August 2018 (0 records).
Regional Offices of the WHO (www.who.int/about/regions/en). Searched 05 September 2017 (0 records) and 12 August 2018 (0 records).
Centers for Disease Control and Prevention (CDC; www.cdc.gov/immpact/resources). Searched 08 September 2017 (0 records) and 12 August 2018 (0 records).
The United Nations Children's Fund (UNICEF; www.unicef.org/nutrition). Searched 07 September 2017 (1 record) and 12 August 2018 (0 records).
The World Food Programme (WFP; www1.wfp.org/nutrition). Searched 06 September 2017 (0 records) and 12 August 2018 (0 records).
Nutrition International (formerly The Micronutrient Initiative (MI); www.nutritionintl.org). Searched 05 September 2017 (0 records) and 12 August 2018 (0 records).
Helen Keller International (HKI; www.hki.org/our‐work/nourishing‐families). Searched 05 September 2017 (5 records) and 13 August 2018 (0 records).
Home Fortification Technical Advisory Group (HFTAG; www.hftag.org/downloads.asp?s=hftag). Searched 08 September 2017 (0 records) and 13 August 2018 (0 records).
The Global Alliance for Improved Nutrition (GAIN; www.gainhealth.org). Searched 06 September 2017 (0 records) and 13 August 2018 (0 records).
Keywords: child growth; child development; ASF; animal source foods; supplementary foods; complementary foods
Appendix 2. 'Risk of bias' domains
Random sequence generation (checking for possible selection bias)
We assessed whether the method used to generate the allocation sequence was described in sufficient detail to determine whether it produced comparable groups, and assigned ratings as follows.
Low risk of bias: any truly random process, e.g. random number table, computer random number generator
High risk of bias: any process that is not strictly random, e.g. odd or even date of birth, hospital or clinic record number
Unclear risk of bias: information about the randomization process not available
Allocation concealment (checking for possible selection bias)
We assessed whether the method used to conceal the allocation sequence was described in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrollment, and assigned ratings as follows.
Low risk of bias: telephone or central randomization; consecutively numbered, sealed, opaque envelopes
High risk of bias: open random allocation, unsealed or non‐opaque envelopes
Unclear risk of bias: information about the allocation process not available
Blinding of participants and personnel (checking for possible performance bias)
We described all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received.
We assessed the risk of performance bias associated with blinding of participants as follows.
Low risk of bias: blinding of caregivers
High risk of bias: non‐blinding likely to have influenced care received throughout the study, e.g. mothers were aware their child was not receiving the treatment intervention
Unclear risk of bias: inadequate information to assess the risk of bias as low or high
We assessed the risk of performance bias associated with blinding of personnel as follows.
Low risk of bias: blinding of all personnel
High risk of bias: non‐blinding likely to have influenced care throughout the study, such as through nutrition counseling at follow‐up visits
Unclear risk of bias: inadequate information to assess the risk of bias as low or high
While we assessed blinding of participants and blinding of personnel separately, we combined the results into a single evaluation of risk of bias associated with blinding of participants and personnel (Higgins 2017).
Blinding of outcome assessment (checking for possible detection bias)
We described all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received, and assigned ratings as follows.
Low risk of bias: outcomes were objective, or participants and key personnel were not blinded, but the outcome assessment was blinded, and the non‐blinding of others was unlikely to have introduced bias
High risk of bias: no blinding of outcome assessment, the measurement was likely to have been influenced by lack of blinding, or blinding could have been broken
Unclear risk of bias: insufficient information to permit a judgement of low or high risk of bias
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We assessed outcomes in each included study for completeness, and assigned ratings as follows.
Low risk of bias: either there were no missing outcome data, or missing outcome data were unlikely to have biased the results based on the following considerations: study authors provided transparent documentation of participant flow throughout the study; the proportion of missing data was similar in the intervention and control groups; the reasons for missing data were provided and balanced across the intervention and control groups or the reasons for missing data were not likely to have biased the results (e.g. moving house)
High risk of bias: missing outcome data were likely to have biased the results, or an 'as‐treated' (per‐protocol) analysis was performed with substantial differences between the intervention received and that assigned at randomization, or potentially inappropriate methods for imputation were used
Unclear risk of bias: insufficient information to assess the risk of bias as low or high
Selective reporting (checking for possible reporting bias)
We stated how the possibility of selective outcome reporting was examined and what was found, and assigned ratings as follows.
Low risk of bias: it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported
High risk of bias: not all of the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; the study failed to include the results of a key outcome that was expected to have been reported
Unclear risk of bias: insufficient information to assess the risk of bias as low or high
Other bias (checking for other potential sources of bias)
We assessed if the study was free of other potential biases, and assigned ratings as follows.
Low risk of bias: there was similarity between outcome measures at baseline or between potential confounding variables at baseline, or there was adequate protection of study arms against contamination
High risk of bias: there was no similarity between outcome measures at baseline or between potential confounding variables at baseline, or there was inadequate protection of study arms against contamination
Unclear risk of bias: insufficient information to assess the risk of bias as low or high
Data and analyses
Comparison 1. Animal‐source foods versus a cereal‐based food or no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Linear growth | 5 | 2972 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.09, 0.56] |
| 2 Linear growth (without Iannotti 2017) | 4 | 2824 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.01, 0.27] |
| 3 Weight gain | 5 | 2972 | Mean Difference (IV, Random, 95% CI) | 0.22 [0.06, 0.39] |
| 4 Weight gain (without Iannotti 2017) | 4 | 2824 | Mean Difference (IV, Random, 95% CI) | 0.12 [0.01, 0.22] |
Comparison 2. Animal‐source foods versus a cereal‐based food or no intervention: sensitivity analysis (ICC = 0.02).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Linear growth | 5 | 2550 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.10, 0.57] |
| 2 Weight gain | 4 | 2148 | Mean Difference (IV, Random, 95% CI) | 0.26 [0.00, 0.52] |
Comparison 3. Animal‐source foods versus a cereal‐based food or no intervention: sensitivity analysis (ICC = 0.05).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Linear growth | 5 | 2260 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.10, 0.57] |
| 2 Weight gain | 4 | 1858 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.01, 0.53] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
He 2005.
