Abstract
Rationale:
Intraductal tubulopapillary neoplasm (ITPN) is a rare type of pancreatic epithelial neoplasm. We report 2 cases of ITPN and detail the imaging findings.
Patient concerns:
The 1st case was a 36-year-old woman who complained of jaundice, yellow urine and diarrhea. She accepted ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) examination before surgery, which all revealed a mass in the pancreatic head. The 2nd case was a 62-year-old woman who was admitted to our hospital for the treatment of a pancreatic tumor. The MRI showed a mass filled the mian pancreatic duct in the head and neck.
Diagnosis:
The ITPN is an intraductal, grossly visible, tubule-forming epithelial neoplasm with high-grade dysplasia and ductal differentiation without overt mucin production.
Interventions:
The 1st patient received percutaneous transhepatic cholangial drainage procedure, endoscopic ultrasound guided fine needle aspiration, pancreatoduodenectomy, cholecystectomy, and lymphadenectomy successively. The 2nd patient received pancreaticoduodenectomy, cholecystectomy, and partial gastrectomy.
Outcomes:
Two months after surgery, the follow-up MRI revealed hepatic metastasis of the 1st patient. She is still alive now. The 2nd patient was lost to follow-up.
Lessons:
The ITPN is a rare pancreatic neoplasm and its clinical symptoms are atypical. It is difficult to make accurate diagnosis of ITPN before surgery even though various imaging modalities are used in combination. When a solid mass growing in the lumen of the pancreatic duct, ITPN should be taken into consideration.
Keywords: computed tomography, intraductal tubulopapillary neoplasm, magnetic resonance imaging, pancreas
1. Introduction
Intraductal tubulopapillary neoplasm (ITPN), a rare epithelial neoplasm of the pancreas, is defined as “an intraductal, grossly visible, tubule-forming epithelial neoplasm with high-grade dysplasia and ductal differentiation without overt mucin production”.[1] It was 1st designated as “intraductal tubulopapillary neoplasms” in 2009,[2] and formally introduced in 2010 WHO classification as a distinct entity which was included in the subgroup of premalignant epithelial tumors of the pancreas.[1] The ITPNs arise from the pancreatic ductal epithelium and usually grow within the lumen of pancreatic duct. If a lesion has a component of invasive carcinoma, it is referred to as an ITPN with an associated invasive carcinoma.[2] In this condition, the tumor can infiltrate the pancreatic parenchyma. According to Yamaguchi's study, ITPNs only account for 0.9% of all pancreatic exocrine tumors and 3% of all pancreatic intraductal neoplasms.[2] Data on this entity is still limited in the published literature. Here we report 2 cases of ITPN with an associated invasive carcinoma, discuss the pitfalls and challenges in the imaging diagnosis of ITPN and review the literature.
2. Case report
2.1. Case 1
