Abstract
Serum albumin (SA) is associated with inflammation and thrombosis, which are involved in acute aortic dissection (AAD). Our aim was to investigate the effect of SA level on survival in patients with AAD.
We analyzed 777 patients with AAD. The patients were divided into hypoalbuminemia and non-hypoalbuminemia groups according to their AAD Stanford classification. Multivariable Cox regression was used to investigate the association between SA levels and in-hospital mortality in type A and B AAD.
A total of 103 (13.3%) patients died in-hospital. The in-hospital mortality in type A and B patients with hypoalbuminemia was higher compared to those without (type A: 34.2% vs 13.9%, P <.001; type B: 7.9% vs 1.6%, P = .001). Kaplan–Meier analysis showed that survival was significantly lower in patients with hypoalbuminemia compared to those without, regardless of AAD type (type A: log-rank χ2 = 14.71; P <.001; Type B: log-rank χ2 = 10.42; P = .001). After adjusting for confounding factors, hypoalbuminemia was an independent predictor of in-hospital mortality in patients with either type A (HR, 2.492; 95% confidence interval [CI], 1.247–4.979; P = .010) or type B (HR, 8.729; 95% CI, 1.825–41.736; P = .007).
SA is independently associated with increased in-hospital mortality in both type A and B AAD.
Keywords: acute aortic dissection, in-hospital mortality, risk stratification, serum albumin
1. Introduction
Acute aortic dissection (AAD) is a rare life-threatening vascular emergency that is associated with high early and late morbidity and mortality rates.[1] The pre-hospital mortality rate of patients with untreated AAD is approximately 48.6%.[2] Dissections confined to the ascending aorta (type A) have worse short-term survival rates than those involving the descending aorta (type B).[1] Historically, the mortality rate was 1% to 2% per hour for the first 24 to 48 hours after symptom onset in ascending AAD.[3] The long-term outcomes of type B AAD are not necessarily better than those of type A AAD. The data from the International Registry of AAD show an in-hospital surgical mortality rate as high as 30% in type A AAD and 13% in type B. For type A patients treated by surgery who survive until hospital discharge, the mortality rates range from 4% to 48% at 1 year and 9% to 63% at 5 years.[4–8] The long-term mortality of surgically treated patients with type B AAD after discharge ranges from 4% to 44% at 1 year and 17% to 52% at 5 years,[4–6,8] similar with those with type A AAD. Therefore, it is critically important to identify the high-risk AAD patients at an early phase.
AAD is associated with an inflammatory reaction, thrombosis, and oxidative stress, evidenced by a significant elevation in inflammatory markers, such as C-reactive protein (CRP), D-dimer, platelets (PLT) count, mean PLT volume to count ratio, and matrix metalloproteinase 9.[9–14] However, a marker or a prognostic score that reflects multiple pathophysiological pathways may be superior to a single biomarker that reflects 1 pathophysiological pathway.[15] Serum albumin (SA), a stable protein in the human serum that is associated with systemic inflammation, thrombosis, and oxidative stress, is reported as a strong independent predictor of long-term mortality in cardiovascular disease.[16] Our previous study found that hypoalbuminemia was predictive for long-term mortality in type B AAD; however, that study was based on a small sample and did not identify the relation between SA and in-hospital mortality in type A and type B AAD.[17] Therefore, the primary goal of this study was to examine the hypothesis that low SA at admission is a good biomarker for early prediction of in-hospital mortality in patients with type A or B AAD.
2. Method
2.1. Study design
This was a single-center, retrospective cohort study, to evaluate the association between SA and the risk of in-hospital mortality in patients with AAD, regardless of type A or type B. The study was performed in accordance with the Helsinki Declaration and was approved by the Human Ethical Committee of West China Hospital of Sichuan University.
2.2. Study population
The study used data from the West China Hospital AAD database. This single-center database respectively included the data of all adults (≥18 years old) patients who were treated for AAD between January 2012 and December 2015. The inclusion criteria were:
-
(1)
AAD presenting within 14 days of symptom onset, and
-
(2)
contrast-enhanced computed tomography confirming a dissected descending aorta containing both a true and false lumen.