| Methods |
Study design: randomized controlled trial
Study duration: 9 months Start date: April 2001 End date: December 2001 |
|
| Participants |
Country and setting: China (LMIC at time of study); preschools in the Fangshan District of Beijing Population: not reported Inclusion criteria:
Exclusion criteria:
Nutritional status: baseline characteristics not available, but study notes that intake of calcium, zinc, and vitamin B2 were roughly 40%, 65%, and 80% of DRIs, respectively Number: 402 (201 in intervention group, 201 in control group) Age: mean age at enrollment: 50.9 months in intervention group, 50.3 months in control group Sex: 43% female in intervention group, 45% female in control group Typical diet: not provided |
|
| Interventions |
Intervention: yogurt; 1 × 125 g cup of yogurt provided 5 days per week (Monday to Friday, while at school) Control: no food |
|
| Outcomes |
Primary:
Secondary:
Measurement:
Time points: baseline, 3 months, 6 months, 9 months for all outcomes except morbidity, which was collected monthly |
|
| Notes |
Funding: not reported Declared conflict of interest: not reported Other notes: attrition not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information to assess |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient information to assess |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: objective outcome assessment unlikely to have introduced bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: attrition rates not reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: all prespecified outcomes or expected outcomes of interest to the review reported |
| Other bias | Low risk | Comment: apparently free of other sources of bias |
Iannotti 2017.
| Methods |
Study design: randomized controlled trial
Study duration: 6 months Start date: March 2015 End date: December 2015 |
|
| Participants |
Country and setting: Ecuador (upper‐middle‐income country); Cotopaxi province Population: Mestizo ethnic majority, 22% self identified as indigenous in 2010 census Inclusion criteria:
Exclusion criteria:
Nutritional status: baseline mean LAZ: −2.09 in intervention group, −1.71 in control group Number: 160 (78 in intervention group, 82 in control group) Age: mean age at enrollment: 7.4 months in intervention group, 7.7 months in control group Sex: 30% female in intervention group, 43% female in control group Typical diet: not described |
|
| Interventions |
Intervention: eggs; 1 medium‐sized egg (approximately 50 g) per day, provided on a weekly basis to children in the treatment group over a 6‐month period Control: no intervention; controls were exposed to social marketing intervention to participate in trial |
|
| Outcomes |
Primary:
Secondary:
Measurement:
Time points: baseline, 6 months |
|
| Notes |
Funding: Mathile Institute for the Advancement of Human Nutrition Declared conflict of interest: "At the time of the study, Drs Reinhart and Palacios worked for The Mathile Institute, which funded the study. The Mathile Institute has no vested interest in the outcome(s) of the study." Other notes: loss to follow‐up: 11, or 7% of total study population (3 in intervention group, 8 in control group). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: block randomization |
| Allocation concealment (selection bias) | Low risk | Comment: use of alpha/beta sealed envelopes during allocation. Field study team blinded except for 1 individual responsible for enrolling and monitoring participants. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: non‐blinding likely to have influenced care in the control group, who were exposed to social media messages around egg consumption; 24‐hour recall frequency of dietary intake showed an increase in egg consumption in both groups between baseline and endline |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: objective outcome assessment unlikely to have introduced bias. Investigators masked to group assignment during analysis. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: low losses to follow‐up (7%), balanced between intervention and control groups |
| Selective reporting (reporting bias) | Low risk | Comment: all prespecified outcomes and expected outcomes of interest to the review were reported |
| Other bias | High risk | Comment: significant baseline imbalances between intervention and control groups, respectively, for LAZ (−2.09 (± 1.08) vs −1.71 (± 0.92)) and WAZ (−0.91 (± 1.24) vs −0.40 (± 0.92)) |
Krebs 2012a (C).
| Methods |
Study design: multisite, cluster‐randomized controlled trial
Study duration: 12 months Start date: July 2008 End date: July 2010 |
|
| Participants |
Country and settings:
Inclusion criteria:
Exclusion criteria:
Nutritional status: baseline mean LAZ: −1.44 in intervention group, −1.32 in control group Number: 1236 infants (618 in intervention group, 618 in control group, with 20 clusters in each group) Age: enrollment at approximately 3 months of age; intervention from 6 to 18 months of age Sex: 53% female in intervention group, 51% female in control group Typical diet: a pilot study, Krebs 2011, indicated that less than 25% of infants' diets included meats, increasing to greater than 60% in toddlers. Use of micronutrient supplements (vitamin A and iron) highly variable |
|
| Interventions |
Intervention: cooked, diced, lyophilized (freeze‐dried) beef product; 15 g at enrollment increasing to 22.5 g per day at 12 months of age
Control: micronutrient (zinc and iron)‐fortified rice‐soy cereal supplement, isocaloric to meat supplement; approximately 70 kilocalories/day in 20 g portion increasing to 30 g at 12 months of age Both groups received 3 educational messages to encourage proper infant and young child feeding:
|
|
| Outcomes |
Primary:
Secondary:
Secondary outcomes were collected from a convenience sample of ˜300 participants per group (60%) of total participants. Measurement:
Time points:
|
|
| Notes |
Funding: "Supported by grants from Eunice Kennedy Shriver National Institute of Child Health and Human Development [HD040657 (UCD), HD043464 (UAB), HD040607 (Drexel), HD043475 (UNC), HD040636 (RTI)], Office of Dietary Supplements, and National Institute of Diabetes and Digestive and Kidney Diseases 9K24 DK083772. The National Cattlemen’s Beef Association partially supported the analyses of the biomarkers for this project and had no input into the study design, implementation, analysis, or interpretation of the data." Declared conflict of interest: none declared Other notes: attrition ˜14%; balanced between groups, both in number (86 in intervention group, 88 in control group) and reason |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐generated randomization algorithm, stratified by stunting rates within communities |
| Allocation concealment (selection bias) | Low risk | Comment: central randomization of clusters after individual participants were recruited within clusters |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: unable to blind due to nature of intervention, but geographic distance between clusters minimized risk of contamination of intervention or communication among study participants in different clusters |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: objective outcome assessment unlikely to have introduced bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition roughly equal between intervention (13.9%) and control (14.2%) groups |
| Selective reporting (reporting bias) | Low risk | Comment: all prespecified outcomes reported |
| Other bias | Unclear risk | Comment: partially supported by National Cattlemen’s Beef Association |
Tang 2014 (C).
