Abstract
The aim of this study was to compare the coagulation tests and blood glucose levels between healthy teenage pregnant patients and healthy adult pregnant patients just before vaginal delivery
In a prospective study, 208 consecutive patients, 3rd trimester healthy pregnant women, underwent blood tests to determine their glucose levels the day before vaginal delivery. Of the 208 patients, 103 also underwent blood coagulation testing performed on the same day.
The median values of the coagulation tests (APTT, prothrombin time, INR, prothrombin activity) and blood glucose were very similar in the healthy pregnant teenagers (32.6; 12.9; 1.02; 97.1; 81) compared with that in the healthy adult pregnant patients (32.45; 13.1; 1.01; 97.5; 81.2). Only the median value for fibrinogen was significantly different in healthy pregnant teenagers (348.9 mg/dL) (interquartile range 21.7) compared with that in healthy adult pregnant patients (359.1 mg/dL) (interquartile range 29.88).
Significantly different median blood glucose levels also occurred in the <20; 20–29; 30–39; >40 age groups, but the glucose levels were still within normal limits.
Even if there was variability between blood values from one age group to another, the median values for coagulation tests and blood glucose were very close in the healthy teenage pregnant patients compared with the median values of the healthy adult pregnant patients, just before vaginal delivery. With very few exceptions, the values for coagulation tests and blood glucose were within normal limits in all age groups of healthy pregnant patients.
Keywords: APTT, blood glucose, coagulation tests, fibrinogen, INR, prothrombin activity, prothrombin time, teenage pregnancy
1. Introduction
Perfect hemostatic balance is required in order to have a successful pregnancy. Obstetric hemorrhage and especially postpartum hemorrhage are responsible for serious maternal and fetal morbidity and mortality. Anticipation of massive postpartum hemorrhage, prompt recognition of the cause, replacement of the lost blood volume, and correction of the washout phenomenon leading to coagulopathy will save lives.[1] Therefore, coagulation tests are required before any cesarean delivery, but not before any vaginal delivery. Elevated blood glucose levels and even gestational diabetes mellitus can influence the outcome of delivery and of the mother's health; therefore, they are determined before any delivery, whether cesarean or vaginal.
The younger the pregnant patient, the healthier she should be. The aim of this study was to compare the coagulation tests and blood glucose levels of healthy teenage pregnant patients with those of healthy adult pregnant patients, just before vaginal delivery.
2. Methods
In a prospective study, 240 Caucasian patients in the 3rd trimester of pregnancy were included initially. They were referred to the hospital to deliver their babies from 2013 to 2017. Upon arrival, they had no indication for cesarean delivery. Patients who were not overweight and did not have diabetes mellitus were considered healthy and therefore included in this study. Sixty patients were selected for each of the four age groups (<20 years old; 20–29 years old; 30–39 years old; 40–49 years old). Ethical approval was not necessary because the standard prenatal protocol included blood glucose determination, and if the gynecologist considered that a patient had a tiny risk of cesarean delivery, coagulation tests were also included. Some patients did in fact require a cesarean delivery; therefore, they were excluded from the study, because we intended to study only patients who delivered vaginally. A total of 208 patients remained to deliver vaginally. They underwent blood tests to determine their glucose levels the day before vaginal delivery. Of the 208 patients, 103 patients underwent blood coagulation testing also. The remaining 105 patients had no significant health issues, and no obstetrical incidents occurred, so that cesarean delivery was not required in their cases; they successfully delivered vaginally; their blood was harvested only for glucose determination and not for coagulation tests.
Coagulation tests were obtained for 59 pregnant patients <20 years old, 17 pregnant patients 20 to 29 years old, 8 pregnant patients 30 to 39 years old, and 19 pregnant patients 40 to 49 years old. The extreme pregnancy ages (less than 20 and over 40) were more likely to require a cesarean delivery than patients between 20 to 39 years of age. In all, 59 pregnant patients <20 years old and 44 pregnant patients >20 years old delivered vaginally and had their coagulation tests determined the day before delivery.
Blood glucose level was determined in 59 pregnant patients <20 years old, 54 pregnant patients 20 to 29 years old, 62 pregnant patients 30 to 39 years old, and 33 pregnant patients 40 to 49 years old. In all, 59 pregnant patients <20 years old and 149 pregnant patients >20 years old delivered vaginally and had their blood glucose determined the day before delivery.