A 36-year-old woman with jaundice, yellow urine and diarrhea for 1 week presented to a local hospital in Nov, 2017. Abdominal computed tomography (CT) revealed a mass in the head of the pancreas. She was therefore admitted to our hospital for further examination and treatment. Laboratory analysis showed increased levels of alanine aminotransferase (267 U/L, normal <32 U/L), aspartate aminotransferase (182 U/L, normal <32 U/L), total bilirubin (125.33 μmol/L; normal range, 5.10–28.00 μmol/l), conjugated bilirubin (109.25 μmol/l; normal range, 0.00∼10.00 μmol/L), total bile acid (36.0 μmol/L; normal range, <10.0 umol/L), γ-glutamyl transpetidase (169 U/L; normal range, 7–32 U/L), glutamate dehydrogenase (78 g/L; normal range, < 6 g/L). Levels of tumor markers, including carcinoembryonic antigen (CEA) and carbohydrate antigen (CA)19-9, were within normal ranges. Endoscopic ultrasonography revealed an ill-defined hypoechoic mass in the head of the pancreas, measuring 3.3×3.1 cm. Unenhanced CT scan showed a low attenuating mass in the head of the pancreas. The mass was relatively hypovascular compared with the normal pancreatic parenchyma on contrast-enhanced CT, sized 4.0×2.5 cm, and the part in the main pancreatic duct showed lower-attenuation, sized 4.0×1.1 cm. The atrophy of the pancreatic parenchyma and dilation of the main pancreatic duct in the body and tail was observed (Fig. 1). Magnetic resonance imaging (MRI) demonstrated the mass was hypointense on T1-weighted imaging (T1WI), slightly hyperintense on T2-weighted imaging (T2WI) and hyperintense on diffusion weighted imaging (DWI) (b = 800 s/mm2). The apparent diffusion coefficient (ADC) value was low. The lesion in the dilated pancreatic duct showed relatively low intensity on all phases of dynamic contrast-enhanced MRI and could be observed more clearly. Magnetic resonance cholangiopancreatography (MRCP) revealed abrupt disruption of the common bile duct and main pancreatic duct in the region of the mass, twist and dilation of the main pancreatic duct in the pancreatic body and tail, and obvious dilation of intrahepatic and extrahepatic bile ducts. The diameter of the main pancreatic duct from the pancreatic body to the tail was up to 7 mm and the common bile duct was dilated up to 16 mm (Fig. 2). These imaging findings explain the symptom of obstructive jaundice. The imaging diagnoses of pancreatic cancer, neuroendocrine carcinoma and malignant solid pseudopapillary tumor were listed. The gallbladder enlarged, wall thickened and the bile density increased on CT. The intensity of bile increased in T1WI and decreased in T2WI. These imaging finds indicate cholecystitis and cholestasis.
Figure 1.
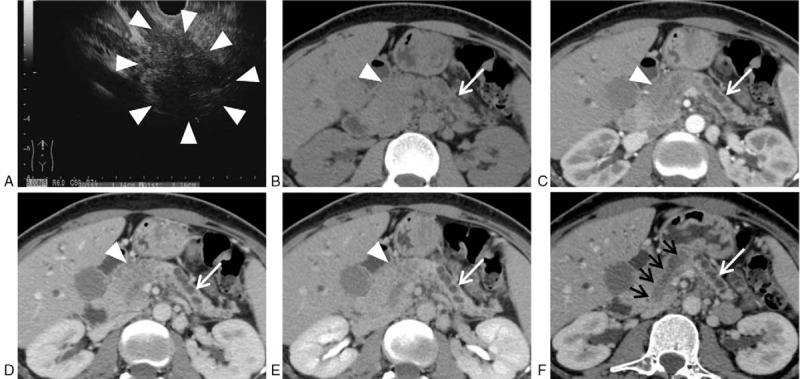
Case 1, female, 36-year-old, intraductal tubulopapillary neoplasm with invasive carcinoma (white arrowheads, the mass; white arrows, the dilated pancreatic duct in the pancreatic body and tail; black arrows, the tumor inside the dilated pancreatic duct). Endoscopic ultrasonography (A) revealed an ill-defined hypoechoic mass in the head of the pancreas. Unenhanced CT scan (B) showed the mass was low attenuating, associated with the atrophy of pancreatic parenchyma and dilation of the main pancreatic duct in the body and tail. Contrast-enhanced CT (C, arterial phase; D, portal phase; E, delayed phase) showed the mass was relatively hypovascular compared with the normal pancreatic parenchyma. The CT curved planar reformation image (F) of the portal phase along the direction of the main pancreatic duct showed the 2-tone duct sign clearly, indicating both the low attenuating tumor and lower attenuating fluid inside the dilated pancreatic duct. CT = computed tomography.
Figure 2.
