Abstract
Our study compared the Ivor-Lewis and Sweet procedures used for treating middle and lower thoracic esophageal squamous cell carcinoma and assessed the associated perioperative complications and long-term survival rates of the patients.
This retrospective study involved 624 middle and lower thoracic esophageal squamous carcinoma patients who received either Ivor-Lewis (n = 325) or Sweet (n = 299) procedures at our hospital. Further, the perioperative conditions and long-term survival rates were analyzed for both groups.
Relative to the Sweet group, the Ivor-Lewis group showed lower volume of drainage within 24 hours after operation (400 (300–500) ml vs 550 (400–658) ml, P = .031). Although we found no significant differences in major postoperative complications between the groups (72 (22.2) vs 65 (21.7), P = .90), there were significant differences observed in minor postoperative complications between the Ivor-Lewis and Sweet groups (59 (18.2) vs 32 (10.7), P = .008). Perioperative death rates remained comparable for the 2 groups (2 (0.6) vs 2 (0.7), P > .99). Further, comparison of the 2 groups revealed that the Ivor-Lewis group had increased number of dissected lymph nodes, (20 (4–42) vs 16 (3–31), P < .001), especially in the upper mediastinum (4 (0–5) vs 2 (0–2), P < .001). The long-term survival rates did not differ significantly between the 2 groups (Kaplan-Meier method, P = .95; Cox regression, P = .20).
These findings suggest that perioperative complications and long-term survival rates were comparable for both patients groups. Patients receiving the Sweet procedure had reduced minor postoperative complications compared to those receiving the Ivor-Lewis procedure. Due to improved quality of lymph node dissection in the upper mediastinum, the Ivor-Lewis procedure may have advantages over the Sweet procedure for treating patients with esophageal cancer with enlarged lymph nodes in the upper mediastinum.
Keywords: Complications, Esophageal cancer, Ivor-Lewis, Long-term survival rate, Sweet
1. Introduction
Esophageal cancer is a common type of malignant disease around the world,[1,2] and it is one of the leading causes of cancer-related deaths worldwide.[3] Currently, about half of the world's esophageal cancer cases occur in China,[4] and most of these patients with esophageal cancer suffer from esophageal squamous cell carcinoma.[5] Patients with middle and lower thoracic esophageal cancers account for the majority of patients with esophageal squamous cell cancer in China.[6]
The Ivor-Lewis esophagectomy (Ivor-Lewis) and left transthoracic approach with anastomosis in the chest (Sweet) are the 2 main open approaches for treating patients with middle and lower esophageal cancer. However, there is still some debate regarding the safety and therapeutic efficacy of these procedures.[7–10] A randomized trial conducted in 2015 showed that both the Ivor-Lewis and Sweet procedures were safe for patients with esophageal cancer, while the long-term survival rate was not analyzed in this study.[7] A recent study demonstrated that the Ivor-Lewis procedure was better than the Sweet procedure in terms of long-term survival outcomes.[8] Some other studies have shown that both procedures are equally effective, resulting in improved long-term survival.[9–10]
Since 2005, the Ivor-Lewis and Sweet procedures have been widely used for the treatment of middle and lower thoracic esophageal squamous cell cancer patients admitted to our department. In this report, we compared the perioperative complications and long-term survival rates of patients with middle and lower thoracic esophageal cancer following the Ivor-Lewis and the Sweet procedures, recorded between January 2010 and December 2015.
2. Methods
2.1. Subjects
We conducted a single-center retrospective study between January 2010 and December 2015. Patients were included in this study if they met the following criteria: First, free of other malignancies. Second, no history of gastric or esophageal surgery. Third, without major organ dysfunction. Fourth, with a Karnofsky Index score of at least 90. Five, no previous preoperative neoadjuvant therapy. Sixth, without distant metastasis. A total of 624 patients with resectable middle and lower thoracic esophageal squamous cell cancer who had undergone the Ivor-Lewis (n = 325) or Sweet (n = 299) procedures in our department were involved in this study. The operations were performed by different skilled surgeons, with each surgeon having performed more than 100 esophagectomies. The surgical procedure chosen was based on the training experience of the attending surgeon. Some of the surgeons preferred to perform the Ivor-Lewis procedure while others had a preference for the Sweet procedure. Informed consent for the surgery was obtained from all patients before surgery. This study was approved by the Clinical Research Ethics Committee of the First People's Hospital of Changzhou. Because of the retrospective nature of the study, patient consent for inclusion was waived.