2.1. Comorbidities
Seven (3.36%) patients in all age groups had gestational elevated blood pressure, and 3 (1.44%) had elevated blood pressure, without proteinuria, which decreased with medication. Two (0.96%) patients had lower limb edema, 1 (0.48%) had lower limb superficial thrombophlebitis, 1 (0.48%) had lower limb varicose veins, and 2 (0.96%) had cicatricial uteruses.
The mean age of these healthy pregnant patients was 28.05 years of age (range 15–45).
The coagulation tests included activated partial prothrombin time (APTT), fibrinogen, prothrombin time, International Normalized Ratio (INR), and prothrombin activity. Coagulation tests were determined using RAYTO 2201C laboratory equipment. Blood glucose was determined using MINDRAY BS200 laboratory equipment.
Because of the small number of patients in the adult groups, determining the median value and interquartile range was considered more appropriate than mean values and standard deviations.
Statistical analysis was performed using IBM SPSS version 18. For descriptive measures, we computed the median, interquartile range, minimum and maximum limits. Multiple group comparison was performed using the Kruskal-Wallis 1-way ANOVA nonparametric test; significance values were adjusted with the Bonferroni correction for multiple post-hoc tests. For two sets, the Mann–Whitney U test was applied. P of .05 was considered statistically significant. Box-plot charts were used to demonstrate the group distributions. We are aware that the small samples sizes denote reduced statistical power. We accept the approximation of similar shape distributions of subgroups; thus, the nonparametric tests reflect the differences between medians. We applied the Kruskal-Wallis instead of the median test because it is more powerful.
3. Results
No significant difference was found between the median values of APTT, prothrombin time, INR in the different age groups. A significant difference was found between the median values of fibrinogen in the four age groups and between the blood glucose median values in the four age groups. There was a significant difference between fibrinogen median values before and after 20 years. Nevertheless, no clinically significant difference was triggered by these differences in fibrinogen and blood glucose, and no medical actions were taken based on the value of one group compared with that of the other group because all the values were within normal limits. (Tables 1–4)
Table 1.
Median values and interquartile range for coagulation tests, blood glucose and significance of Kruskal-Wallis 1 way ANOVA nonparametric test.
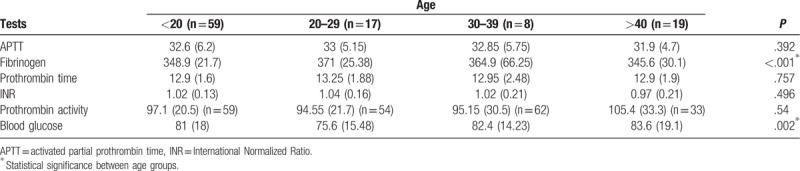
Table 4.
Median values and interquartile range for coagulation tests, blood glucose and significance of Mann–Whitney U for two samples nonparametric test. Fibrinogen values are significantly lower in healthy pregnant women <20 years than in healthy pregnant women over 20 years of age.
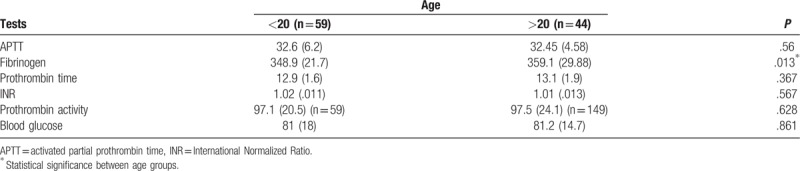
Table 2.
Pairwise comparisons of AGE groups for fibrinogen.
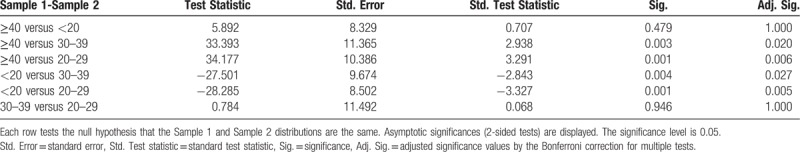
Table 3.
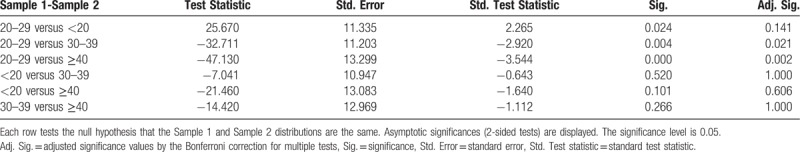
Pairwise Comparisons of AGE groups for blood glucose.