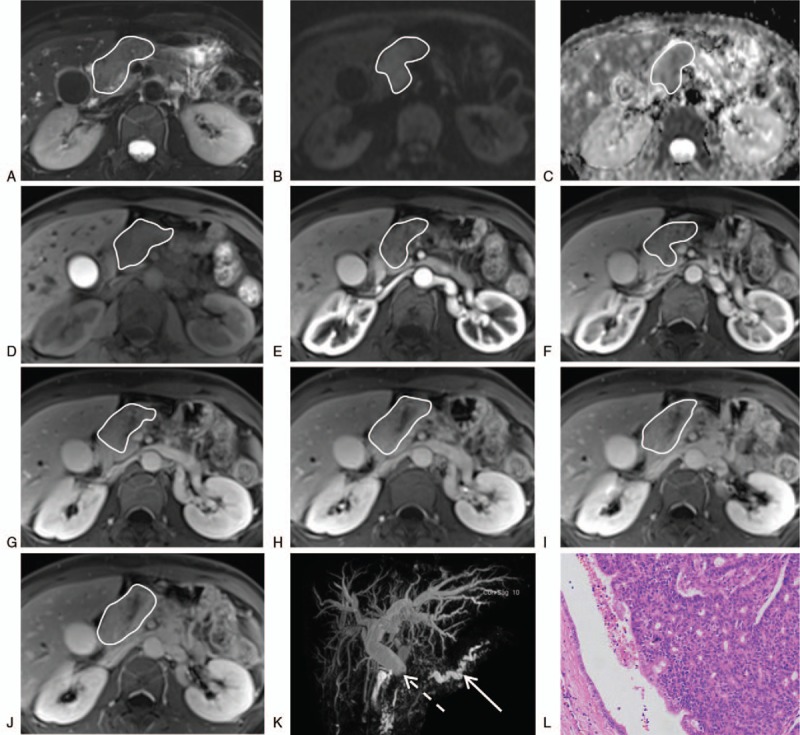
Case 1, female, 36-year-old, intraductal tubulopapillary neoplasm with invasive carcinoma (curves, the mass; dotted arrow, the common bile duct; solid arrow, the dilated pancreatic duct). The MRI (A, fat suppression [FS]-T2WI; B, DWI; C, ADC; D, unenhanced FS-T1WI; E–J, dynamic contrast-enhanced FS-T1WI in sequential order; J, DWI; K, MRCP) demonstrated the mass was slightly hypointense on FS-T1WI, slightly hyperintense on FS-T2WI, hyperintense on DWI and hypovascular compared with the normal pancreatic parenchyma. The ADC value was low. The MRCP revealed abrupt disruption of the common bile duct in the region of the mass, twist and dilation of the main pancreatic duct in the pancreatic body and tail, and obvious dilation of intrahepatic and extrahepatic bile ducts. Microscopic image of haematoxylin-eosin staining (L, magnification x100) showed the tumor consisted of closely apposed tubules forming complex cribriform structures in dilated pancreatic ducts with focal areas of papillary architecture. The normal ductal epithelium can be seen surrounding the tumor. ADC = apparent diffusion coefficient, DWI = diffusion weighted imaging, FS = fat suppression, MRCP = magnetic resonance cholangiopancreatography, MRI = magnetic resonance imaging, T1WI = T1-weighted imaging, T2WI = T2-weighted imaging.
The patient received percutaneous transhepatic cholangial drainage procedure on Dec 5th and 50 mL black bile drained out from drainage tube. Percutaneous transhepatic cholangiography showed the bottom of the Department of common bile duct was truncated. Endoscopic ultrasound guided fine needle aspiration was performed on Dec 18th. Histopathology showed glandular, cribriform, and sheet structures and low-grade dysplastic with mitotic figures. On immunohistochemistry, the tumor cells expressed cytokeratin (CK)7(++), CK19(+) and Villin(+++), and were negative for CA199, CEA, CK20, synaptophysin and chromogranin A. The Ki-67 proliferation index was 10–30%. Because of the limited tissues examined, a certain diagnosis could not be given and intraductal tubulopapillary neoplasm, neuroendocrine neoplasm and solid pseudopapillary tumor were suspected. The patient received pancreatoduodenectomy, cholecystectomy and lymphadenectomy on Jan 4th, 2018.