The exclusion criteria were:
-
(1)
pregnancy;
-
(2)
trauma-induced AAD; and
-
(3)
patients with infection, cancers, and other diseases related to the cardiovascular, urinary, endocrine, and immune systems.
2.3. Data collection and classifications
The clinical data of each patient with AAD was extracted from their clinical notes and charts available at the hospital. The database included demographic data, cardiovascular risk factors, medical history, results of blood tests at admission, inflammatory and thrombotic biomarkers, renal function, electrocardiographic, and computed tomography findings, details of medical treatment at the hospital, and the occurrence of in-hospital death. The Modification of Diet in Renal Disease equation was used to estimate the glomerular filtration rate (eGFR) in milliliters per minute per 1.73 m2.[18] The SA levels at admission were analyzed using an Architect c16000 analyzer (Abbott Diagnostics) and were categorized into 2 categories SA 34 g/L and SA >34 g/L.
2.4. Outcomes
The primary outcome was all-cause in-hospital mortality.
2.5. Statistical analysis
All statistical analysis was performed using IBM SPSS Statistics 21 software (IBM Corp., Armonk, NY). Patient characteristics were presented as frequencies and percentages for categorical variables and compared using the χ2 test or Fisher exact test. Normally distributed continuous variables were presented as mean and standard deviation and compared using Student t test while abnormally distributed variables were presented as median and 25th and 75th percentiles (Q1 and Q3, respectively) and compared using the Mann–Whitney U test. Kaplan–Meier curves were constructed and stratified according to different SA levels. In addition, a multivariable Cox regression analysis (forward: LR) was used to investigate the relationship between SA and in-hospital mortality. We performed prespecified subgroup analyses to explore the effects of confounding factors on the association between SA and in-hospital mortality in AAD. Subgroup analyses were performed with the use of Kaplan–Meier curves and log-rank tests. All tests were 2-sided and considered statistically significant at P <.05.
3. Results
3.1. Baseline patient characteristics
The initial cohort included 854 patients with AAD. We excluded 3 pregnant women, 34 patients with potentially confounding co-morbidity (e.g., active infection and chronic inflammatory disease), 16 with trauma-induced AAD, and 37 with no SA levels at admission. Finally, 777 patients were included in the study. Of these, 305 (39.3%) patients had type A, and 472 (40.7%) had type B AAD. The mean age of the study cohort was 51 ± 13 years, 686 (88.3%) were males, and 129 (16.6%) died in hospital. The mean SA levels at admission were 34 ± 6 g/L with a median of 34 (30–38) g/L.
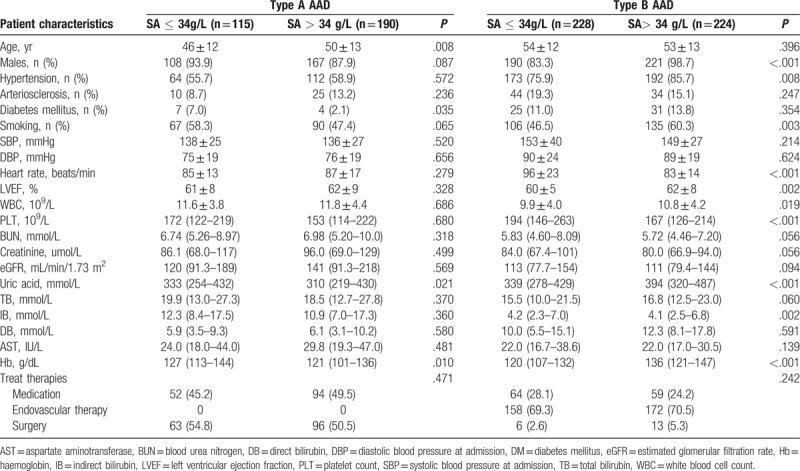
The baseline characteristics of the study cohort are shown in Table 1. Among the type A patients with AAD, 115 (37.7%) were hypoalbuminemic, defined as SA ≤34 g/L. Patients with Type A AAD and hypoalbuminemia were younger and had higher hemoglobin (Hb) and uric acid (UA) levels compared with those with SA >34 g/L. No differences were found in the other parameters between patients with and without hypoalbuminemia (Table 1). For patients with type B AAD, those with SA >34 g/L were comprised of more males, had higher rates of hypertension and smoking and increased left ventricular fractions, white blood cells (WBCs), UA, and Hb compared to those with hypoalbuminemia. The patients who had hypoalbuminemia had more PLT and higher indirect bilirubin and heart rate compared to those with SA >34 g/L. There were no differences in the other parameters between patients with and without hypoalbuminemia (Table 1).