| Methods |
Study design: cluster‐randomized controlled trial
Study duration: 12 months Start date: not provided End date: not provided |
|
| Participants |
Country and setting: China (upper‐middle‐income country); Xichou County, Yunnan Province, rural community with a 30% rate of stunting Population: 60 administrative villages (clustered) in 9 domains in Xichou County, Yunnan Province, China, a rural community with a 30% rate of stunting Inclusion criteria:
Exclusion criteria: not provided Nutritional status: baseline mean LAZ: −0.89 in intervention group, −1.02 in control group Number: 1471 (514 in intervention group, 957 in control group) Age: 6 months at enrollment Sex: not provided Typical diet: not provided |
|
| Interventions |
Intervention: pork; 60‐gram aliquots of fresh, certified‐safe pork, minced and stored frozen, distributed every other day to children's homes Control: cereal; 2 arms: a multiple‐micronutrient‐fortified cereal or locally produced, non‐fortified cereal; both arms isocaloric to meat arm |
|
| Outcomes |
Primary:
Measurement:
If measurements differed by more than 0.4 cm for length, a third measurement was taken. Time points: 6, 7, 9, 12, 15, and 18 months of age |
|
| Notes |
Funding: not provided Declared conflict of interest: not provided Other notes: The study authors note that the "two cereal groups were essentially indistinguishable in terms of macro‐ and micronutrient contents and were therefore combined to assess linear growth" (Tang 2014 (C)). No attrition was reported in the article, which was confirmed via email communication with the author (Tang 2014 (C)). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐generated cluster randomization |
| Allocation concealment (selection bias) | Low risk | Comment: central randomization of clusters after individual participants recruited within clusters |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: unable to blind but unlikely to have affected outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: objective outcome assessment unlikely to have introduced bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: study authors reported no attrition via personal communication (Tang 2018b) |
| Selective reporting (reporting bias) | High risk | Comment: micronutrient status described as an outcome of interest in study text but not reported in results; results obtained after contacting study authors (Tang 2018a) |
| Other bias | High risk |
Quote: "Observed differences between the groups at baseline, including infant length and length‐for‐age z‐score (LAZ) and maternal education, work status, height, and weight, were adjusted between groups for the primary analysis" Comment: significant (P = 0.02) imbalance in baseline LAZ between meat (−0.89 (± 0.97)) and cereal (−1.02 (± 0.99)) groups |
Tang 2018a.
| Methods |
Study design: randomized controlled trial
Study duration: 7 months Start date: September 2013 End date: August 2016 |
|
| Participants |
Country and setting: USA (high‐income country); Denver, Colorado metro area Population: not reported Inclusion criteria:
Exclusion criteria:
Nutritional status: baseline weight: 7.37 (± 0.67) in meat intervention group, 7.35 (± 0.74) in dairy control group; no significant differences between groups Number: 64 (32 in meat intervention group, 32 in dairy control group) Age: 5 months at enrollment Sex: 45% male in meat intervention group, 48% male in dairy control group Typical diet: formula‐fed. Fruit and vegetable intake were not restricted. |
|
| Interventions |
Intervention: meat; commercially available puréed meats Control: dairy; infant yogurt, cheese, and a powdered concentrate of 80% whey protein In both groups, either a meat or dairy‐based suite of foods were provided to parents. Parents were provided with tailored feeding guidelines and were encouraged to let the infant's appetite dictate their total intake. |
|
| Outcomes |
Primary:
Secondary:
Measurement:
Time points:
|
|
| Notes |
Funding: supported by the National Institutes of Health (NIH) (National Institute of Diabetes and Digestive and Kidney Diseases; 1K01DK111665), NIH/National Center for Advancing Translational Sciences (NCATS) Colorado Clinical and Translational Science Awards (CTSA; grant UL1 TR001082), and (alphabetically) Abbott Nutrition, the American Heart Association, the Beef Checkoff through the National Cattlemen’s Beef Association, Leprino Foods, and the National Pork Board Declared conflict of interest: none declared Other notes: "Only exclusively formula‐fed infants were chosen 1) to increase internal validity because breast‐ and formula‐fed infants pose different risks to rapid weight gain and may respond differently to complementary feeding, 2) because formula‐fed infants are at higher risk of excessive weight gain, and 3) because the majority of infants in the United States are formula‐fed, especially after 3 mo of age" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐generated assignment |
| Allocation concealment (selection bias) | Low risk | Quote: "Upon recruitment to the study, participants were matched to another participant with the use of 10 race/ethnicity categories. The treatment assignment for the first participant in each matched pair was randomly assigned in Microsoft Excel" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: unable to blind but unlikely to have affected outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: growth measurements conducted by nurses blinded to infants' feeding group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition rate ˜15% and relatively even between groups |
| Selective reporting (reporting bias) | Low risk | Comment: all stated outcomes reported |
| Other bias | Unclear risk | Comment: partially supported the National Cattlemen's Association, Leprino Foods, and the National Pork Board |
Tang and Krebs 2014.