Detailed history-taking and physical examinations were conducted for each patient before the surgery. Additionally, we performed lung function test, echocardiography, arterial blood gas analysis, blood biochemistry, and coagulation test. The Child-Pugh score was used to evaluate liver function. Gastric endoscopy, gastrointestinal barium swallow, and computed tomography (CT) scans of the cervical, thorax, and upper abdomen were also performed for each patient before operation.
2.2. Surgical procedures
The Ivor-Lewis procedure was carried out as described previously.[6] Under general anesthesia by tracheal cannula, the supine position was adopted for the dissociation of the stomach and lymphadenectomy of the abdominal lymph nodes via a median incision in the upper abdomen. The patients were assigned to the left lateral position for dissociation of the thoracic esophagus to clear the way for access to the thoracic lymph nodes via a posterolateral incision in the 5th intercostal space. With the help of an anastomat, esophagogastric anastomosis was performed at the top of the thorax.
Patients who received the Sweet procedure were placed in the right lateral position. A posterolateral incision in the 6th intercostal space on the left side of the chest was made to dissociate the esophagus and clear the way to access the lymph nodes in the thoracic region. The diaphragm was opened for dissociation of the stomach to clear the way for access to the abdominal lymph nodes. Using an anastomat, anastomosis was performed at the top of the thorax.
2.3. Pathological examination
Routine pathology was performed to assess the infiltration depth and lymphatic metastasis. The 7th edition of the American Joint Committee on Cancer TNM classification for esophageal cancer was used for postoperative pathological staging.
2.4. Statistical analysis
The perioperative data of these patients were obtained from the electronic medical records system of our hospital. Until October 2016, the patients were followed up by re-diagnosis, telephone calls, and letters, and a database was established for further analysis. Continuous data were presented as medians and interquartile ranges, and discrete data were presented as numbers and percentages. Overall survival (OS) was considered as the primary end-point in this study. A commercially available statistical software package, SPSS 22.0 (SPSS, Inc, IL), was used for statistical analysis. T-test and Chi-squared test were used for analyzing numerical variables and categorical variables, respectively. The long-term survival rate was determined by the Kaplan-Meier and Cox regression methods. Statistical significance was defined as P value < .05.
3. Results
3.1. Patients’ characteristics and laboratory findings
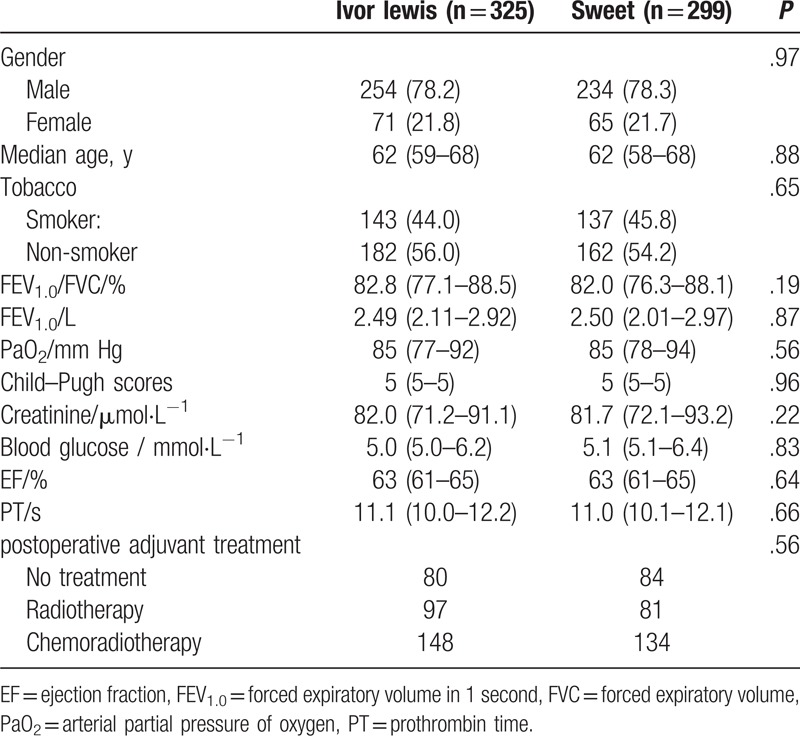
In this study, we included a total of 624 esophageal cancer patients who received the Ivor-Lewis (n = 325) or Sweet (n = 299) procedures in our department. The baseline levels of patient characteristics and laboratory findings are presented in Table 1. Before operation, the 2 patient groups did not differ significantly in gender (P = .97), median age (P = .88), tobacco use (P = .65), ratio of forced expiratory volume in one second (FEV1.0) to forced vital capacity (P = .19), FEV1.0 (P = .87), arterial partial pressure of oxygen (P = .56), Child-Pugh scores (P = .96), creatinine levels (P = .22), blood glucose levels (P = .83), ejection fraction (P = .64), prothrombin time (P = .66) and postoperative adjuvant treatment (P = .56).