Table 1.
Baseline characteristics of patients with acute aortic dissection.
3.2. SA and in-hospital mortality
In type, A AAD, the in-hospital mortality was higher in patients with hypoalbuminemia compared to those without (34.2% vs. 13.9%, P <.001; Fig. 1A). Kaplan–Meier survival analysis showed that survival was significantly lower in patients with hypoalbuminemia compared to those without (log-rank χ2 = 14.71; P <.001; Fig. 1B), irrespective of medication (log-rank χ2 = 10.77; P = .001; Fig. 1C) or surgical therapy (log-rank χ2 = 4.34; P = .037; Fig. 1D).
Figure 1.
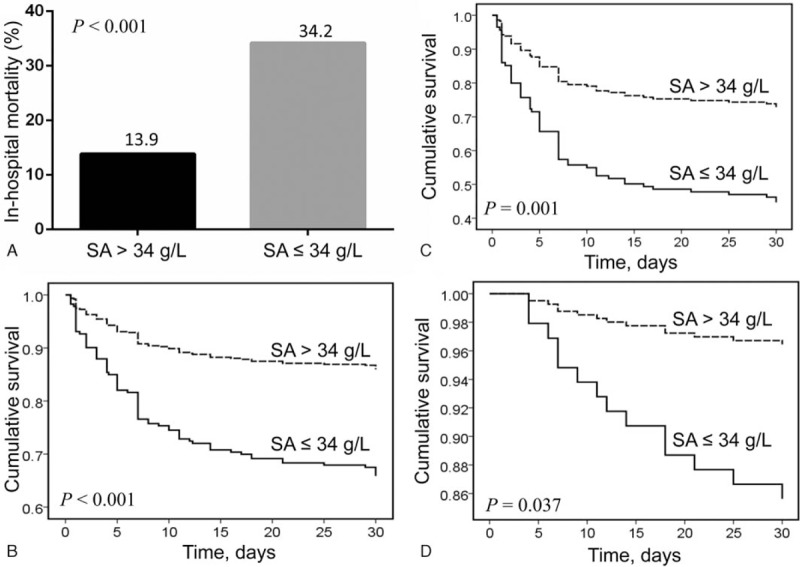
(A) The in-hospital mortality in different levels of serum albumin in type A acute aortic dissection; (B) Kaplan–Meier analysis survival curve according to different levels of serum albumin in all patients with type A acute aortic dissection; (C) Kaplan–Meier analysis survival curve according to levels of serum albumin in patients with type A acute aortic dissection receiving medication only; (D) Kaplan–Meier analysis survival curve according to levels of serum albumin in patients with type A acute aortic dissection receiving surgery.
Among patients with type B AAD, those with hypoalbuminemia had higher in-hospital mortality rates than those without (7.9% vs 1.6%, P = 0.001; Fig. 2A). Kaplan–Meier survival analysis indicated the cumulative survival rates of patients with hypoalbuminemia were lower compared to those without (log-rank χ2 = 10.42; P = .001; Fig. 2B), subgroup analysis showed a similar result in patients receiving medication (log-rank χ2 = 5.53; P = .019; Fig. 2C) and endovascular therapy (log-rank χ2 = 4.07; P = .044; Fig. 2D). None of the patients treated by surgery died.
Figure 2.