| Methods |
Study design: randomized controlled trial
Study duration: 5 months Start date: January 2008 End date: September 2010 |
|
| Participants |
Country and setting: USA (high‐income country); Denver, Colorado metro area Population: not reported Inclusion criteria:
Exclusion criteria:
Nutritional status: baseline mean LAZ: −0.66 in intervention group, −0.10 in control group Number: 42 Age: 6 months at beginning of intervention Sex: 17 boys, 25 girls (differentiation between intervention and control groups not provided) Typical diet: not provided |
|
| Interventions |
Intervention: puréed meat and gravy; provision of 1 jar (71 g in total) by 7 months of age and 1 to 2 jars/d by 9 months of age Control: zinc‐ and iron‐fortified cereal; provision of 1 serving/d (15 g) by 7 months of age and 2 servings/d by 9 months of age |
|
| Outcomes |
Primary:
Reported in separate studies (Krebs 2012b; Krebs 2013) Secondary:
Measurement:
Time points: 5, 6, 7, 8, 9 months of age |
|
| Notes |
Funding: supported by the Beef Checkoff through the National Cattlemen’s Beef Association and the National Institutes of Health (K24 DK083772) Declared conflict of interest: none declared Other notes: infants in the cereal group were instructed to avoid single‐ingredient meats. The authors note that this study was a secondary analysis on growth of a randomized controlled trial designed to compare the effects of fortified cereal and meat on zinc homeostasis and iron status in breastfed‐only infants (Krebs 2012b; Krebs 2013). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomization and assignment to study group was accomplished using a random number generating program by statistician with no contact with field team |
| Allocation concealment (selection bias) | Low risk | Comment: central assignment by statistician who had no contact with field team |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: unable to blind but unlikely to have affected outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: anthropometric outcome measures obtained by research nurses with no knowledge of group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition rates were balanced between groups (approximately 14%), and all reasons specified and balanced between groups |
| Selective reporting (reporting bias) | Low risk | Comment: all prespecified outcomes reported |
| Other bias | Unclear risk | Comment: funding partially provided by National Cattlemen's Beef Association |
CDC: Centers for Disease Control and Prevention DRI: dietary reference intake HAZ: height‐for‐age z score LAZ: length‐for‐age z score LMIC: low‐ and middle‐income country (or countries) SAM: severe acute malnutrition WAZ: weight‐for‐age z score WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baker 1978 | Severely malnourished children |
| Batra 2016 | Did not meet requirement for animal‐source food |
| Bauserman 2015 | Did not meet requirement for animal‐source food |
| Bhandari 2001 | Did not meet requirement for animal‐source food |
| de Oliveira 1966 | Severely malnourished children |
| Dube 2010 | Did not meet requirement for animal‐source food |
| Engelmann 1998 | Did not meet requirement for animal‐source food |
| Jalil 2013 | Did not meet requirement for animal‐source food |
| Lartey 1999 | Did not meet requirement for animal‐source food |
| Lin 2008 | Did not meet requirement for animal‐source food |
| Long 2012 | Did not meet requirement for animal‐source food |
| NCT02272543 | Did not meet requirement for animal‐source food |
| NCT02516852 | Did not provide food |
| NCT02791100 | Did not provide food |
| Rosado 2011 | Did not meet requirement for animal‐source food |
| Schlossman 2015 | Did not meet requirement for animal‐source food |
| Skau 2015 | Did not meet requirement for animal‐source food |
| Tang 2016 | Did not provide food |
| Tavill 1969 | Not an RCT or quasi‐RCT |
RCT: randomized controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
NCT02496247.
| Methods |
Study design: randomized controlled trial Start date: May 2015 Current status: completed |
| Participants |
Number: 1200 children Age: 2 to 5 years |
| Interventions |
Intervention: immediate provision of canned herring for 8‐to‐10‐week period Control: delayed provision of canned herring; specifically, no intervention during 8‐to‐10‐week period when immediate‐herring families received weekly rations, then equal amount distribution following the first 8‐to‐10‐week period |
| Outcomes |
Primary:
Secondary:
Measurement: not reported Time point: baseline; 8 to 10 weeks |
| Notes |
Funding: Global Food & Nutrition Inc, Alaska Seafood Marketing Institute, International Partnership for Human Development Contact details:www.globalfoodandnutrition.com, principal investigator contacted at nina@globalfoodandnutrition.com |
HAZ: height‐for‐age z score MUAC: mid‐upper arm circumference WAZ: weight‐for‐age z score
Differences between protocol and review
We were unable to use all of our preplanned methods as per our published protocol (Eaton 2017). These have been archived in Table 3 for use in further updates of the review.
-
In our protocol, Eaton 2017, we specified that we would include children between the ages of 6 and 59 months. However, we included two studies that enrolled infants between five and six months of age (Tang 2018a; Tang and Krebs 2014), as the studies took place in a high‐income setting where the likelihood of food‐related infection is lower, and the majority of the intervention occurred in the complementary‐feeding window (between 5 and 10 months of age).
-
We planned to assess primary and secondary outcomes at 6 months' and 12 months' duration of intervention. However, as these time points were not uniformly reported, we reported baseline and endline measurements instead.
-
Assessment of risk of bias in included studies
We did not specify in our protocol how we would assess the overall risk of bias for each study (Eaton 2017). We included this in the review to be incorporated into judgments about the quality of the evidence provided in the 'Summary of findings' tables and to inform our confidence in the results of the review. We considered overall risk of bias as the presence of bias in the following key domains: random sequence generation, incomplete outcome data, selective reporting, and other risk (specifically, baseline imbalances in primary outcomes between intervention and control groups or the presence of funding from industries with an interest in the results). Where we rated a study at unclear risk of bias on one of these domains, we considered that study to be at unclear risk of bias overall. Where we rated a study at high risk of bias on one of these domains, we considered that study to be at high risk of bias overall. If a study appeared at both unclear and high risk of bias on two or more of the domains, we considered it to be at high risk of bias overall. Following the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017), we considered unclear risk of bias in a study to be a plausible bias that raises some doubt about the results. We considered high risk of bias in a study to be a plausible bias that seriously weakness the confidence in the results.
-
Dichotomous data. We planned to present any dichotomous data (e.g. anemia or morbidity) as odds ratios (OR) with 95% confidence intervals (CI) (Deeks 2011). However, due to differences in the ways that studies reported morbidity outcomes, we provided a narrative review of these outcomes instead.
-
Continuous data.
We planned to use the standardized mean difference (SMD) with 95% CI to combine trials that measured the same outcome using different measurement methods (Deeks 2011). We did not find applicable results, and so only used the mean difference.
Where some studies reported endpoint data and others reported change from baseline data (with errors), we planned to combine outcomes in the meta‐analysis providing the outcomes were reported using the same scale. However, we instead sought mean change in baseline data for primary outcomes (length‐for‐age z (LAZ) or height‐for‐age z (HAZ) scores), weight‐for‐age z scores (WAZ)) from the studies in which they were not provided by contacting the study authors.
-
We did not employ our preplanned methods to adjust for missing data (Eaton 2017), as loss to follow‐up was low in all studies (< 15%), and where it was present, it was balanced between groups with detailed reporting of reasons for the missing data.
We did not conduct a sensitivity analysis to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect, as none of the included studies had high levels of missing data (see Sensitivity analysis).
-
Had continuous measures not been available for primary outcomes (such as HAZ and LAZ scores), and the data not been available from the study authors, we planned to use dichotomous outcomes and re‐express ORs as SMD (or vice versa) and combine the results using the generic inverse variance method, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). However, we did not need to do this as we were able to obtain continuous measures for LAZ and WAZ.
-
Subgroup analysis and investigation of heterogeneity
We did not conduct subgroup analyses because we did not include more than 10 studies in the review.
-
We did not conduct a sensitivity analysis to examine the effects of removing studies at high risk of bias (those with high or unclear risk of bias for allocation concealment, similarity of baseline outcome measurements, incomplete outcome data), as we included only five studies in the meta‐analysis.
Contributions of authors
JE wrote the protocol and the review and performed all statistical analysis, with extensive input from PRS. All review authors read and reviewed the final manuscript. JE is the guarantor for the review.