Table 1.
Patient characteristics and laboratory findings.
3.2. Perioperative conditions
The perioperative conditions of the patients are listed in Table 2. The Ivor-Lewis and Sweet groups did not present significant differences in the following clinical outcomes: operative time (P = .48), hemorrhage during operation (P = .18), retaining time of thoracic drainage tube (P = .86), postoperative hospital stay (P = .11), anastomotic fistula (P = .27), deep venous thrombosis (P = .25), pulmonary infection (P = .25), heart failure (P = .89), chylothorax (P = .81), pneumothorax (P = .29), laryngeal recurrent nerve injury (P = .46), arrhythmia (P = .18), incision infection (P = .77), ICU stay (P = .41), blood transfusion (P = .77), cost of treatment (P = .33), and second operation (P = .29). Compared with the Sweet group, the Ivor-Lewis group showed lower volume of drainage within 24 hours after surgery (400 (300–500) ml vs 550 (400–658) ml, P = .031) and higher incidence of gastric retention (24 (7.4) vs 5 (1.7), P = .001).
Table 2.
Perioperative conditions of the patients.
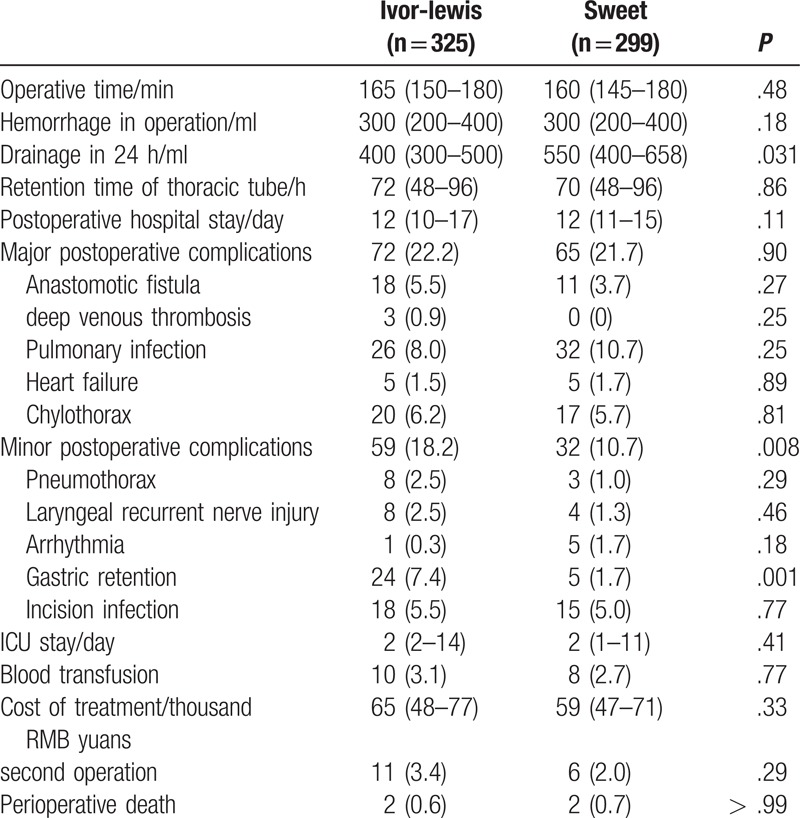
Postoperative complications were evaluated as previously described.[11] No significant difference was found in major postoperative complications between the Ivor-Lewis and Sweet groups (P = .90); however, the Ivor-Lewis group presented increased minor postoperative complications [59 (18.2) vs 32 (10.7), P = .008]. The incidence of recurrent nerve injury may be biased as laryngoscopy was not performed during the postoperative period. Perioperative deaths did not differ significantly between the Ivor-Lewis and Sweet groups (P > .99).