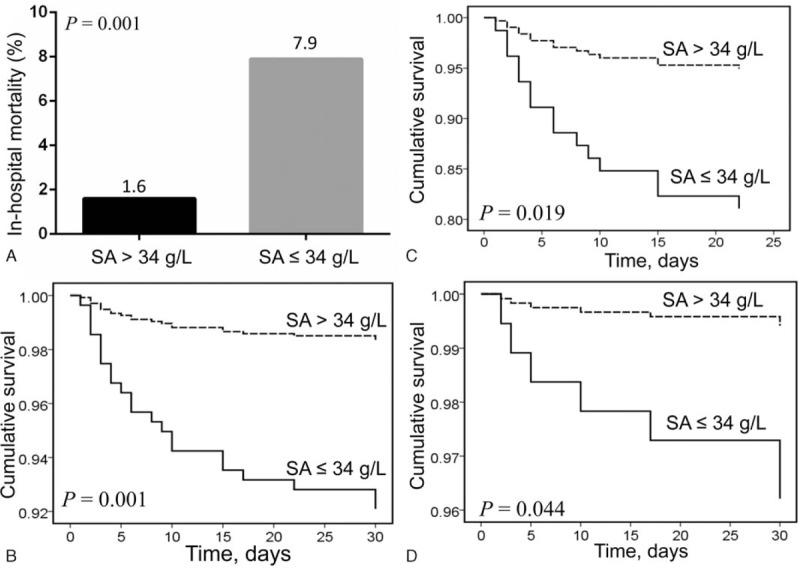
(A) The in-hospital mortality in different levels of serum albumin in type B acute aortic dissection; (B) Kaplan–Meier analysis survival curve according to different levels of serum albumin in all patients with type B acute aortic dissection; (C) Kaplan–Meier analysis survival curve according to different levels of serum albumin in patients with type B acute aortic dissection receiving medication therapy; (D) Kaplan–Meier analysis survival curve according to different levels of serum albumin in patients with type B acute aortic dissection receiving endovascular therapy.
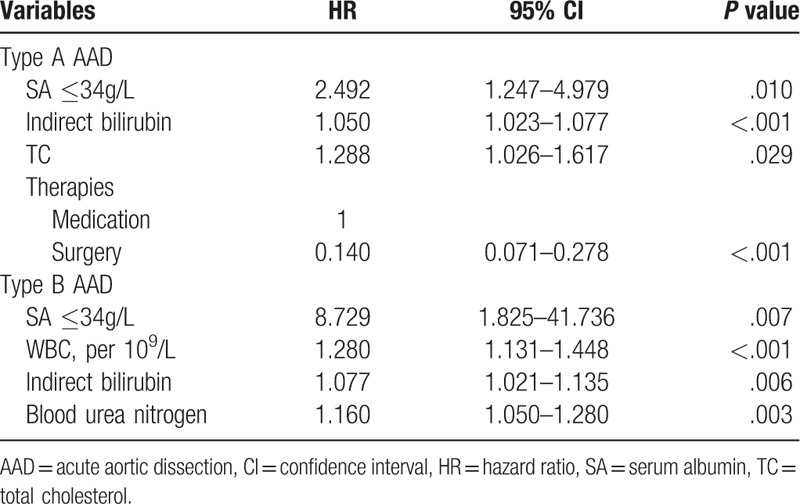
The unadjusted hazard ratio (HR) of hypoalbuminemia for predicting in-hospital mortality in the univariate Cox regression model was 2.763 [95% confidence interval (CI), 1.599–4.776; P <.001) for type A and 4.976 (95% CI, 1.684–14.704; P = .004) for type B. After adjusting for confounding factors, hypoalbuminemia was an independent risk factor of in-hospital mortality in patients with either type A (HR, 2.492l 95% CI, 1.247–4.979; P = .010) or type B (HR, 8.729; 95% CI, 1.825–41.736; P = .007) (Table 2).
Table 2.
Multivariable Cox regression of in-hospital mortality for patients with acute aortic dissection.