No statistical difference was found (P = .392) between APTT median values in the different age groups. Six (5.82%) patients (4 [3.88%] <20 years; 2 [1.94%] >40 years) had abnormally low APTT levels (21.6, 22.4, 23.4, 23.5, 23.5; 19.8 seconds), which showed the tendency towards hypercoagulability.
A statistical difference was found (P < .001) between fibrinogen median values in the four different age groups. Eight patients (n = 8, 7.76%) (2 [1.94%] <20 years old, 2 [1.94%] 20 to 29 years old, 3 [2.91%] 30 to 39 years old, and 1 [0.97%] >40 years old) had elevated fibrinogen levels (411.1; 422.2; 484.4; 414.7; 456; 484.4; 484.4; 435.3 mg/dL), which showed the tendency towards hypercoagulability. In the 30 to 39 and 20 to 29 age groups, distribution was strongly asymmetrical. The median value for fibrinogen was significantly different in pregnant patients <20 years of age (348.9 mg/dL) (IQR 21.7) compared with pregnant patients >20 years of age (359.1 mg/dL) (IQR 29.88).
There was no statistical difference (P = .757) between prothrombin time values in the different age groups. Six patients (n = 6, 5.82%) (2 [1.94%] <20 years old, 1 [0.97%] 20 to 29 years old, 2 [1.94%] 30 to 39 years old, and 1 [0.97%] >40 years old) had elevated prothrombin time (15.2; 17.9; 15.5; 15.1; 16.1; 15.7 seconds). The median value for prothrombin time was close in pregnant patients <20 years of age (12.9 seconds) (IQR 1.6) and pregnant patients >20 years of age (13.1 seconds) (IQR 1.9).
No statistical difference (P = .496) was reported between INR values in the different age groups. One patient (n = 1, 0.97%) had decreased INR (0.78) and another patient (n = 1, 0.97%) had elevated INR (1.42), both were <20 years of age. The median value for INR was close in healthy pregnant patients <20 years of age (1.02) compared with that in healthy pregnant patients >20 years of age (1.01).
No statistical difference (P = .54) occurred between prothrombin activity values in the different age groups. Nine patients (n = 9, 8.73%) (5 [4.85%] <20 years old, 1 [0.97%] between 30 and 39 years old, and 3 [2.91%] >40 years old) had abnormally elevated values for prothrombin activity (123.9–144.7%). One patient (n = 1, 0.97%) <20 years old had a decreased value of prothrombin activity (56.3%). The median value for prothrombin activity was close in healthy pregnant patients <20 years of age (97.1%) (IQR 20.5) compared with healthy pregnant patients >20 years of age (97.5%) (IQR 24.1).
A statistical difference (P = .002) did occur between blood glucose values in the different age groups. Four patients (n = 4, 1.92%) had elevated blood glucose levels. Of the 4, 2 (0.96%) were <20 years old, 1 (0.48%, 209.6 mg/dL) was 30 to 39 years old, and 1 (0.48%, 117.7 mg/dL) was >40 years old. Three (1.44%) patients had decreased blood glucose (1 [0.48%, 60.4 mg/dL] was <20 years old, and 2 [0.96%; 64; 63.7 mg/dL] were 20–29 years old). Blood glucose values were significantly lower in the 20 to 29 age group compared with the other age groups, but the values were still within normal limits (Table 1). This showed a tendency towards hypoglycemia in patients 20 to 29 years of age. The median value for blood glucose was very close in healthy pregnant patients <20 years of age (81 mg/dL) (IQR 18) compared with healthy pregnant patients >20 years of age (81.2 mg/dL) (IQR 14.7).
Neither a significant increase in coagulation test values nor a significant increase in blood glucose values was found in these healthy pregnant patients who were able to deliver vaginally.
4. Discussion
The World Health Organization considers persons less than 20 years of age as teenagers. Because they are the youngest, they are assumed to be the healthiest and to adapt better to different situations, including pregnancy, than the other age groups. We studied only healthy pregnant patients who were able to deliver vaginally. This study showed that even if there was variability between blood values from one age group to another, the median values for coagulation tests and blood glucose were very close in the healthy teenage pregnant patients compared with the average values of all the healthy adult pregnant patients. With very few exceptions, the values for coagulation tests and blood glucose were within normal limits in all age groups of healthy pregnant patients.