Sources of support
Internal sources
-
World Health Organization (WHO), Switzerland.
Pura Rayco‐Solon is a full‐time member of staff at the WHO.
External sources
-
WHO, Switzerland.
The Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development, provided financial support to Jacob Eaton for his work in the preparation of the protocol and review.
-
Bill & Melinda Gates Foundation, USA.
The World Health Organization acknowledges the financial support of Bill & Melinda Gates Foundation for its work in building and maintaining updated systematic reviews on the effects of nutrition interventions.
Declarations of interest
Jacob Eaton received financial support from the World Health Organization (WHO) for his work on this review and for travel to attend the Cochrane/Cornell/WHO Summer Collaborative for Systematic Reviews in Nutrition. Pamela Rothpletz‐Puglia: none known. Margaret R Dreker: none known. Lora Iannotti and Chessa Lutter are authors of one of the studies included in this review (Iannotti 2017). Neither review author was involved in study selection, assessment of risk of bias, data extraction, or assessment of the quality of the evidence. Joyceline Kaganda: none known. Pura Rayco‐Solon is a full‐time member of staff at the WHO.
Disclaimer: the review authors alone are responsible for the views expressed in this publication, and they do not necessarily represent the official position, decisions, policy, or views of the WHO. The WHO gratefully acknowledges the financial contribution of the Bill & Melinda Gates Foundation, Nutrition International (NI; formerly Micronutrient Initiative (MI)), the Centers for Disease Control and Prevention (CDC), the US Agency for International development (USAID), and the Global Alliance for Improved Nutrition (GAIN) towards work in the area of nutrition. Donors do not fund specific guidelines and do not participate in any decision related to the guideline development process, including the composition of research questions, membership of the guideline groups, conduct and interpretation of systematic reviews, or formulation of recommendations.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
He 2005 {published data only}
- He M, Yang YX, Han H, Men J‐H, Bian L‐H, Wang GD. Effects of yogurt supplementation on the growth of preschool children in Beijing suburbs. Biomedical and Environmental Sciences 2005;18(3):192‐7. [PUBMED: 16131023] [PubMed] [Google Scholar]
Iannotti 2017 {published data only}
- Iannotti LL, Lutter CK, Stewart CP, Gallegos Riofrío CA, Malo C, Reinhart G, et al. Eggs in early complementary feeding and child growth: a randomized controlled trial. Pediatrics 2017;140(1):1‐9. [DOI: 10.1542/peds.2016-3459; PUBMED: 28588101] [DOI] [PubMed] [Google Scholar]
- Iannotti LL, Lutter CK, Waters WF, Gallegos Riofrio CA, Malo C, Reinhart G, et al. Eggs early in complementary feeding increase choline pathway biomarkers and DHA: a randomized controlled trial in Ecuador. American Journal of Clinical Nutrition 2017;106(6):1482‐9. [DOI: 10.3945/ajcn.117.160515] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krebs 2012a (C) {published and unpublished data}
- Krebs NF. Cochrane review ‐ request for information [personal communication]. Email to: J Eaton 26 January 2018.
- Krebs NF, Hambidge KM, Mazariegos M, Westcott J, Goco N, Wright LL, et al. Complementary feeding: a global network cluster randomized controlled trial. BMC Pediatrics 2011;11(4):1‐10. [DOI: 10.1186/1471-2431-11-4; PMC3032692; PUBMED: 21232139] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs NF, Mazariegos M, Chomba E, Sami N, Pasha O, Tshefu A, et al. Randomized controlled trial of meat compared with multimicronutrient‐fortified cereal in infants and toddlers with high stunting rates in diverse settings. American Journal of Clinical Nutrition 2012;96(4):840‐7. [DOI: 10.3945/ajcn.112.041962; PMC3441111; PUBMED: 22952176] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2014 (C) {published and unpublished data}
- Tang M. Cochrane review ‐ request for information [personal communication]. Email to: J Eaton 10 January 2018.
- Tang M. Micronutrient status in pork study in China [personal communication]. Email to: J Eaton 22 February 2018.
- Tang M, Sheng X‐Y, Krebs NF, Hambridge KM. Meat as complementary food for older breastfed infants and toddlers: a randomized, controlled trial in rural China. Food and Nutrition Bulletin 2014;35(4 Suppl):S188‐92. [DOI: 10.1177/15648265140354S304; PMC4490582; PUBMED: 25639137] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2018a {published data only}
- Tang M. Cochrane review ‐ request for information [personal communication]. Email to: J Eaton 10 January 2018.