Macroscopically, a solid tumor sized 4.0×1.3×1.2 cm was identified in the head of the pancreas. On cross-section, the tumor was located in the dilated pancreatic duct manifesting as soft, granular and grayish yellow. No mucin was observed. Microscopically, the tumor consisted of closely apposed tubules forming complex cribriform structures in dilated pancreatic ducts with focal areas of papillary architecture (Fig. 2). The vascular space in the resection margin of common bile duct was involved. Metastasis was present in 1 lymph node among the 29 lymph nodes dissected. The normal ductal epithelium can be seen surrounding the tumor. Immunohistochemical staining showed the neoplastic cells were CK7(+), CK8(+++), CK18(+++), Villin(+++), CK20(−), synaptophysin(−), chromogranin A(−). The lesion was focally positive for CK19 and p53. The Ki-67 index reached 40%. The final diagnosis was pancreatic ITPN with an associated invasive carcinoma (adenocarcinoma) and the staging was T2N1cM0. The pathology result of resected gallbladder was chronic cholecystitis.
One month after the surgery, the patient received gemcitabine in combination with capecitabine as chemotherapy. On Mar 7th, the follow-up MRI revealed multiple abnormal nodules in the liver which were supposed as metastasis.
2.2. Case 2
A 62-year-old woman was admitted to our hospital for the treatment of a pancreatic tumor in 2012. The MRI showed a mass filled the mian pancreatic duct in the head and neck, sized 3.2×1.0 cm. It was hypointense on T1WI, slightly hyperintense on T2WI, hyperintense on DWI (b = 800 s/mm2) and hypovascular compared with the normal pancreatic parenchyma. The ADC value was low (Fig. 3). The patient received pancreaticoduodenectomy, cholecystectomy, and partial gastrectomy. The tumor was taupe, fragile and consisted of long glandular glands. Microscopically, closely arranged tubules grow in the way of back to back, forming nodular structures in the dilated pancreatic duct. The tumor cells were cuboidal and low columnar in shape with a moderate amount of eosinophilic or amphophilic cytoplasm and round to oval nuclei of moderate to severe atypia (Fig. 3). Immunohistochemical staining showed the tumor cells were EGFR(++++), P53(++), TOPO II(+), TOPO I(−), VEGF(−), synaptophysin(−), chromogranin A(−), CD56(−). The Ki-67 index was 30%. The pathologic diagnosis was pancreatic ITPN with microinvasive carcinoma. Tumor staging was T2N0Mx.
Figure 3.
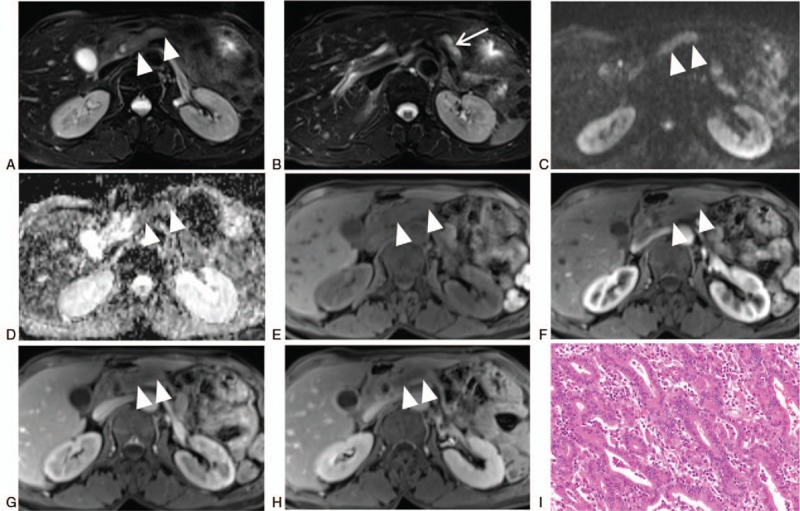
Female, 62-year-old, intraductal tubulopapillary neoplasm with microinvasive carcinoma (arrowheads, the mass; arrows, the dilated pancreatic duct). The MRI (A and B, FS-T2WI; C, DWI; D, ADC; E–H, FS-TIWI [E, unenhanced scan; F, arterial phase; G, portal phase; H, delayed phase]) showed the mass was located in the dilated main pancreatic duct, hypointense on FS-T1WI, slightly hyperintense on FS-T2WI, hyperintense on DWI and hypovascular compared with the normal pancreatic parenchyma. The ADC value was low. The FS-T2WI showed the mass was markedly hyperintense and the fluid was slightly hyperintense inside the dilated pancreatic duct, which constituted the 2-tone duct sign. Microscopic image of haematoxylin-eosin staining (I, magnification x400) showed closely arranged tubules grow in the way of back to back, forming nodular structures in the dilated pancreatic duct. The tumor cells were cuboidal and low columnar in shape with a moderate amount of eosinophilic or amphophilic cytoplasm and round to oval nuclei of moderate to severe atypia. ADC = apparent diffusion coefficient, DWI = diffusion weighted imaging, FS = fat suppression, MRI = magnetic resonance imaging, T1WI = T1-weighted imaging, T2WI = T2-weighted imaging.
