Abstract
Background:
This systematic review aims to assess the efficacy and safety of transsphenoidal surgery (TPS) for patients with a pituitary tumor (PT).
Methods:
We will retrieve the following electronic databases for randomized controlled trials or case–control studies to assess the effect and safety of TPS for PT: Cochrane Library, EMBASE, MEDLINE, Cumulative Index to Nursing and Allied Health Literature, Web of Science, Allied and Complementary Medicine Database, and Chinese Biomedical Literature Database. Each database will be retrieved from the inception to December 20, 2018. The entire process consists of the study selection, data collection, methodology quality assessment, data pooled, and meta-analysis performance. The methodology quality will be assessed by using Cochrane risk of bias tool. The data pooled and meta-analysis will be conducted by using RevMan 5.3 software.
Results:
This study will evaluate the efficacy and safety of TPS for PT. The primary outcome includes total tumor resection rate. The secondary outcomes consist of quality of life, total tumor resection rate, postoperative complication rate, and the rate of functional tumor hormone levels.
Conclusion:
The expected results may provide up-to-date evidence of TPS for the treatment of PT.
PROSPERO registration number:
PROSPERO CRD42018120194.
Keywords: case–control study, efficacy, pituitary tumor, randomized controlled trial, safety, systematic review, transsphenoidal surgery
1. Introduction
Pituitary tumor (PT) is a very common disorder of brain diseases, which often contributes to approximately 15% of all brain tumors.[1,2] With the growth of this tumor, it can affect optic chiasm, and thus can cause visual function impairment with the presentation of the visual field defects, decreased visual acuity, and decreased color vision.[3–6] In addition, it can also further affect the visual function by pressing on the anterior visual pathway. In such situation, it often manifests with headache, vomiting, dizziness, diplopia, and so on.[7–9] Of these, visual field defect is the most frequent symptoms,[10–12] which is often recognized as 1 of the primary indications for surgery on PT.[13,14]
Transsphenoidal surgery (TPS) has reported to treat PT effectively and safely by through reduction the pressure on the anterior visual pathway.[15–22] Although several clinical studies have addressed the efficacy and safety of TPS for the treatment of patients with PT,[16–22] and have achieved satisfied outcome results, no systematic review has evaluated its efficacy for PT. Thus, in this systematic review, we will conduct a systematic review to investigate the efficacy and safety of TPS for PT.
2. Methods and materials
2.1. Study registration
This systematic review has been registered on PROSPERO with number of CRD42018120194. It follows the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocol statement guidelines.[23]
2.2. Inclusion criteria for study selection
2.2.1. Type of studies
This study will only include randomized controlled trials and case–control studies for TPS in patients with PT. However, the studies of non-clinical trial, case reports, case series, and crossover studies will all be excluded.
2.2.2. Type of participants
Patients with a confirmed diagnosis of PT, male or female, of any age will be considered to include in this study. However, patients will be excluded if they also have other conditions that may affect the efficacy of TPS.
2.2.3. Type of interventions
The interventions of the experimental group will include TPS only. The studies will be excluded if they include the combination therapies of TPS and other treatments. The interventions in the control group can consist of any treatments, except the TPS.
2.2.4. Types of outcomes
The primary outcome is the total tumor resection rate. The secondary outcomes include quality of life, total tumor resection rate, postoperative complication rate, and the rate of functional tumor hormone levels.
2.3. Search methods for the identification of studies
2.3.1. Search strategy
We will be searching the following bibliographic databases for relevant studies from the inception to the December 20, 2018 without restrictions: Cochrane Library, EMBASE, MEDLINE, Cumulative Index to Nursing and Allied Health Literature, Web of Science, Allied and Complementary Medicine Database, and Chinese Biomedical Literature Database. In addition, Google Scholar, clinical registration website, and reference lists of relevant trials will also be searched. The sample of retrieval strategy for Cochrane Library is showen in Table 1. Similar retrieval strategy will also be built and be applied to the other literature sources.
Table 1.
Search strategy applied in Cochrane Library database.