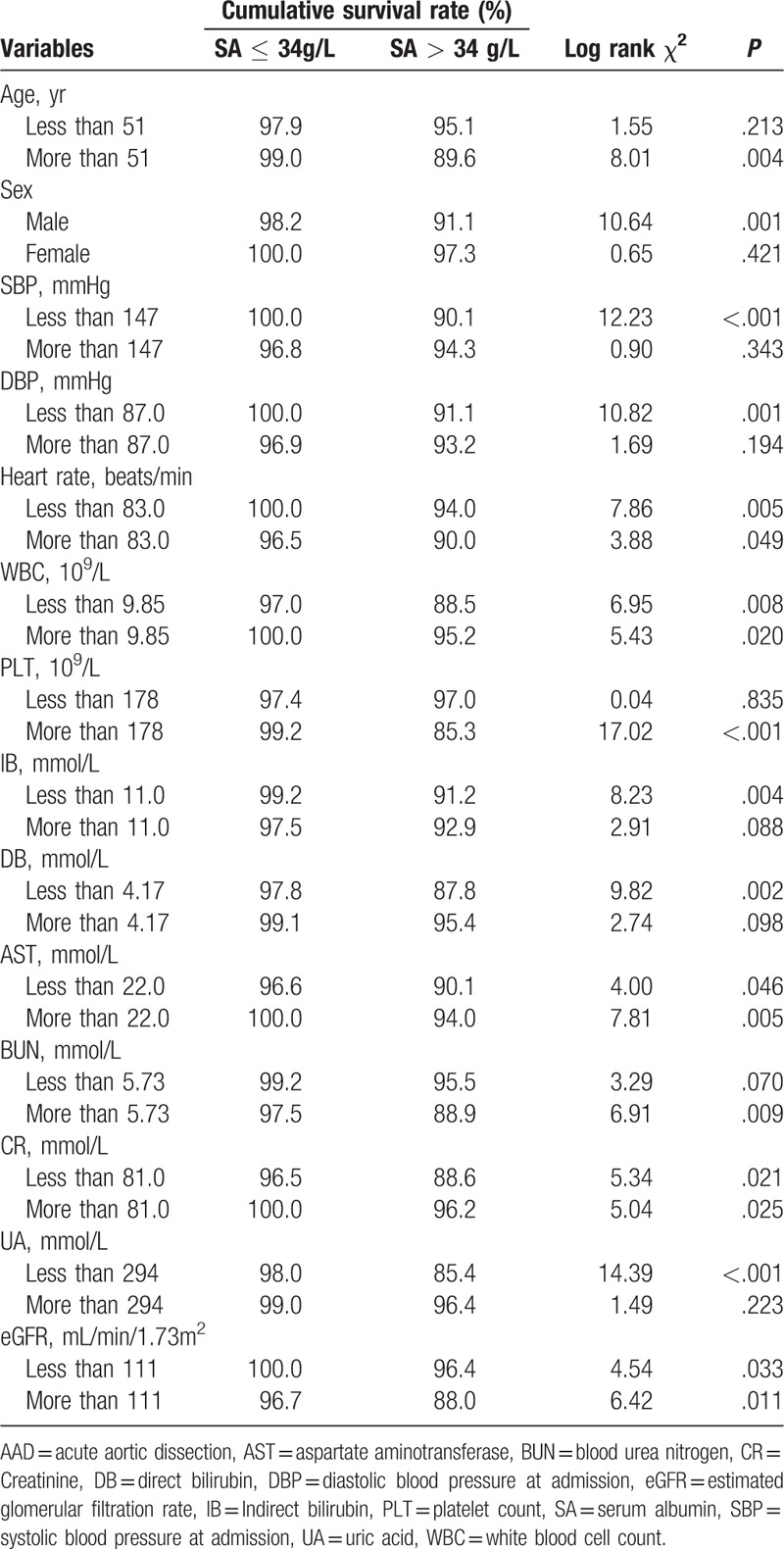
In type A AAD, subgroup analysis showed that hypoalbuminemia was independently associated with in-hospital mortality in subgroups with different ages and levels of systolic and diastolic blood pressure, heart rate, WBCs, PLTs, aspartate aminotransferase (AST), blood urea nitrogen, creatinine (Cr), and eGFR (Table 3). In type B AAD, hypoalbuminemia was also associated with the risk of in-hospital mortality in patients with different heart rates, AST, Cr, and eGFR (Table 4).