3. Discussion
3.1. Imaging findings of ITPN
The ITPN is a rare and newly defined entity of the pancreas. Yamaguchi et al[2] 1st reported 10 cases of pancreatic ITPN. Its clinical symptoms are atypical, which makes the diagnosis quite challenging for clinicians. Due to limited literature elaborating on the imaging characterization of ITPN, it is also a tough work for radiologists to precisely diagnose ITPN preoperatively. An electronic search was performed for English literature of ITPN on PubMed before May 31st, 2018. After screening, 30 articles were included from which 71 different cases with detail information were extracted (Furuhata and Someya reported the same case and nine cases were the same in 2 articles of Yamaguchi).[2–31] A total of 73 cases were analyzed (Table 1), including 2 cases from our report. The ITPN can grow in any part of the pancreas and some cases were diffuse (6/61). About half of the tumors located in the head of the pancreas (51%, 31/61). The tumor size varied form 0.5 cm to 15 cm and the mean size was 4.6 cm. Among the 36 cases that the texture of tumors were described, 78% (28/36) were solid and only 22% (8/36) were cystic. The solid tumors were hypoechoic on ultrasound images, low density on CT images, hypointense on T1WI, slightly hyperintense on T2WI and hyperintense on DWI. After the contrast agent injected, the solid ITPNs were hypovascular except one[16] which was early intense enhancement, resembling a neuroendocrine tumor. The main pancreatic duct of ITPN often dilated because of the occlusion of tumor inside. Only 1 article was a series study about imaging, which introduced 2 typical imaging findings of ITPN, “2-tone duct sign” and “cork-of-wine-bottle sign”.[32] Two-tone duct sign describes the tumor (slightly higher density on CT image and slightly high-intensity on T2WI) and fluid (lower density on CT image and strikingly high-intensity on T2WI) in the dilated pancreatic duct show 2 different colors.[32] Cork-of-wine-bottle sign represents the tumor is surrounded by pancreatic fluid in the dilated duct[32] and MRCP is the best noninvasive examination to show this sign. Our cases showed the typical characteristics of 2-tone duct sign, especially in case 2. However, the area of tumor in case 1 was bigger in imaging than the real size in the gross specimen, which makes it more difficult to get accurate diagnosis. We assume that it is because the tumor is associated with an invasive carcinoma. By postprocessing technique of CT images, curved planar reformation image of the portal phase along the direction of the main pancreatic duct showed the 2-tone duct sign clearly, indicating both the low attenuating tumor and lower attenuating fluid inside the dilated pancreatic duct. The tumor in case 2 was restricted to the pancreatic duct. The T2WI showed the mass was markedly hyperintense and the fluid was slightly hyperintense, which constituted the 2-tone duct sign. As MRI can provide more detailed information, we suggested MRI as important imaging examination for the diagnosis of ITPN.
Table 1.
Reported cases of intraductal tubulopapillary neoplasm of the pancreas.