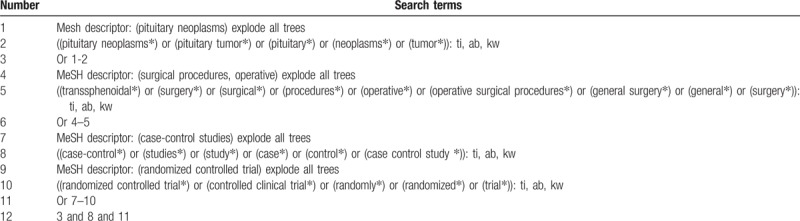
2.3.2. Missing data and insufficient information
If the essential information and/or data are missing or insufficient, we will contact the original authors to request those information or data. If we will not get response, the only available data will be pooled and analyzed.
2.3.3. Study identification and data extraction
Two independent authors will select all potential studies by scanning the titles, abstracts, and reading the full texts based on the predefined eligibility criteria. The same 2 independent authors will extract the data from included studies according to the predesigned form of data extraction. Any disagreements regarding the study selection and data extraction will be resolved with a third author through discussion. The procedure of study selection is showen in Figure 1.
Figure 1.
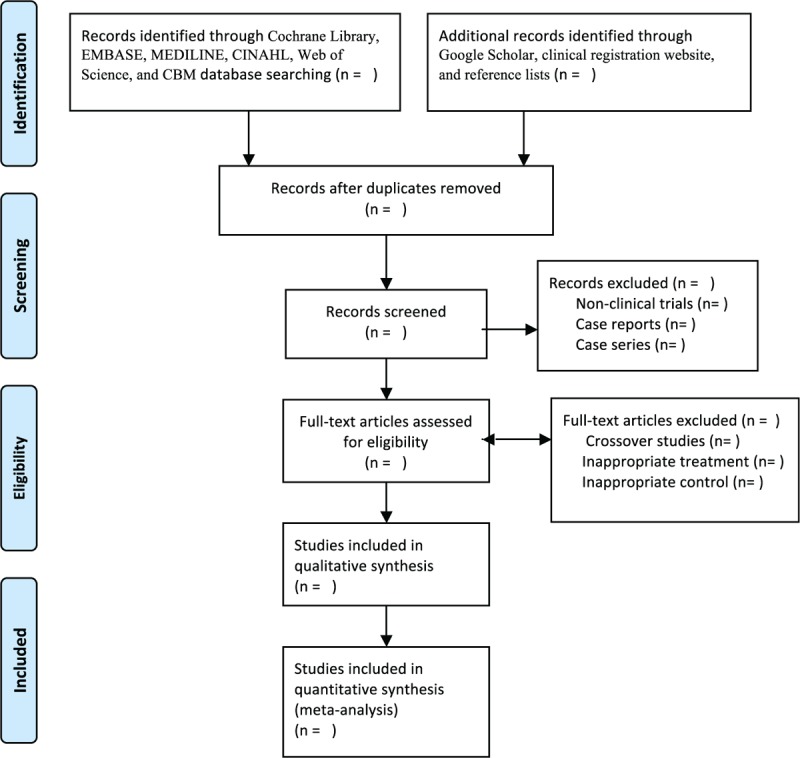
Procedure of study selection.
2.3.4. Methodology quality assessment
Cochrane risk of bias tool will be utilized to assess the methodology quality of all included studies. This tool consists of 7 items and each item will be classified as high, unclear, or low risk of bias. Two independent authors assess the methodology quality of each item for each included study. The disagreements between 2 authors will be solved with a third author by discussion.
2.4. Data synthesis and analysis
2.4.1. Measurement of treatment effect
For continuous values, data will be presented with a mean difference and 95% confidence intervals (CIs). For dichotomous values, data will be presented with risk ratio and 95% CIs. A value of P < .05 is set as having statistically significant.
2.4.2. Assessment of heterogeneity and data synthesis
We use Cochrane Q statistic and I2 tests to assess the heterogeneity. If I2 ≤50% or/and Q statistic test ≥0.10, the heterogeneity is acceptable, and fixed-effect model will be used to pool the data, and meta-analysis will be performed by RevMan 5.3 software. Otherwise, the heterogeneity is considered as substantial, random-effect model will be used to pool the data, and subgroup analysis will be conducted to identify any potential factors that may result in the heterogeneity. If the heterogeneity is still significant after the subgroup analysis, then the data will not be pooled. A narrative summary will be performed.