3.3. Postoperative pathological findings
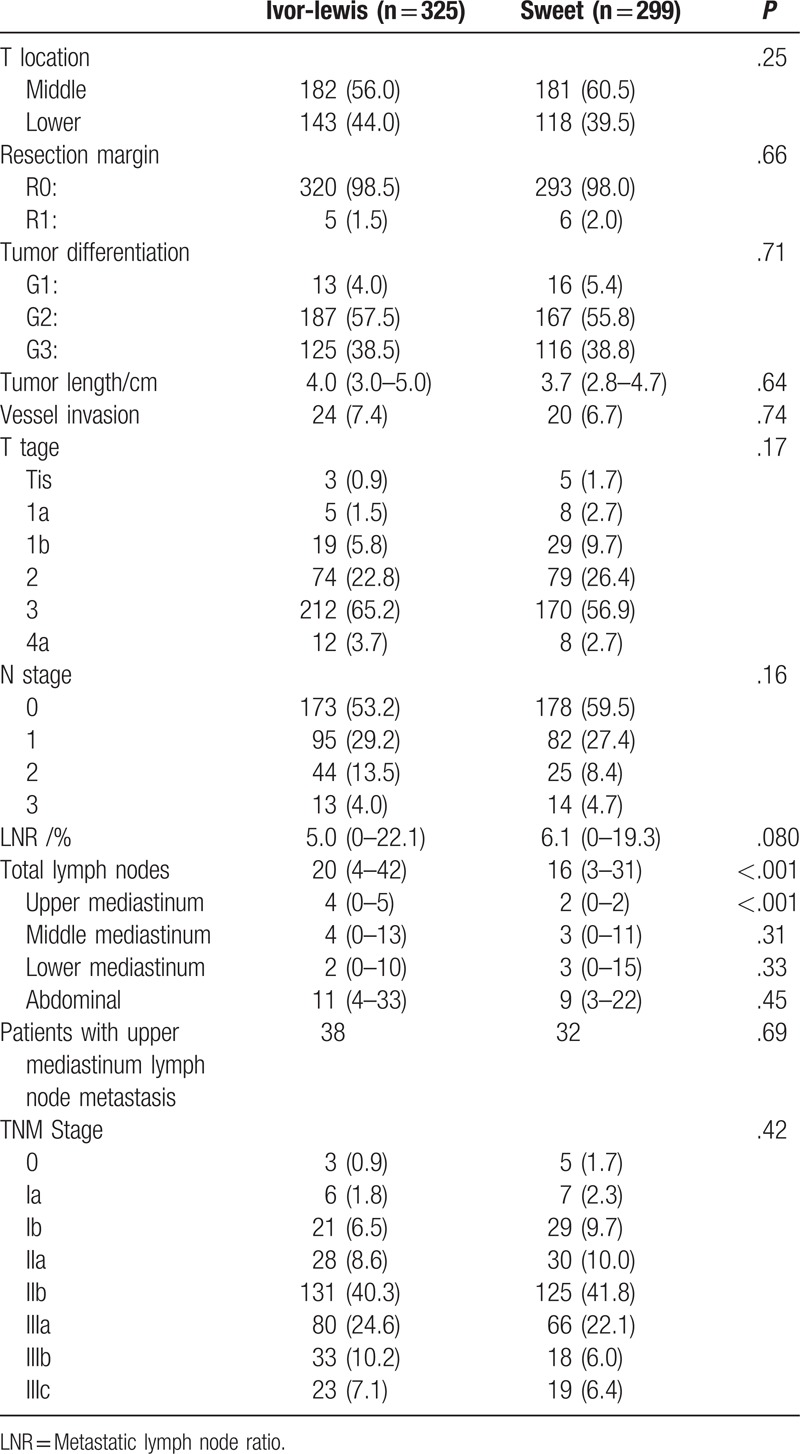
The postoperative pathological findings are described in Table 3. Between the two patient groups, we observed no significant differences in tumor location (P = .25), resection margin (P = .66), tumor differentiation (P = .71), tumor length (P = .64), vessel invasion (P = .74), T stage (P = .17), N stage (P = .16), metastatic lymph node ratio (P = .080), patients with upper mediastinum lymph node metastasis (P = .69) and TNM stage (P = .42). Relative to the Sweet group, the Ivor-Lewis group had increased number of dissected lymph nodes (20 (4–42) vs 16 (3–31), P < .001) and upper mediastinal lymph nodes (4 (0–5) vs 2 (0–2), P < .001). No significant differences were found in the number of dissected lymph nodes in the middle (P = .31), lower (P = .33) or abdominal areas (P = .45).