This study included 208 Caucasian patients. It was not intentional to exclude other populations. In Romania, the vast majority of the population is Caucasian, with very few persons of other races/ethnicities. From 2013 to 2017, only one person of Asian origin delivered a baby in our hospital, and it was by cesarean delivery. Therefore, this study may not be generalizable to other populations because of lack of diversity and because of the small number of subjects in some age groups (over 20 years).
Pregnancy induces a hypercoagulability risk. But not all pregnant patients experience hypercoagulability. Cui[2] reported reference intervals for APTT in the third trimester of pregnancy of healthy Han patients 25.6 to 34.9 seconds, decreasing from the first to the third trimester of pregnancy. We reported confidence intervals for APTT of 30.23 to 32.60 seconds for healthy pregnant patients less than 20 years of age and of 30.81 to 33.14 seconds for healthy pregnant patients over 20 years of age. These values are all within normal limits even for patients who are not pregnant. These pregnant patients are healthy. Pregnancy did not alter their blood values.
When APTT values are modified, they accompany other diseases. Gao[3] reported APTT and prothrombin time as potential factors influencing adverse maternal outcomes in acute fatty liver during pregnancy, and APTT and frequency of fetal distress were significantly higher in pregnancies with dead fetuses than in those where viable fetuses.
Cui[2] also reported reference intervals for fibrinogen in the third trimester of pregnancy of healthy Han patients 2.79 to 5.91 g/L (279–591 mg/dL), increasing from the first to the third trimester of pregnancy.
We reported confidence intervals for fibrinogen of 346.43 to 357.23 mg/dL for healthy pregnant patients less than 20 years of age and 356.46 to 379.33 mg/dL for healthy pregnant patients over 20 years of age. These values are all within normal limits even for patients who are not pregnant. These pregnant patients were healthy. Pregnancy did not alter their blood values.
Jin[4] reported significant increases in fibrinogen, D-dimer reference intervals, and clear decreases in platelet counts and thrombin time reference intervals. We did not find increases in fibrinogen values in healthy pregnant patients before vaginal delivery.
Fibrinogen was reported as a biomarker suggestive of preeclampsia in the third trimester of pregnancy, with a potential cutoff value of 2.87 g/L (287 mg/dL) for screening preeclampsia.[5] Our patients had a median value of fibrinogen of 348.9 mg/dL for healthy pregnant patients less than 20 years of age, and 359.1 mg/dL for healthy pregnant patients over 20 years of age, higher than the 287 mg/dL cutoff, and they all delivered vaginally. No cesarean delivery was required, and no preeclampsia was found, which means that fibrinogen alone is not enough to be a biomarker suggestive for preeclampsia in pregnant patients.
Joly[6] showed that although fibrinogen and prothrombin fragments 1 and 2 increased significantly throughout pregnancy, there was no difference between high-risk venous thrombosis pregnancies and normal pregnancies. We did not find a significant increase in fibrinogen values in healthy pregnant patients before vaginal delivery. Joly also reported an increase in thrombin generation, but no correlation between thrombin generation parameters and coagulation activation markers.
Romagnuolo[7] found significantly higher fibrinogen, F VIII, and plasminogen activator inhibitor 1 levels, and thrombin generation, in patients with recurrent pregnancy losses, with respect to those observed in patients with uneventful pregnancies. No fetal loss occurred in our study subjects.
Cui[2] also reported reference intervals for prothrombin time in the third trimester of pregnancy of healthy Han patients 8.6 to 12.4 seconds, and a decrease from the first to the third trimester of pregnancy. We reported confidence intervals for prothrombin time of 12.60 to 13.26 seconds for healthy pregnant patients less than 20 years of age and of 12.81 to 13.52 seconds for healthy pregnant patients over 20 years of age. These values are all within normal limits even for patients who are not pregnant. These pregnant patients are healthy. Pregnancy did not alter their blood values.
It has been demonstrated that thrombin (resulting from prothrombin) generation increased during pregnancy, starting with the very early stages of the first trimester of normal pregnancy.[8] We did not determine thrombin values, but prothrombin time was within normal limits in healthy pregnant patients.
Ratsch[9] reported that even bone marrow function was influenced by pregnancy: the immature platelet fraction, as a percentage of all thrombocytes, had the highest levels near term, whereas thrombocyte counts decreased slowly during this period. Kumar[10] demonstrated that total protein, prealbumin, folate, and triceps skin fold could significantly predict prothrombin time in hepatitis E infection in pregnancy. Our patients were healthy.