3.2. Clinical and histopathologic features of ITPN
Among the 73 patients, 38 were males, 33 were females, and 2 were not available. There was no gender difference of ITPN. The mean age of the reported patients was 58 years (range, 25–82 years). Near half of the patients were asymptomatic (47%, 31/66). The most common symptoms were abdominal pain, and other symptoms were emesis, appetite loss, weight loss, jaundice, and exacerbation of diabetes mellitus. The tumor consisted of closely arranged back-to-back tubular glands with occasional papillary elements, and the tumor cells were cuboidal in shape with scant mucin production. Immunohistochemical study showed all ITPNs were positive for CK7, CK19, and mucin (MUC)1 and negative for trypsin and MUC2. Most ITPN are positive for MUC6 (17/25, 68%). Almost all ITPNs are negative for MUC5AC except that 1 case was focally positive for MUC5AC, in which 8.2% of total neoplasm cells were positively stained.[13] More than half of the tumors had a component of invasive carcinoma (55%, 36/65), which is consistent with Kolby's[14] study. Age and gender did not affect the existence of invasive component. Larger tumor had a higher risk of invasive growth (P = .002). Surgery is the only way to cure ITPN. Date's[3] study showed curative surgery achieved excellent outcome for patients, even if ITPN had an invasive component.
3.3. Differential diagnosis
The ITPN should mainly be differentiated from intraductal papillary mucinous neoplasm (IPMN), the most common intraductal tumor. The IPMN are typically cystic and have a marked dilated pancreatic duct filled with abundant mucin. The ITPN are usually solid and have merely mucin secretion. Most IPMN are positive for MUC5AC and negative for MUC6 on immunohistochemical staining. The ITPN are usually positive for MUC6 and negative for MUC5AC.[33] When ITPN are accompanied with invasive component, it is difficult to distinguish it from pancreatic cancer. The 2 entity are hypo-enhanced after contrast medium injection. Pancreatic cancer are malignant and prone to involve blood vessels, nerves, and lymph nodes. The differential diagnosis also includes neuroendocrine neoplasms and acinar cell carcinomas with an intraductal growth pattern. Contrast-enhanced imaging is helpful because neuroendocrine neoplasms and acinar cell carcinomas show early and marked enhancement, while ITPN are almost hypo-enhanced.[32]
3.4. Limitation
We focus on imaging findings of the 2 cases. The patient data was incomplete, especially case 2. The patient only accepted MRI examination before surgery. We are failed to follow up, so the outcome information was missing.
4. Conclusion
The ITPN is a rare type of pancreatic epithelial neoplasm. It is difficult to make accurate diagnosis of ITPN even though various imaging modalities are used in combination. Further imaging characterization of ITPN based on more cases may improve diagnostic accuracy.
Author contributions
Conceptualization: Jingjing Zhang.
Data curation: Jingjing Zhang, Jianhua Wang.
Formal analysis: Jingjing Zhang.
Methodology: Jingjing Zhang, Shuai Ren, Wenli Qiu.
Validation: Dandan Ye, Huifeng Zhang.
Writing – original draft: Jingjing Zhang.
Writing – review & editing: Jingjing Zhang, Zhongqiu Wang.
Zhongqiu Wang orcid: 0000-0003-3722-6663.
Footnotes
Abbreviations: ADC = apparent diffusion coefficient, CA = carbohydrate antigen, CEA = carcinoembryonic antigen, CK = cytokeratin, CT = computed tomography, DWI = diffusion weighted imaging, IPMN = intraductal papillary mucinous neoplasm, ITPN = intraductal tubulopapillary neoplasm, MRCP = magnetic resonance cholangiopancreatography, MRI = magnetic resonance imaging, MUC = mucin, T1WI = T1-weighted imaging, T2WI = T2-weighted imaging.
Patient Consent Statement: The patients have provided informed consent for publication of the 2 cases.
The authors have no conflicts of interest to disclose.
This study was supported by the Major Program of Research and Development of Jiangsu Province (BE2017772) and the National Natural Science Foundation of China (81771899).