Table 3.
Postoperative pathological findings.
3.4. Long-term outcomes
All patients were followed up until their death or end of the study period (i.e., October 2016). The Kaplan-Meier method was used for the analysis of survival data (Fig. 1). The median follow-up duration of the patients was 27 months (15–51). The 5-year OS rates were 45.6 and 47.0% for patients in the Ivor-Lewis and Sweet groups (P = .95), respectively. Following the Ivor-Lewis and Sweet procedures, the median survival time was found to be 50 months for both groups (Fig. 1).
Figure 1.
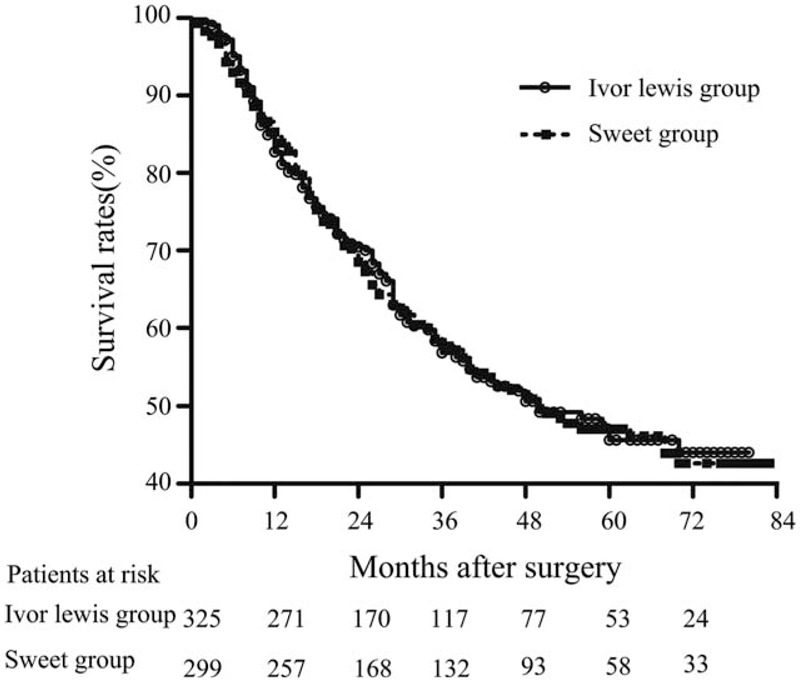
Overall survival (OS) curves for the Ivor-Lewis and Sweet patient groups. The 5-year OS rates for the Ivor-Lewis and Sweet groups were 45.6 and 47%, respectively. The long-term survival rates of the 2 patient groups did not differ significantly (P = .95). The median survival time for both groups was 50 months.
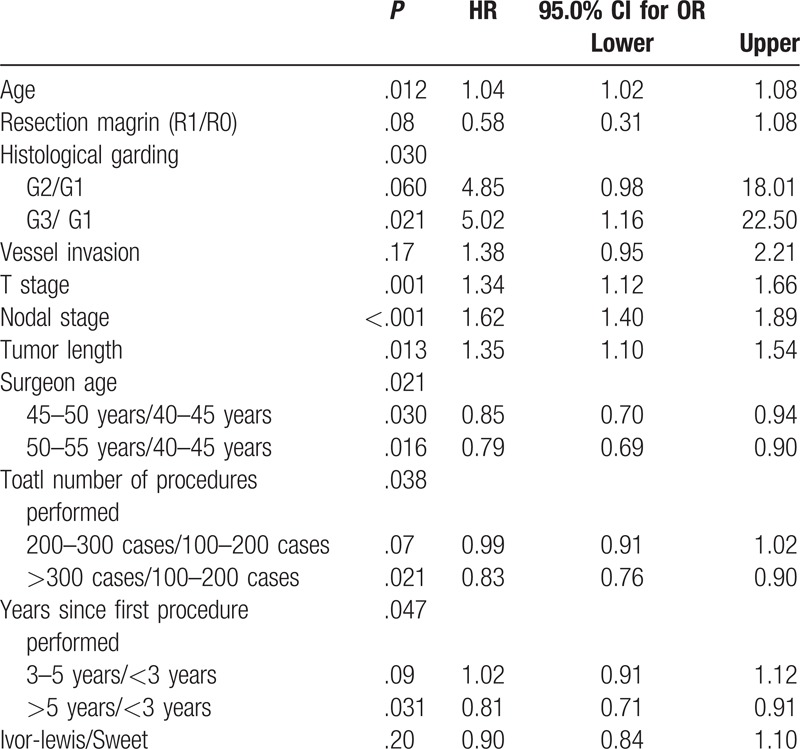
Cox regression analysis was performed to determine OS, and this was achieved by adjusting the following clinically relevant variables chosen in advance: age, resection margin, histological grading, vessel invasion, T stage, N stage, tumor length, surgeon's experience, and surgical procedure. The surgeon's experience was assessed by his age, total number of procedures performed, and years since first procedure performed. Multivariate Cox regression analysis showed that age (P = .012), histological grading (P = .030), T stage (P = .001), N stage (P < .001), tumor length (P = .013), surgeon's age (P = .021), total number of procedures performed (P = .038), and years since first procedure performed (P = .047) were independent prognostic factors for the patients with esophageal squamous cell carcinoma. These results suggested that the surgical method was not an independent prognostic factor for these patients (P = .20, Table 4).