Table 3.
Subgroup analysis of Kaplan–Meier survival analysis of the association between hypoalbuminaemia and in-hospital mortality of patients with type A acute aortic dissection.
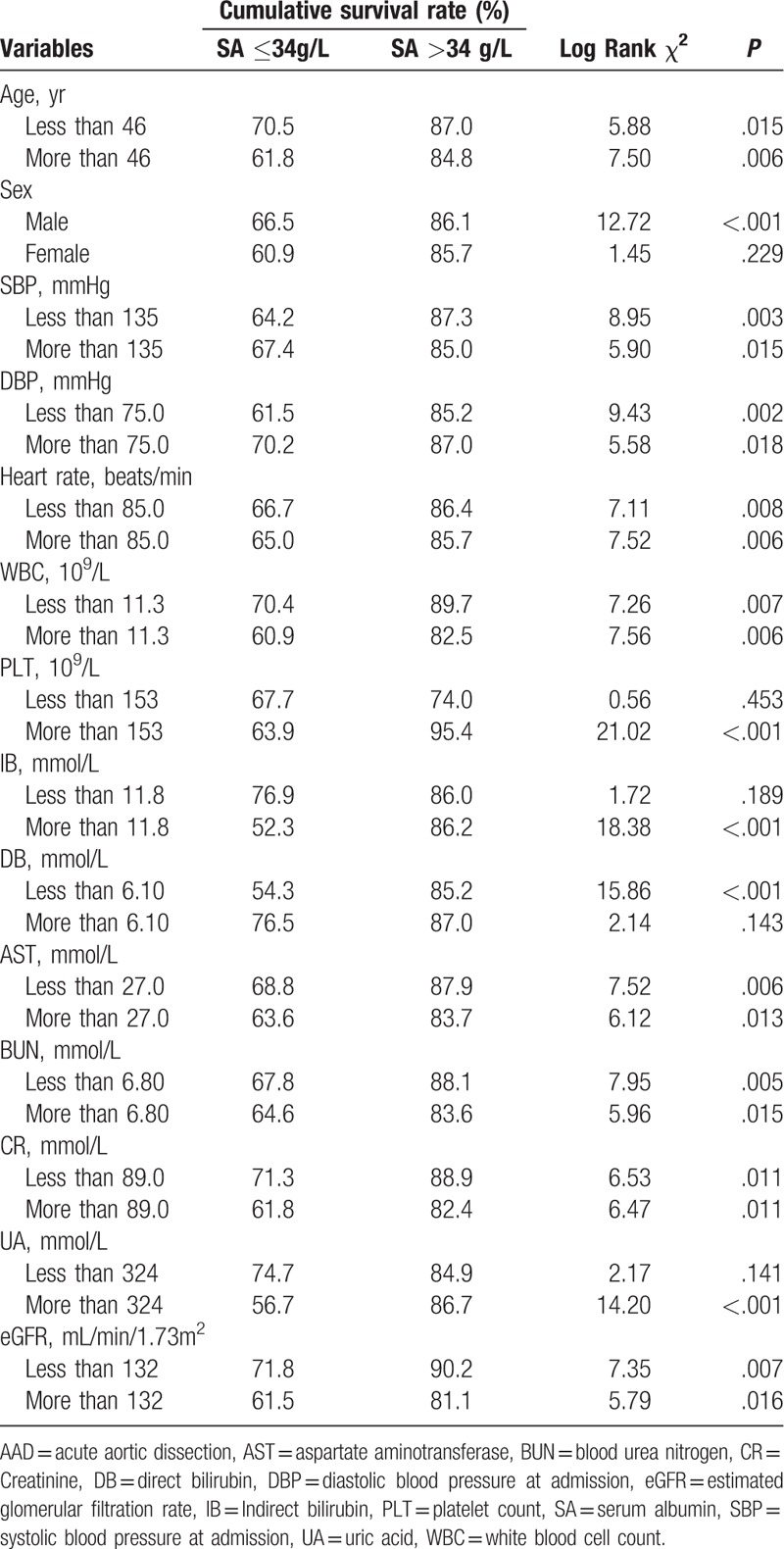
Table 4.