References
- [1].WHO, Bosman FT, Carneiro F, Hruban RH, et al. WHO classification of tumours of the digestive system. 2010. [Google Scholar]
- [2].Yamaguchi H, Shimizu M, Ban S, et al. Intraductal tubulopapillary neoplasms of the pancreas distinct from pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol 2009;33:1164–72. [DOI] [PubMed] [Google Scholar]
- [3].Date K, Okabayashi T, Shima Y, et al. Clinicopathological features and surgical outcomes of intraductal tubulopapillary neoplasm of the pancreas: a systematic review. Langenbecks Arch Surg 2016;401:439–47. [DOI] [PubMed] [Google Scholar]
- [4].Basturk O, Adsay V, Askan G, et al. Intraductal tubulopapillary neoplasm of the pancreas: a clinicopathologic and immunohistochemical analysis of 33 cases. Am J Surg Pathol 2017;41:313–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Inomata K, Kitago M, Obara H, et al. Concurrent presentation of an intraductal tubulopapillary neoplasm and intraductal papillary mucinous neoplasm in the branch duct of the pancreas, with a superior mesenteric artery aneurysm: a case report. World J Surg Oncol 2018;16:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Umemura A, Ishida K, Nitta H, et al. A rare case of intraductal tubulopapillary neoplasm of the pancreas rupturing and causing acute peritonitis. Case Rep Gastroenterol 2017;11:661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Maghrebi H, Makni A, Rhaeim R, et al. Intraductal tubulopapillary neoplasm: a new entity in the spectrum of pancreatic intraductal neoplasms. J Clin Diagn Res 2017;11:D14–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kuscher S, Steinle H, Soleiman A, et al. Intraductal tubulopapillary neoplasm (ITPN) of the pancreas associated with an invasive component: a case report with review of the literature. World J Surg Oncol 2017;15:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kovacevic B, Añó PL, Toxværd A, et al. Intraductal tubulopapillary neoplasm of the pancreas diagnosed by endoscopic ultrasonography-guided fine needle aspiration. Endoscopy 2017;49:E266–7. [DOI] [PubMed] [Google Scholar]
- [10].Fujimoto Y, Tomimaru Y, Tamura H, et al. Pancreatic intraductal tubulopapillary neoplasm with associated invasive cancer successfully treated by total pancreatectomy: a case report. Oncol Lett 2017;14:153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Niu L, Xu Z, Liu H, et al. Intraductal tubulopapillary neoplasm accompanied by invasive carcinoma of the pancreas: a case report and review of the literature. Mol Clin Oncol 2017;6:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Savant D, Lee L, Das K. Intraductal tubulopapillary neoplasm of the pancreas masquerading as pancreatic neuroendocrine carcinoma: review of the literature with a case report. Acta Cytologica 2016;60:267–74. [DOI] [PubMed] [Google Scholar]
- [13].Muraki T, Uehara T, Sano K, et al. A case of MUC5AC-positive intraductal neoplasm of the pancreas classified as an intraductal tubulopapillary neoplasm? Pathol Res Pract 2015;211:1034–9. [DOI] [PubMed] [Google Scholar]
- [14].Kolby D, Thilen J, Andersson R, et al. Multifocal intraductal tubulopapillary neoplasm of the pancreas with total pancreatectomy: report of a case and review of literature. Int J Clin Exp Pathol 2015;8:9672–80. [PMC free article] [PubMed] [Google Scholar]
- [15].Tajima S. Intraductal tubulopapillary neoplasm of the pancreas suspected by endoscopic ultrasonography-fine-needle aspiration cytology: report of a case confirmed by surgical specimen histology. Diagn Cytopathol 2015;43:1003–6. [DOI] [PubMed] [Google Scholar]
- [16].Takayama S, Maeda T, Nishihara M, et al. A case of intraductal tubulopapillary neoplasm of pancreas with severe calcification, a potential pitfall in diagnostic imaging. Pathol Int 2015;65:501–6. [DOI] [PubMed] [Google Scholar]