Table 4.
Results of Cox regression model for prognosis of esophageal squamous cell carcinoma.
4. Discussion
Esophageal squamous cell carcinoma is associated with high morbidity and mortality, and it remains the 4th leading cause of cancer-related mortality in China.[4] To date, esophagectomy is regarded as the primary treatment option for esophageal squamous cell carcinoma patients. Since 2005, the Ivor-Lewis and Sweet procedures have been widely performed for treating patients in our department. This study compared the perioperative complications and long-term survival rates of 2 groups of patients who underwent either the Ivor-Lewis or Sweet procedure.
According to previous studies, patients who underwent the Sweet procedure showed shorter operative time than those who received the Ivor-Lewis procedure.[9–10] In the Ivor-Lewis procedure, the supine position was adopted for the operation of the abdomen followed by the adoption of the left lateral position for the operation of the thorax. It is critical that the body position is changed for patients receiving the Ivor-Lewis procedure, and the operation area in the thoracic and abdominal regions is prepared and draped separately. Changing positions is not needed during the Sweet procedure, and the surgical field can be prepared and draped simultaneously. In our study, the operative time did not differ between our patient groups. The Ivor-Lewis procedure has been widely used in our department since 2005. With the extensive use of the Ivor-Lewis procedure, the operative time can be reduced. As compared to the Sweet approach, better exposure of the thorax and abdomen surgical areas during Ivor-Lewis approach may help in saving operative time.
A comparison of the 2 treatment procedures demonstrated that the Ivor-Lewis option had the advantage of reduced drainage within 24 hours after operation. This approach was also more convenient in improving the visualization of the surgical field, and good exposure to the surgical field helped stop the bleeding. In contrast, the posterolateral incision could not fully expose the surgical field during the Sweet procedure; hence, it was more difficult to manage the surgery and bleeding process.
It has previously been reported that the incidence of gastric retention is higher in patients who underwent the Ivor-Lewis procedure than the Sweet group patients.[12] Consistently, our Ivor-Lewis group showed increased gastric retention than the Sweet group. The stomach is fixed to the left thorax in the Sweet procedure, but to the right thorax in the Ivor-Lewis procedure.[9] Fixing the stomach to the right thorax induces an inappropriate angle between the pyloric region and stomach;[6] this may explain for the delay in gastric emptying.
Esophagectomy, a complex procedure with a high risk for complications, is often performed to treat patients with esophageal cancer.[13] In this study, postoperative complications were evaluated as described previously.[10] There were no differences found in the major postoperative complications between the patient groups included in our study. While the Ivor-Lewis group showed increased minor postoperative complications, the Sweet approach appeared to have advantages of reducing the minor postoperative complications.
Previous studies have suggested that esophagectomy is associated with a high risk of mortality ranging from 2 to 3%.[14,15] In our study, there were 4 patients who died perioperatively, and the perioperative mortality was about 0.7%. The causes of death of these patients were tracheoesophageal fistula formation (1/4), aortoesophageal fistula formation (1/4), pulmonary embolism (1/4), and pneumonia (1/4). Esophageal fistula accounted for half of the perioperative deaths. There were 29 cases with symptoms of anastomotic leakage, and the incidence of anastomotic leaks was about 5% in our study. Of the four deaths, 2 cases were associated with anastomotic fistula; one case resulted from aortoesophageal fistula, and another from tracheoesophageal fistula. Development of an aortoesophageal fistula is a fatal condition that can lead to life-threatening hemorrhage and has limited treatment options available. It has been proposed that thoracic endovascular aortic repair may be a method for treating aortoesophageal fistula.[16] Tracheoesophageal fistula is also associated with severe complications and increased mortality.[17] This condition leads to the settlement of digestive juices into the lungs, causing severe lung infections.[18] Emergency removal of the stomach by esophageal exclusion may have a beneficial palliative effect in patients with tracheoesophageal fistula;[19] however, the effectiveness of this approach remains debatable. A previous study reported that esophageal exclusion was ineffective in treating patients with established sepsis.[20] Airway stenting is another way to close the fistula between the esophagus and airway,[21] especially for patients who are intolerant to a second operation.