Subgroup analysis of Kaplan-Meier survival analysis of the association between hypoalbuminaemia and in-hospital mortality of patients with type B acute aortic dissection.
4. Discussion
The present study indicated that SA was independently associated with the in-hospital mortality of patient with AAD, irrespective of the different types and therapies. In addition, hypoalbuminemia was an independent predictor of in-hospital mortality among AAD patients with different inflammatory statuses and impaired renal function. Therefore, SA was a useful biomarker to stratify the high-risk patients with type A or B AAD. To the best of our knowledge, no studies had researched the prognostic value of SA in all types of AAD patients.
Prior studies had indicated that decreased SA levels were associated with an increased risk of cardiovascular mortality and other adverse outcomes.[16] Hypoalbuminemia was an independent predictor of in-hospital and long-term adverse outcomes of acute myocardial infarction (AMI), and was associated with the development of advanced heart failure in patients with AMI undergoing primary PCI.[19,20] Furthermore, a reduction in SA over time, even 1 within the normal range, was also associated with an increased incidence of cardiovascular disease.[16] Plakht et al[21] found that SA at admission is an independent prognostic marker for long-term (up to 10 years) all-cause mortality in hospital survivors with AMI. In addition, reduced SA, even within the clinically normal range, is a strong biomarker of increased long-term mortality with a gradual “dose-response” association. In addition, Nakayama et al have found that a decreasing trend in SA level in patients with heart failure was correlated with a worse long-term prognosis, and that change of SA levels was also associated with changes in cachectic factors.[22] Therefore, SA may be a good marker to stratify high-risk patients with cardiovascular diseases.
In Takeuchi et al's study, the mean albumin level of patients with AAD was significantly lower than that of healthy controls. Low SA levels and smoking, in addition to hypertension, are significantly associated with AAD.[23] Our previous study showed that hypoalbuminemia was present in 47% of patients with type B AAD and that it was associated with long-term mortality.[17] However, the study was based on a small sample and we did not investigate the relationship between SA and in-hospital outcomes in patients with type A AAD.[17] Furthermore, in subgroup analysis according to different therapies, inflammatory marker (WBC) levels, and renal function, hypoalbuminemia was also associated with an increased risk of in-hospital mortality in patients with type A or B AAD. Subgroup analysis also showed that the association between SA and the risk of in-hospital mortality was not influenced by potential confounding factors. Therefore, SA was a useful biomarker for early risk stratification of patients with AAD.