- [17].Yoshida Y, Matsubayashi H, Sasaki K, et al. Intraductal tubulopapillary neoplasm of the pancreatic branch duct showing atypical images. J Dig Dis 2015;16:357–61. [DOI] [PubMed] [Google Scholar]
- [18].Kitaguchi K, Kato Y, Kojima M, et al. A Resected case of intraductal tubulopapillary neoplasm of the pancreas: report of a case. Int Surg 2015;100:281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Matthews Y, McKenzie C, Byrne C, et al. Intraductal tubulopapillary neoplasm of pancreas with associated invasive carcinoma, lymph node, rectal and hepatic metastases. Pathology 2015;47:169–71. [DOI] [PubMed] [Google Scholar]
- [20].Zhao L, Hart J, Xiao SY, et al. Cytological features of pancreatic intraductal tubulopapillary neoplasm and an unexpected immunohistochemical profile. Pathology 2014;46:662–5. [DOI] [PubMed] [Google Scholar]
- [21].Ahls M, Niedergethmann M, Dinter D, et al. Case report: intraductal tubulopapillary neoplasm of the pancreas with unique clear cell phenotype. Diagn Pathol 2014;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Furuhata A, Minamiguchi S, Mikami Y, et al. Intraductal tubulopapillary neoplasm with expansile invasive carcinoma of the pancreas diagnosed by endoscopic ultrasonography-guided fine needle aspiration: a case report. Diagn Cytopathol 2014;42:314–20. [DOI] [PubMed] [Google Scholar]
- [23].Someya Y, Nakamoto Y, Nakatani K, et al. 18F-FDG uptake in intraductal tubulopapillary neoplasm of the pancreas. Clin Nucl Med 2014;39:e277–80. [DOI] [PubMed] [Google Scholar]
- [24].Guan H, Gurda G, Lennon AM, et al. Intraductal tubulopapillary neoplasm of the pancreas on fine needle aspiration: case report with differential diagnosis. Diagn Cytopathol 2014;42:156–60. [DOI] [PubMed] [Google Scholar]
- [25].Del CM, Mucelli RP, Blomberg J, et al. Is intraductal tubulopapillary neoplasia a new entity in the spectrum of familial pancreatic cancer syndrome? Fam Cancer 2014;13:227–9. [DOI] [PubMed] [Google Scholar]
- [26].Kasugai H, Tajiri T, Takehara Y, et al. Intraductal tubulopapillary neoplasms of the pancreas: case report and review of the literature. J Nippon Med Sch 2013;80:224–9. [DOI] [PubMed] [Google Scholar]
- [27].Urata T, Naito Y, Nagamine M, et al. Intraductal tubulopapillary neoplasm of the pancreas with somatic BRAF mutation. Clin J Gastroenterol 2012;5:413–20. [DOI] [PubMed] [Google Scholar]
- [28].Tajiri T, Tate G, Matsumoto K, et al. Diagnostic challenge: intraductal neoplasms of the pancreatobiliary system. Pathol Res Pract 2012;208:691–6. [DOI] [PubMed] [Google Scholar]
- [29].Jokoji R, Tsuji H, Tsujimoto M, et al. Intraductal tubulopapillary neoplasm of pancreas with stromal osseous and cartilaginous metaplasia; a case report. Pathol Int 2012;62:339–43. [DOI] [PubMed] [Google Scholar]
- [30].Bhuva N, Wasan H, Spalding D, et al. Intraductal tubulopapillary neoplasm of the pancreas as a radiation induced malignancy. BMJ Case Reports 2011;2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yamaguchi H, Kuboki Y, Hatori T, et al. Somatic mutations in PIK3CA and activation of AKT in intraductal tubulopapillary neoplasms of the pancreas. Am J Surg Pathol 2011;35:1812–7. [DOI] [PubMed] [Google Scholar]
- [32].Motosugi U, Yamaguchi H, Furukawa T, et al. Imaging studies of intraductal tubulopapillary neoplasms of the pancreas: 2-tone duct sign and cork-of-wine-bottle sign as indicators of intraductal tumor growth. J Comput Assist Tomogr 2012;36:710–7. [DOI] [PubMed] [Google Scholar]
- [33].Rooney SL, Shi J. Intraductal tubulopapillary neoplasm of the pancreas: an update from a pathologist's perspective. Arch Pathol Lab Med 2016;140:1068–73. [DOI] [PubMed] [Google Scholar]