- Tang M, Hendricks AE, Krebs NF. A meat‐ or dairy‐based complementary diet leads to distinct growth patterns in formula‐fed infants: a randomized controlled trial. American Journal of Clinical Nutrition 2018;107(5):734‐42. [DOI: 10.1093/ajcn/nqy038; PMC6128676; PUBMED: 29722841] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang and Krebs 2014 {published and unpublished data}
- Tang M, Krebs NF. High protein intake from meat as complementary food increases growth but not adiposity in breastfed infants: a randomized trial. American Journal of Clinical Nutrition 2014;100(5):1322‐8. [DOI: 10.3945/ajcn.114.088807; PMC4196483; PUBMED: 25332329] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Baker 1978 {published data only}
- Baker RD, Baker SS, Margo GM, Reuter HH. Succesful use of a soya‐maize mixture in the treatment of kwashiorkor. South African Medical Journal 1978;53(17):674‐7. [PUBMED: 354046] [PubMed] [Google Scholar]
Batra 2016 {published data only}
- Batra P, Schlossman N, Balan I, Pruzensky W, Balan A, Brown C, et al. A randomized controlled trial offering higher‐ compared with lower‐dairy second meals daily in preschools in Guinea‐Bissau demonstrates an attendance‐dependent increase in weight gain for both meal types and an increase in mid‐upper arm circumference for the higher‐dairy meal. Journal of Nutrition 2016;146(1):124‐32. [DOI: 10.3945/jn.115.218917; PUBMED: 26609172] [DOI] [PubMed] [Google Scholar]
Bauserman 2015 {published data only}
- Bauserman M, Lokangaka A, Gado J, Close K, Wallace D, Kodondi KK, et al. A cluster‐randomized trial determining the efficacy of caterpillar cereal as a locally available and sustainable complementary food to prevent stunting and anaemia. Public Health Nutrition 2015;18(10):1785‐92. [DOI: 10.1017/S1368980014003334; PUBMED: 25631295] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhandari 2001 {published data only}
- Bhandari N, Bahl R, Nayyar B, Khokhar P, Rohde JE, Bhan MK. Food supplementation with encouragement to feed it to infants from 4 to 12 months of age has a small impact on weight gain. Journal of Nutrition 2001;131(7):1946‐51. [DOI: 10.1093/jn/131.7.1946; PUBMED: 11435512] [DOI] [PubMed] [Google Scholar]
de Oliveira 1966 {published data only}
- Oliveira JE, Scatena L, Netto Nde O, Duarte GG. The nutritive value of soya milk and cow's milk in malnourished children: a comparative study. Journal of Pediatrics 1966;69(4):670‐5. [PUBMED: 5953306] [DOI] [PubMed] [Google Scholar]
Dube 2010 {published data only}
- Dube K, Schwartz J, Mueller MJ, Kalhoff H, Kersting M. Complementary food with low (8%) or high (12%) meat content as source of dietary iron: a double‐blinded randomized controlled trial. European Journal of Nutrition 2010;49(1):11‐8. [DOI: 10.1007/s00394-009-0043-9; PUBMED: 19618230] [DOI] [PubMed] [Google Scholar]
Engelmann 1998 {published data only}
- Engelmann MD, Sandström B, Michaelsen KF. Meat intake and iron status in late infancy: an intervention study. Journal of Pediatric Gastroenterology and Nutrition 1998;26(1):26‐33. [PUBMED: 9443116] [DOI] [PubMed] [Google Scholar]
Jalil 2013 {published data only}
- Jalil R, Naser I, Wan Muda WM, Wan Nik WS, Shariff Z, Adbullah MR. Effect of animal source food (ASF) provision on the growth of malnourished children in Kelantan, Malaysia: a randomized controlled trial. Annals of Nutrition and Metabolism 2013;63(Suppl 1):391. [P0282] [Google Scholar]
Lartey 1999 {published data only}
- Lartey A, Manu A, Brown KH, Peerson JM, Dewey KG. A randomized, community‐based trial of the effects of improved, centrally processed complementary foods on growth and micronutrient status of Ghanaian infants from 6 to 12 mo of age. American Journal of Clinical Nutrition 1999;70(3):391‐404. [DOI: 10.1093/ajcn/70.3.391; PUBMED: 10479202] [DOI] [PubMed] [Google Scholar]
Lin 2008 {published data only}
- Lin CA, Manary MJ, Maleta K, Briend A, Ashorn P. An energy‐dense complementary food is associated with a modest increase in weight gain when compared with a fortified porridge in Malawian children aged 6‐18 months. Journal of Nutrition 2008;138(3):593‐8. [DOI: 10.1093/jn/138.3.593; PUBMED: 18287372] [DOI] [PubMed] [Google Scholar]
Long 2012 {published data only}
- Long JK, Murphy SP, Weiss RE, Nyerere S, Bwibo NO, Neumann CG. Meat and milk intakes and toddler growth: a comparison feeding intervention of animal‐source foods in rural Kenya. Public Health Nutrition 2012;15(6):1100‐7. [DOI: 10.1017/S1368980011002746; PUBMED: 22152430] [DOI] [PubMed] [Google Scholar]
NCT02272543 {published data only}
- NCT02272543. Acceptability and efficacy of 'fish surimi peptide' in under five children suffering from moderate acute malnutrition. clinicaltrials.gov/ct2/show/NCT02272543 (first received 23 October 2014).
NCT02516852 {published data only}
- NCT02516852. Assessing the effect of sustainable small‐scale egg production on maternal and child nutrition in rural Zambia [Assessing the effect of sustainable small‐scale egg production on maternal and child nutrition in rural Zambia]. clinicaltrials.gov/ct2/show/NCT02516852 (first received 6 August 2015).
NCT02791100 {published data only}
- NCT02791100. Promotion of egg and eggshell powder consumption improve nutritional status of children in Halaba Ethiopia [Promotion of egg and eggshell powder consumption improve nutritional status of children of under two years of age: a cluster randomized controlled community trial in Halaba Sp. Woreda, Southern Nations and Nationalities Peoples Region (SNNPR), Ethiopia]. clinicaltrials.gov/ct2/show/NCT02791100 (first received 6 June 2016).
Rosado 2011 {published data only}
- Rosado JL, López P, García OP, Alatorre J, Alvarado C. Effectiveness of the nutritional supplement used in the Mexican Oportunidades programme on growth, anaemia, morbidity and cognitive development in children aged 12‐24 months. Public Health Nutrition 2011;14(5):931‐7. [DOI: 10.1017/S1368980010003344; PUBMED: 21205399] [DOI] [PubMed] [Google Scholar]
Schlossman 2015 {published data only}
- Schlossman N, Batra P, Balan E, Pruzensky W, Shae K, Schleicher M, et al. The effectiveness of two ready to use supplementary foods (RUSFs) differing in dairy protein content on growth and nutritional status of young children: a pilot study in preschools in Guinea‐Bissau. FASEB Journal 2015;29(1 Suppl):898.15. [DOI: 10.1096/fasebj.29.1_supplement.898.15] [DOI] [Google Scholar]
Skau 2015 {published data only}
- Skau JKH, Touch B, Chhoun C, Chea M, Unni US, Makurat J, et al. Effects of animal source food and micronutrient fortification in complementary food products on body composition, iron status, and linear growth: a randomized trial in Cambodia. American Journal of Clinical Nutrition 2015;101(4):742‐51. [DOI: 10.3945/ajcn.114.084889; PUBMED: 25833972] [DOI] [PubMed] [Google Scholar]
Tang 2016 {published data only}
- Tang M, Griese KE, Krebs NF. Dietary intakes of formula‐fed infants consuming a meat‐ or dairy‐based complementary diet: a semi‐controlled feeding trial. FASEB Journal 2016;30(1 Suppl):151.8. [DOI: 10.1096/fasebj.30.1_supplement.151.8] [DOI] [Google Scholar]
Tavill 1969 {published data only}
- Tavill F, Gonik A. Use of fish protein concentrate in the diets of weanling infants. A study of 88 infants from a low socioeconomic population, Casablanca, Morocco. American Journal of Clinical Nutrition 1969;22(12):1571‐6. [DOI: 10.1093/ajcn/22.12.1571; PUBMED: 5395473 ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT02496247 {published data only (unpublished sought but not used)}
- Eaton J. Canned herring for prevention of childhood malnutrition during the early rainy season in rural Guinea‐Bissau [personal communication]. Email to: N Schlossman 28 November 2017.