In our study, more dissected lymph nodes were found in the Ivor-Lewis group than the Sweet group (20(4–42) vs 16(3–31), P < .001), especially in the upper mediastinal region (4(0–5) vs 2(0–2), P < .001). A previous meta-analysis of esophagectomy also showed that the Ivor-Lewis procedure could dissect more lymph nodes than the Sweet procedure.[14] Another study showed that more superior mediastinal lymph nodes were removed during Ivor-Lewis procedure than during the Sweet procedure with a statistically significant difference.[7] During the Sweet procedure, posterolateral incision in the 6th intercostal space on the left side of the chest could not fully expose the abdominal and thoracic surgical fields for the lymph node dissection. The aortic arch may also have disturbed the lymph node dissections in the thorax. This might have limited the lymph node dissection during the Sweet procedure. In contrast, good exposure of the thorax and abdomen regions facilitated lymph node resection during the Ivor-Lewis procedure. Thus, the Ivor-Lewis procedure may be a better choice for patients with esophageal cancer with enlarged lymph nodes in the upper mediastinum.
The Kaplan-Meier method was used to evaluate the long-term survival of patients involved in our study. In both patient groups, no significant differences were observed in the long-term survival rates (P = .95). In addition, Cox regression analyses revealed that the surgical method was not an independent prognostic factor for survival of these patients (P = .20). These results indicated that the Sweet procedure was inferior to the Ivor-Lewis procedure in terms of lymph node dissection, while both procedures resulted in comparable long-term survival outcomes. However, patients with middle or lower third esophageal cancer might not benefit from the improved upper mediastinal lymph node dissection with the Ivor-Lewis procedure.[22]
5. Limitations
This was a retrospective case control study performed in a single-center, and many of the participating patients were diagnosed with advanced stages of cancer. The disease-free survival of these patients was not analyzed in this study. Thus, there is a need for conducting randomized controlled multi-center clinical trials in the future.
6. Conclusions
Our findings suggest that following either the Sweet or Ivor-Lewis procedure, perioperative complications and long-term survival rates remained comparable in patients with middle and lower thoracic esophageal squamous cell cancer. Given that fewer minor postoperative complications were observed in the Sweet group, this approach was favored over the Ivor-Lewis procedure. Due to extensive lymph node dissection in the upper mediastinum, the Ivor-Lewis procedure has advantages over the Sweet procedure for esophageal cancer patients with enlarged lymph nodes in the upper mediastinum.
Author contributions
Conceptualization: Jun Wang, Ning Wei, Yiming Lu, Xiaoying Zhang.
Data curation: Jun Wang, Yiming Lu, Ning Wei, Xiaoying Zhang.
Formal analysis: Jun Wang, Ning Wei, Nanqing Jiang, Yiming Lu.
Funding acquisition: Xiaoying Zhang.
Investigation: Jun Wang, Ning Wei, Nanqing Jiang, Yimin Lu
Methodology: Yiming Lu, Xiaoying Zhang, Jun Wang.
Project administration: Nanqing Jiang, Xiaoying Zhang.
Resources: Xiaoying Zhang
Supervision: Nanqing Jiang and Xiaoying Zhang
Validation: Xiaoying Zhang.
Writing – original draft: Jun Wang.
Writing – review & editing: Ning Wei, Nanqing Jiang, Yiming Lu, Xiaoying Zhang, Jun Wang
Footnotes
Abbreviations: FEV1.0 = forced expiratory volume in 1 second, OS = overall survival.
The authors have no conflicts of interest to disclose.