2.4.3. Subgroup analysis
If the heterogeneity is significant after the data pooled, we will conduct the subgroup analysis to detect the possible factors that may lead to the high heterogeneity. It will be performed in accordance with the different forms of experimental and control treatments, different outcome measurement tools.
2.4.4. Sensitivity analysis
If the data can be pooled, then sensitivity analysis will conduct to detect the robustness and stability of pooled results data, and methodological quality.
2.4.5. Assessment of publication bias
Funnel plot will be performed if more than 10 studies will be included.[24] In such situation, funnel plot asymmetry will also be detected by using Egg regression test.[25]
3. Discussion
This protocol of systematic review will summarize the latest data to assess the efficacy and safety of TPS for patients with PT. The findings of this study will provide the up-to-date evidence whether TPS will achieve promising efficacy and acceptable safety. In addition, the findings will also provide a useful reference for implementation of PT treatment and collection of patient-produced data for both clinical practitioners, researchers, as well as the health policy makers.
Author contributions
Conceptualization: Wei-Feng Wang, Lin-Hong Yang, Jian-Qi Xiao.
Data curation: Wei-Feng Wang, Lin-Hong Yang, Lin Han, Jian-Qi Xiao.
Formal analysis: Wei-Feng Wang, Lin-Hong Yang.
Funding acquisition: Wei-Feng Wang.
Investigation: Jian-Qi Xiao.
Methodology: Wei-Feng Wang, Lin-Hong Yang, Lin Han, Ming-Jun Li, Jian-Qi Xiao.
Project administration: Jian-Qi Xiao.
Resources: Wei-Feng Wang, Lin-Hong Yang, Lin Han, Ming-Jun Li.
Software: Wei-Feng Wang, Lin-Hong Yang, Lin Han, Ming-Jun Li.
Supervision: Lin Han, Jian-Qi Xiao.
Validation: Lin-Hong Yang, Lin Han, Ming-Jun Li, Jian-Qi Xiao.
Visualization: Lin-Hong Yang, Lin Han, Ming-Jun Li, Jian-Qi Xiao.
Writing – original draft: Wei-Feng Wang, Lin-Hong Yang, Ming-Jun Li, Jian-Qi Xiao.
Writing – review and editing: Wei-Feng Wang, Lin-Hong Yang, Lin Han, Ming-Jun Li, Jian-Qi Xiao.
Footnotes
Abbreviations: PT = pituitary tumor, TPS = transsphenoidal surgery.
L-HY and W-FW contributed equally to this study.
No patient and public will be involved in this study.
This systematic review does not requires ethic approval, because all the data used in this study has already been published. The results of this study will be determined to publish at peer-reviewed journals.
This study is supported by the Heilongjiang Provincial Health Department Project (2018023). The funder is not allowed to join in the conduction of this study.
The authors have no conflicts of interest to disclose.
References
- [1].Colao A, Auriemma RS, Pivonello R. The effects of somatostatin analogue therapy on pituitary tumor volume in patients with acromegaly. Pituitary 2016;19:210–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Johnston PC, Hamrahian AH, Weil RJ, et al. Pituitary tumor apoplexy. J Clin Neurosci 2015;22:939–44. [DOI] [PubMed] [Google Scholar]
- [3].Araujo PB, Vieira Neto L, Gadelha MR. Pituitary tumor management in pregnancy. Endocrinol Metab Clin North Am 2015;44:181–97. [DOI] [PubMed] [Google Scholar]
- [4].Carija R, Vucina D. Frequency of pituitary tumor apoplexy during treatment of prolactinomas with dopamine agonists: a systematic review. CNS Neurol Disord Drug Targets 2012;11:1012–4. [DOI] [PubMed] [Google Scholar]
- [5].Winder MJ, Mayberg MR. Recent advances in pituitary tumor management. Curr Opin Endocrinol Diabetes Obes 2011;18:278–88. [DOI] [PubMed] [Google Scholar]
- [6].Kerrison JB, Lynn MJ, Baer CA, et al. Stages of improvement in visual fields after pituitary tumor resection. Am J Ophthalmol 2000;130:813–20. [DOI] [PubMed] [Google Scholar]