- NCT02496247. Canned herring for prevention of childhood malnutrition during the early rainy season in rural Guinea‐Bissau [Effectiveness of canned herring for prevention of childhood malnutrition during the early rainy season in rural Guinea‐Bissau]. clinicaltrials.gov/ct2/show/NCT02496247 (first received 14 July 2015).
Additional references
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI: 10.1016/j.jclinepi.2010.07.015; PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
Bes‐Rastrollo 2013
- Bes‐Rastrollo M, Schulze MB, Ruiz‐Canela M, Martinez‐Gonzalez MA. Financial conflicts of interest and reporting bias regarding the association between sugar‐sweetened beverages and weight gain: a systematic review of systematic reviews. PLoS Medicine 2013;10(12):e1001578. [DOI: 10.1371/journal.pmed.1001578; PUBMED: PMC3876974] [DOI] [PMC free article] [PubMed] [Google Scholar]
Black 2008
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371(9608):243‐60. [DOI: 10.1016/S0140-6736(07)61690-0; PUBMED: 18207566] [DOI] [PubMed] [Google Scholar]
Black 2013
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, Onis M, et al. Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet 2013;382(9890):427‐51. [DOI: 10.1016/S0140-6736(13)60937-X; PUBMED: 23746772] [DOI] [PubMed] [Google Scholar]
Bradlee 2010
- Bradlee ML, Singer MR, Qureshi MM, Moore LL. Food group intake and central obesity among children and adolescents in the Third National Health and Nutrition Examination Survey (NHANES III). Public Health Nutrition 2010;13(6):797‐805. [DOI: 10.1017/S1368980009991546; PUBMED: 19772691] [DOI] [PubMed] [Google Scholar]
Brazilian MoH 2015
- Ministry of Health of Brazil. Dietary guidelines for the Brazilian population. bvsms.saude.gov.br/bvs/publicacoes/dietary_guidelines_brazilian_population.pdf (accessed 13 February 2017).
Chandler 1980
- Chandler LS, Andrews MS, Swanson MW, Larson AH. Movement assessment of infants: a manual. depts.washington.edu/dbpeds/Clinics%20and%20Activities/Forms‐HRIF%20(MAI‐1980).pdf (accessed 3 August 2017).
De‐Regil 2011
- De‐Regil LM, Suchdev PS, Vist GE, Walleser S, Peña‐Rosas JP. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD008959.pub2] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analysis. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Demment 2003
- Demment MW, Young MM, Sensenig RL. Providing micronutrients through food‐based solutions: a key to human and national development. Journal of Nutrition 2003;133(11 Suppl 2):3879S‐85S. [DOI: 10.1093/jn/133.11.3879S; PUBMED: 14672285] [DOI] [PubMed] [Google Scholar]
Dewey 2008
- Dewey KG, Adu‐Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Maternal & Child Nutrition 2008;4(Suppl 1):24‐85. [DOI: 10.1111/j.1740-8709.2007.00124.x; PUBMED: 18289157] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dror 2011
- Dror DK, Allen LH. The importance of milk and other animal‐source foods for children in low‐income countries. Food and Nutrition Bulletin 2011;32(3):227‐43. [DOI: 10.1177/156482651103200307; PUBMED: 22073797] [DOI] [PubMed] [Google Scholar]
Eaton 2017 [pers comm]
- Eaton J. Canned herring for prevention of childhood malnutrition during the early rainy season in rural Guinea‐Bissau [personal communication]. Email to: N Schlossman 28 November 2017.
EPOC 2013
- Cochrane Effective Practice, Organisation of Care (EPOC). Data collection form. EPOC resources for review authors, 2017. epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/Resources‐for‐authors2017/good_practice_data_extraction_form.doc (accessed 1 August 2016).
FAO 1995
- Loftas T, editor. Dimensions of Need: An Atlas of Food and Agriculture. Rome: Food and Agriculture Organization of the United Nations, 1995. [Google Scholar]
FAO/WHO 1998
- Food, Agriculture Organization of the United Nations. World Health Organization. Preparation and Use of Food‐Based Dietary Guidelines: Report of a Joint FAO/WHO Consultation. Geneva: World Health Organization, 1998. [Google Scholar]
Fardet 2015
- Fardet A, Rock E, Bassama J, Bohuon P, Prabhasankar P, Monteiro C, et al. Current food classifications in epidemiological studies do not enable solid nutritional recommendations for preventing diet‐related chronic diseases: the impact of food processing. Advances in Nutrition 2015;6(6):629‐38. [DOI: 10.3945/an.115.008789; PMC4642417] [DOI] [PMC free article] [PubMed] [Google Scholar]
Folio 1983
- Folio MR, Fewell RR. Peabody Developmental Motor Scales and Activity Cards. Austin (TX): Riverside Publishing Company, 1983. [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version (accessed 3 August 2017). Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Headey 2016
- Headey D, Hirvonen K. Is exposure to poultry harmful to child nutrition? An observational analysis for rural Ethiopia. PLOS ONE 2016;11(8):e0160590. [DOI: 10.1371/journal.pone.0160590; PMC4986937; PUBMED: 27529178] [DOI] [PMC free article] [PubMed] [Google Scholar]
Headey 2017
- Headey D, Hirvonen K, Hoddinott J. Animal sourced foods and child stunting. ebrary.ifpri.org/utils/getfile/collection/p15738coll2/id/132232/filename/132446.pdf (accessed 15 September 2018). [DOI] [PMC free article] [PubMed]
Higgins 2011
- Higgins JPT, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpson MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available at www.training.cochrane.org/handbook.