He[11] proved that hyperglycemia in pregnancy induced dysfunctional pulmonary cell apoptosis and proliferation, and aberrant structure of the alveolar wall in mice. Nakano[12] demonstrated how maternal hyperglycemia was associated with congenital heart disease: high glucose suppressed cardiac maturation. Our patients (except for 4, 1.92%) did not have hyperglycemia. And all of them were able to deliver vaginally.
Severe liver disease that is associated with elevated prothrombin activity and elevated fasting blood glucose has been described in pregnancy.[13] We found no healthy pregnant patient with concomitant elevated prothrombin activity and elevated blood glucose; though we did find 9 patients (8.73%) with elevated prothrombin activity, and 4 (1.92%) with elevated blood glucose.
A significant relationship between platelet indices and gestational diabetes mellitus (GDM) has been found.[14] We did not study platelet indices. In our hospital, patients with gestational diabetes had a cesarean delivery.
Patients with histories of GDM had the same levels of fibrinogen and tissue-plasminogen activator as patients without GDM had.[15] Our study group did not include patients with GDM.
In nondiabetic patients, with a history of parental type 2 diabetes, fibrinogen level was associated with previous GDM.[16] We did not study the diabetic or nondiabetic status of the pregnant patient's parents.
Blood samples from the umbilical cord in placentas from patients with GDM showed elevated glucose and fibrinogen levels.[17] We reported two patients 26 and 24 years old who had increased fibrinogen levels (484.4 and 414.7 mg/dL) associated with decreased blood glucose levels (64 and 63.73 mg/dL). No patient had elevated glucose and fibrinogen levels, although 8 (7.76%) patients had elevated fibrinogen levels and 4 (1.92%) others had elevated blood glucose levels.
A significant difference in fibrinogen levels was noticed between pregnant patients with normal glucose tolerance and pregnant patients with impaired glucose tolerance or GDM.[18] We did not evaluate the glucose tolerance in these healthy pregnant patients who were about to deliver.
Overweight pregnant patients had higher glucose concentrations and higher fibrinogen and prothrombin levels compared with healthy pregnant patients.[19] We only studied healthy pregnant patients.
But none of these studies focused on very young teenage pregnant patients. Because maternal age has increased dramatically lately, especially due to assisted reproductive technologies, corresponding blood values are calculated for an average pregnancy age of late 20s or even early 30s. Moreover, the increase in the incidence of overweight worldwide has resulted in an increase in reported blood values. To our knowledge, neither coagulation test values nor blood glucose values in healthy teenage pregnant patients have been reported separately.
5. Conclusions
This article shows that, even if variability existed between blood values from one age group to another, the median values for coagulation tests and blood glucose were very close in the healthy teenage group of pregnant patients compared with the median values of the healthy adult pregnant patients, just before vaginal delivery. With very few exceptions, the values for coagulation tests and blood glucose were within normal limits, in all age groups of healthy pregnant patients.
The median values for coagulation tests (APTT, prothrombin time, INR, prothrombin activity) and blood glucose were very close in healthy pregnant teenagers compared with healthy adult pregnant patients. Only the median value for fibrinogen was significantly different in healthy pregnant teenagers (348.9 mg/dL) (IQR 21.7) compared with healthy adult pregnant patients (359.1 mg/dL) (IQR 29.88).
There also was a significantly lower blood glucose value in the age group 20 to 29 compared with that in the other age groups, but it was still within normal limits.
Neither a significant increase in coagulation test values nor a significant increase in blood glucose values was found. Further studies in larger groups of healthy pregnant patients are required to establish the normal value for coagulation tests and blood glucose in every age group.
Acknowledgments
Many thanks to Mr. Lucian Boiculese, Associate Professor, Department of Statistics, “Grigore T. Popa” University of Medicine and Pharmacy Iasi, for contributing to this study.
Author contributions
Conceptualization: Roxana Covali, Demetra Socolov.
Data curation: Roxana Covali.
Methodology: Razvan Socolov.
Supervision: Demetra Socolov, Razvan Socolov.
Validation: Demetra Socolov, Razvan Socolov.
Writing – original draft: Roxana Covali.
Writing – review & editing: Roxana Covali.
Footnotes
Abbreviations: APTT = activated partial prothrombin time, GDM = gestational diabetes mellitus, INR = International Normalized Ratio, IQR= interquartile range.