- [7].Dekkers OM, Hammer S, de Keizer RJ, et al. The natural course of non-functioning pituitary macroadenomas. Eur J Endocrinol 2007;156:217–24. [DOI] [PubMed] [Google Scholar]
- [8].Shimatsu A. Diagnosis and testing of hypothalamo-hypophysial (pituitary) tumor: general considerations. Nihon Rinsho 2011;69Suppl 2:146–9. [PubMed] [Google Scholar]
- [9].Elston MS, Clifton-Bligh RJ. Identification of Wnt family inhibitors: a pituitary tumor directed whole genome approach. Mol Cell Endocrinol 2010;326:48–54. [DOI] [PubMed] [Google Scholar]
- [10].Monteiro ML, Moura FC, Cunha LP. Frequency doubling perimetry in patients with mild and moderate pituitary tumor-associated visual field defects detected by conventional perimetry. Arq Bras Oftalmol 2007;70:323–9. [DOI] [PubMed] [Google Scholar]
- [11].Yorgason JG, Arthur AS, Orlandi RR, et al. Endoscopic decompression of tension pneumosella following transsphenoidal pituitary tumor resection. Pituitary 2004;7:171–7. [DOI] [PubMed] [Google Scholar]
- [12].Dirr LY, Troost BT, Elster AD, et al. Amaurosis fugax due to pituitary tumor. J Clin Neuroophthalmol 1991;11:254–8. [PubMed] [Google Scholar]
- [13].Zoli M, Milanese L, Faustini-Fustini M, et al. Endoscopic endonasal surgery for pituitary apoplexy: evidence on a 75-case series from a tertiary care center. World Neurosurg 2017;106:331–8. [DOI] [PubMed] [Google Scholar]
- [14].Ogiwara T, Horiuchi T, Nagm A, et al. Significance of surgical management for cystic prolactinoma. Pituitary 2017;20:225–30. [DOI] [PubMed] [Google Scholar]
- [15].Mortini P, Losa M, Barzaghi R, et al. Results of transsphenoidal surgery in a large series of patients with pituitary adenoma. Neurosurgery 2005;56:1222–33. [DOI] [PubMed] [Google Scholar]
- [16].Muhlestein WE, Akagi DS, McManus AR, et al. Machine learning ensemble models predict total charges and drivers of cost for transsphenoidal surgery for pituitary tumor. J Neurosurg 2018;21:1–0. [DOI] [PubMed] [Google Scholar]
- [17].Li CZ, Li CC, Hsieh CC, et al. Fatal antiphospholipid syndrome following endoscopic transnasal-transsphenoidal surgery for a pituitary tumor: a case report. Medicine (Baltimore) 2017;96:e5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee CH, Chen SM, Lui TN. Posterior cerebral artery pseudoaneurysm, a rare complication of pituitary tumor transsphenoidal surgery: case report and literature review. World Neurosurg 2015;84:1493.e1–3. [DOI] [PubMed] [Google Scholar]
- [19].Cho JM, Min KT, Kim EH, et al. Sudden asystole due to trigeminocardiac reflex during transsphenoidal surgery for pituitary tumor. World Neurosurg 2011;76: 477. e11–5. [DOI] [PubMed] [Google Scholar]
- [20].Choudhry OJ, Choudhry AJ, Nunez EA, et al. Pituitary tumor apoplexy in patients with Cushing's disease: endocrinologic and visual outcomes after transsphenoidal surgery. Pituitary 2012;15:428–35. [DOI] [PubMed] [Google Scholar]
- [21].Sciarretta V, Mazzatenta D, Ciarpaglini R, et al. Surgical repair of persisting CSF leaks following standard or extended endoscopic transsphenoidal surgery for pituitary tumor. Minim Invasive Neurosurg 2010;53:55–9. [DOI] [PubMed] [Google Scholar]
- [22].Arai T, Okada K, Yamaguchi T, et al. Endonasal transsphenoidal surgery under local anesthesia for elderly patient with pituitary tumor: case report. No Shinkei Geka 2000;28:991–5. [PubMed] [Google Scholar]
- [23].Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sutton AJ, Duval SJ, Tweedie RL, et al. Empirical assessment of effect of publication bias on meta-analyses. BMJ 2000;320:1574–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