Iannotti 2014
- Iannotti LL, Lutter CK, Bunn DA, Stewart CP. Eggs: the uncracked potential for improving maternal and young child nutrition among the world's poor. Nutrition Reviews 2014;72(6):355‐68. [DOI: 10.1111/nure.12107; PUBMED: 24807641] [DOI] [PubMed] [Google Scholar]
Jacobs 2009
- Jacobs DR Jr, Gross MD, Tapsell LC. Food synergy: an operational concept for understanding nutrition. American Journal of Clinical Nutrition 2009;89(5):1543S‐8S. [DOI: 10.3945/ajcn.2009.26736B; PMC2731586; PUBMED: 19279083] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kramer 2002
- Kramer MS, Kakuma R. The Optimal Duration of Exclusive Breastfeeding: A Systematic Review. Geneva: World Health Organization, 2002. [DOI] [PubMed] [Google Scholar]
Krebs 2011
- Krebs NF, Mazariegos M, Tshefu A, Bose C, Sami N, Chomba E, et al. Meat consumption is associated with less stunting among toddlers in four diverse low‐income settings. Food and Nutrition Bulletin 2011;32(3):185‐91. [DOI: 10.1177/156482651103200301; PMC3918945; PUBMED: 22073791] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krebs 2012b
- Krebs NF, Westcott JE, Culbertson DL, Sian L, Miller LV, Hambidge KM. Comparison of complementary feeding strategies to meet zinc requirements of older breastfed infants. American Journal of Clinical Nutrition 2012;96(1):30‐5. [DOI: 10.3945/ajcn.112.036046; PMC3374732; PUBMED: 22648720] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krebs 2013
- Krebs NF, Sherlock LG, Westcott J, Culbertson D, Hambidge KM, Feazel LM, et al. Effects of different complementary feeding regimens on iron status and enteric microbiota in breastfed infants. Journal of Pediatrics 2013;163(2):416‐23. [DOI: 10.1016/j.jpeds.2013.01.024; PMC3674183; PUBMED: 23452586] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leroy 2007
- Leroy JL, Frongillo EA. Can interventions to promote animal production ameliorate undernutrition?. Journal of Nutrition 2007;137(10):2311‐6. [DOI: 10.1093/jn/137.10.2311] [DOI] [PubMed] [Google Scholar]
Michaelsen 1998
- Michaelsen KF, Friis H. Complementary feeding: a global perspective. Nutrition 1998;14(10):763‐6. [PUBMED: 9785357] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097; PMC2707599; PUBMED: 19621072] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nyaradi 2013
- Nyaradi A, Li J, Hickling S, Foster J, Oddy WH. The role of nutrition in children's neurocognitive development, from pregnancy through childhood. Frontiers in Human Neuroscience 2013;7(97):1‐16. [DOI: 10.3389/fnhum.2013.00097; PMC3607807; PUBMED: 23532379] [DOI] [PMC free article] [PubMed] [Google Scholar]
PAHO/WHO 2003
- Pan American Health Organization, World Health Organization. Guiding principles for complementary feeding of the breastfed child. www.who.int/nutrition/publications/guiding_principles_compfeeding_breastfed.pdf (accessed 13 February 2017).
Pingali 2007
- Pingali P. Westernization of Asian diets and the transformation of food systems: implications for research and programming. Food Policy 2007;32(3):281‐98. [DOI: 10.1016/j.foodpol.2006.08.001] [DOI] [Google Scholar]
Popkin 2001
- Popkin BM. The nutrition transition and obesity in the developing world. Journal of Nutrition 2001;131(3):871S‐3S. [PUBMED: 11238777] [DOI] [PubMed] [Google Scholar]
Popkin 2014
- Popkin BM. Nutrition, agriculture and the global food system in low and middle income countries. Food Policy 2014;47:91‐6. [DOI: 10.1016/j.foodpol.2014.05.001; PMC4053196; PUBMED: 24932059] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prudhon 2006
- Prudhon C, Prinzo ZW, Briend A, Daelmans BM, Mason JB. Proceedings of the WHO, UNICEF, and SCN informal consultation on community‐based management of severe malnutrition in children. Food and Nutrition Bulletin 2006; Vol. 27, issue 3 Suppl:S99‐104. [DOI: 10.1177/15648265060273S307; PUBMED: 17076216] [DOI] [PubMed]
Rauber 2015
- Rauber F, Campagnolo PD, Hoffman DJ, Vitolo MR. Consumption of ultra‐processed food products and its effects on children's lipid profiles: a longitudinal study. Nutrition, Metabolism and Cardiovascular Diseases 2015;25(1):116‐22. [DOI: 10.1016/j.numecd.2014.08.001; PUBMED: 25240690] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sguassero 2012
- Sguassero Y, Onis M, Bonotti AM, Carroli G. Community‐based supplementary feeding for promoting the growth of children under five years of age in low and middle income countries. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD005039.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Speakman 2013
- Speakman JR. Evolutionary perspectives on the obesity epidemic: adaptive, maladaptive, and neutral viewpoints. Annual Review of Nutrition 2013;33:289‐317. [DOI: 10.1146/annurev-nutr-071811-150711; PUBMED: 23862645] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D, editor(s). Chapter 10: Addressing reporting bias. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Tang 2018b
- Tang M. Cochrane review ‐ request for information [personal communication]. Email to: J Eaton 10 January 2018.
Victora 2010
- Victora CG, Onis M, Hallal PC, Blössner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics 2010;125(3):e473‐80. [DOI: 10.1542/peds.2009-1519; PUBMED: 20156903] [DOI] [PubMed] [Google Scholar]
Wang 2009
- Wang Y, Beydoun MA. Meat consumption is associated with obesity and central obesity among US adults. International Journal of Obesity 2009;33(6):621‐8. [DOI: 10.1038/ijo.2009.45; PMC2697260; PUBMED: 19308071] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2003
- World Health Organization. Global Strategy for Infant and Young Child Feeding. Geneva: World Health Organization, 2003. [Google Scholar]
WHO 2004
- World Health Organization. Global Strategy on Diet, Physical Activity and Health. Geneva: World Health Organization, 2004. [Google Scholar]
WHO 2006
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatrica 2006;95(S450):76‐85. [DOI: 10.1111/j.1651-2227.2006.tb02378.x; PUBMED: 16817681] [DOI] [PubMed] [Google Scholar]
WHO 2016
- UNICEF, WHO, World Bank Group. Levels and trends in child malnutrition. UNICEF/WHO/World Bank Group joint child malnutrition estimates. Key findings of the 2016 edition. www.who.int/nutgrowthdb/jme_brochure2016.pdf (accessed 12 June 2017).
References to other published versions of this review
Eaton 2017
- Eaton JC, Rothpletz‐Puglia P, Dreker MR, Kaganda J, Iannotti L, Lutter C, et al. Effectiveness of provision of animal‐source foods for supporting optimal growth and development in children 6 to 59 months of age. Cochrane Database of Systematic Reviews 2017, Issue 10. [DOI: 10.1002/14651858.CD012818] [DOI] [PMC free article] [PubMed] [Google Scholar]