The authors contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Sebghati M, Chandraharan E. An update on the risk factors for and management of obstetric haemorrhage. Womens Health (Lond) 2017;13:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cui C, Yang S, Zhang J, et al. Trimester-specific coagulation and anticoagulation reference intervals for healthy pregnancy. Thromb Res 2017;156:82–6. [DOI] [PubMed] [Google Scholar]
- [3].Gao Q, Qu X, Chen X, et al. Outcomes and risk factors of patients with acute fatty liver of pregnancy: a multicentre retrospective study. Singapore Med J 2018;59:425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jin Y, Lu J, Jin H, et al. Reference intervals for biochemical, haemostatic and haematological parameters in healthy Chinese women during early and late pregnancy. Clin Chem Lab Med 2018;56:973–9. [DOI] [PubMed] [Google Scholar]
- [5].Chen Y, Lin L. Potential value of coagulation parameters for suggesting preeclampsia during the third trimester of pregnancy. Am J Med Sci 2017;354:39–43. [DOI] [PubMed] [Google Scholar]
- [6].Joly BS, Sudrie-Arnaud B, Barbay V, et al. Thrombin generation test as a marker for high risk venous thrombosis pregnancies. J Thromb Thrombolysis 2018;45:114–21. [DOI] [PubMed] [Google Scholar]
- [7].Romagnuolo I, Attanasio M, Cozzolino M, et al. Thrombin potential and traditional coagulation assay: are they useful in exploring recurrent pregnancy loss risk? Blood Coagul Fibrinolysis 2018;29:160–6. [DOI] [PubMed] [Google Scholar]
- [8].Bagot CN, Leishman E, Onyaodike CC, et al. Normal pregnancy is associated with an increase in thrombin generation from the very early stages of the first trimester. Thromb Res 2017;157:49–54. [DOI] [PubMed] [Google Scholar]
- [9].Ratsch U, Kaiser T, Stepan H, et al. Evaluation of bone marrow function with immature platelet fraction in normal pregnancy. Pregnancy Hypertens 2017;10:70–3. [DOI] [PubMed] [Google Scholar]
- [10].Kumar A, Sharma S, Kar P, et al. Impact of maternal nutrition in hepatitis E infection in pregnancy. Arch Gynecol Obstet 2017;296:885–95. [DOI] [PubMed] [Google Scholar]
- [11].He MY, Wang G, Han SS, et al. Negative impact of hyperglycaemia on mouse alveolar development. Cell Cycle 2018;17:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nakano H, Minami I, Braas D, et al. Glucose inhibits cardiac muscle maturation through nucleotide biosynthesis. Elife 2017;6: pii: e29330. doi: 10.7554/eLife.29330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wei X, Lü H, An Y, et al. Application of plasmapheresis combined with hemofiltration in the treatment of severe liver disease in middle and late pregnancy. Zhonghua Yi Xue Za Zhi 2015;95:3607–10. [PubMed] [Google Scholar]
- [14].Sahbaz A, Cicekler H, Aynioglu O, et al. Comparison of the predictive value of plateletcrit with various other blood parameters in gestational diabetes development. J Obstet Gynaecol 2016;36:589–93. [DOI] [PubMed] [Google Scholar]
- [15].Kim C, Christophi CA, Goldberg RB, et al. Adiponectin, C-reactive protein, fibrinogen and tissue plasminogen activator antigen levels among glucose-intolerant women with and without histories of gestational diabetes. Diabet Med 2016;33:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sokup A, Ruszkowska-Ciastek B, Walentowicz-Sadlecka M, et al. Gestational diabetes mellitus worsens the profile of cardiometabolic risk markers and decreases indexes of beta-cell function independently of insulin resistance in nondiabetic women with a parental history of type 2 diabetes. J Diabets Res 2014;2014:743495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lopez Morales CM, Brito Zurita OR, Gonzalez Heredia R, et al. Placental atherosclerosis and markers of endothelial disfunction in infants born to mothers with gestational diabetes. Med Clin (Barc) 2016;147:95–100. [DOI] [PubMed] [Google Scholar]
- [18].Özier S, Engin-Üstün Y, Uzunlar Ö, et al. Inflammation and glycemic tolerance status in pregnancy: the role of maternal adiposity. Gynecol Obstet Invest 2014;78:53–8. [DOI] [PubMed] [Google Scholar]
- [19].Loukidi B, Merzouk H, Merzouk SA, et al. Thrombosis factors and oxidant/antioxidant markers in obese and hypertensive women during pregnancy. Blood Press 2015;24:242–9. [DOI] [PubMed] [Google Scholar]