References
- [1].Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11–30. [DOI] [PubMed] [Google Scholar]
- [2].Abbas G, Krasna M. Overview of esophageal cancer. Ann Cardiothorac Surg 2017;6:131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- [4].Chen W, Zheng R, Zeng H, et al. Annual report on status of cancer in China, 2011. Chin J Cancer Res 2015;27:2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang J, Jiang NQ, Jiang B, et al. Mediastinoscopy-assisted oesophagectomy in T1 oesophageal cancer patients with serious comorbidities: a 5-year long-term follow-up. Interact Cardiovasc Thorac Surg 2015;20:477–81. [DOI] [PubMed] [Google Scholar]
- [6].Wang J, Wei N, Lu Y, et al. Mediastinoscopy-assisted esophagectomy for T2 middle and lower thoracic esophageal squamous cell carcinoma patients. World J Surg Oncol 2018;16:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li B, Xiang J, Zhang Y, et al. Comparison of Ivor-Lewis vs Sweet esophagectomy for esophageal squamous cell carcinoma: a randomized clinical trial. JAMA Surg 2015;150:292–8. [DOI] [PubMed] [Google Scholar]
- [8].Li B, Hu H, Zhang Y, et al. Extended right thoracic approach compared with limited left thoracic approach for patients with middle and lower esophageal squamous cell carcinoma: three-year survival of a prospective, randomized, open-label trial. Ann Surg 2018;267:826–32. [DOI] [PubMed] [Google Scholar]
- [9].Ma J, Zhan C, Wang L, et al. The sweet approach is still worthwhile in modern esophagectomy. Ann Thorac Surg 2014;97:1728–33. [DOI] [PubMed] [Google Scholar]
- [10].Mu JW, Gao SG, Xue Q, et al. The impact of operative approaches on outcomes of middle and lower third esophageal squamous cell carcinoma. J Thorac Dis 2016;8:3588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lerut T, Moons J, Coosemans W, et al. Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: role of systematic grading of complications using the modified Clavien classification. Ann Surg 2009;250:798–807. [DOI] [PubMed] [Google Scholar]
- [12].Yu Y, Wang Z, Liu XY, et al. Therapeutic efficacy comparison of two surgical procedures to treat middle thoracic esophageal carcinoma. World J Surg 2010;34:272–6. [DOI] [PubMed] [Google Scholar]
- [13].Wightman SC, Posner MC, Patti MG, et al. Extremes of body mass index and postoperative complications after esophagectomy. Dis Esophagus 2017;30:1–6. [DOI] [PubMed] [Google Scholar]
- [14].Zhang H, Wang J, Wang W, et al. A meta-analysis of esophagectomy: the comparative study of Ivor-Lewis operation and Sweet operation. Zhonghua Wei Chang Wai Ke Za Zhi 2014;17:892–7. [PubMed] [Google Scholar]
- [15].Raymond DP, Seder CW, Wright CD, et al. Predictors of major morbidity or mortality after resection for esophageal cancer: a society of thoracic surgeons general thoracic surgery database risk adjustment model. Ann Thorac Surg 2016;102:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ren W, He Y, Wang X, et al. Secondary aorto-esophageal fistula after esophagectomy treated with endovascular treatment: A case report. Medicine (Baltimore) 2017;96:e6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lindner K, Lübbe L, Müller AK, et al. Potential risk factors and outcomes of fistulas between the upper intestinal tract and the airway following Ivor-Lewis esophagectomy. Dis Esophagus 2017;30:1–8. [DOI] [PubMed] [Google Scholar]
- [18].Wu WC, Wang Y, Wang X, et al. Clinical analysis of acute lung injury after esophagectomy. J Cancer Res Ther 2014;10Suppl:314–8. [DOI] [PubMed] [Google Scholar]
- [19].Yu YK, Zhang RX, Sun HB, et al. Tracheoesophageal fistula treated with esophageal exclusion and cervical esophago-gastric anastomosis via a retrosternal approach. Chin Med J (Engl) 2013;126:1800. [PubMed] [Google Scholar]
- [20].Hause DW, Kagan AR, Fleischman E, et al. Tracheo-esophageal fistula complicating carcinoma of the esophagus. Am Surg 1992;58:441–2. [PubMed] [Google Scholar]
- [21].Chung FT, Lin HC, Chou CL, et al. Airway ultraflex stenting in esophageal cancer with esophagorespiratory fistula. Am J Med Sci 2012;344:105–9. [DOI] [PubMed] [Google Scholar]
- [22].van der Schaaf M, Johar A, Wijnhoven B, et al. Extent of lymph node removal during esophageal cancer surgery and survival. J Natl Cancer Inst 2015;107:djv043. [DOI] [PubMed] [Google Scholar]