The mechanisms underlying the association between reduced SA and mortality in patients with AAD are not clear; however, several explanations could be considered. First, the decreased synthesis and increased catabolism of SA in relation to inflammation.[24] Inflammation plays an important and crucial role along the entire continuum of AAD pathophysiology, and the severity of inflammation has been shown to be associated with rupture of aortic dissection.[25] Inflammatory markers, such as CRP[26] and WBCs,[10,27] have also been shown as predictors of short and long-term mortality in patients with AAD. The SA levels of patients with type B AAD who died were lower than those of survivors, and low SA was also associated with in-hospital adverse outcomes and long-term mortality.[17] Second, SA is a significant inhibitor of PLT activation and aggregation and a mediator of PLT-induced coronary artery disease. Therefore, reduced SA levels could be associated with an increased risk for thrombotic events related to increased PLT activation and aggregation.[28] PLTs play an important role in thrombosis, which is involved in AAD.[9] The thrombus status is associated with the severity of AAD and could reflect the prognosis at an early phase.[9] Some thrombus biomarkers, including D-dimer[29,30] and fibrinogen,[31] were reported to be useful for stratification of high-risk patients with AAD. In previous studies, the mean PLT volume to count ratio,[12] PLT count,[14] and markers of PLT activity, were independent predictors of mortality in patients with type A AAD. Therefore, low SA was associated with increased risks of mortality in association with PLT activation and thrombosis in AAD. Finally, oxidative stress is also involved in AAD patients with reduced SA.[32] Increased oxidative stress was reported to be associated with deleterious long-term outcomes in patients following AMI.[33] Thus, 1 possible explanation for why hypoalbuminemia is associated with in-hospital mortality after AAD is the antioxidant activity of albumin. In our previous study, no sufficient evidence was found to support the concept that decreased nutritional intake and liver and renal dysfunction were associated with decreased SA in AAD patients.[16,17] Therefore, low SA most frequently arises from the inflammatory response, oxidative stress, and PLT activity. In the present study, low SA was predictive of in-hospital mortality in AAD, regardless of inflammatory status and PLT activity. SA may be a biomarker that reflects multiple pathophysiological processes that are involved in the severity of AAD.
4.1. Limitations
This study has several limitations that should be considered when interpreting its findings. First, the present study was a single-center, respective observational study. A prospective, large-scale multicenter study is needed to confirm our conclusions. Second, some inflammatory and thrombotic biomarkers, such as CRP and D-dimer, could not be detected, and in-hospital adverse outcomes were not collected in the present study. We could not analyze the relationship between some inflammatory and thrombotic biomarkers or in-hospital adverse outcomes and SA. Third, we did not analyze SA variation at different time points during hospitalization.
4.2. Future directions
For future research, several aspects should be considered. First, the relationship between AAD with some other inflammatory and thrombotic biomarkers or combination of these indicators could be analyzed. Second, articles about the molecular and genetic mechanisms of SA in the progress of pathophysiology of AAD formation and development are needed directly. Third, whether the intervention for the hypoalbuminemia AAD patients could improve the prognosis of these patients effectively need to be verified.
5. Conclusions
The results from this study indicate that reduced SA is associated with a high risk of in-hospital mortality in patients with AAD, irrespective of confounding factors. SA was a useful biomarker for early stratification of high-risk patients with AAD.
Author contributions
Conceptualization: Yongli Gao, Dongze Li, Li Tang.
Data curation: Yongli Gao, Dongze Li.
Formal analysis: Yongli Gao, Dongze Li.
Funding acquisition: Yu Cao, Li Tang.
Investigation: Yongli Gao, Dongze Li, Yu Cao, Xingyu Zhu.
Methodology: Yongli Gao, Dongze Li.
Project administration: Yongli Gao, Dongze Li.
Resources: Dongze Li.
Software: Dongze Li.
Validation: Yongli Gao.
Writing – original draft: Yongli Gao, Dongze Li.
Writing – review & editing: Zhi Zeng, Li Tang.
Footnotes
Abbreviations: AAD = acute aortic dissection, AMI = acute myocardial infarction, AST = aspartate aminotransferase, CI = confidence interval, CR = creatinine, CRP = c-reactive protein, eGFR = estimated glomerular filtration rate, Hb = hemoglobin, HR = hazard ratio, PLT = platelets, SA = serum albumin, UA = uric acid, WBCs = white blood cells.
YG and DL authors contributed equally to this work.
This work was supported financially by grants from the Science Foundation of Science and Technology Department of Chengdu (No. 2016-HM02-00099-SF) and Sichuan (No. 2018RZ0139,19MZGC0097, 2018JY0577 and 2017SZ0190), 1•3•5 project for disciplines of excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (No. 2018HXFH001, 2018HXFH027), and Health and Family Planning Commission of Sichuan Province (No.16PJ305).
The authors have no conflicts of interest to disclose.
References
- [1].Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266–369. [DOI] [PubMed] [Google Scholar]
- [2].Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897–903. [DOI] [PubMed] [Google Scholar]
- [3].Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management: part I: from etiology to diagnostic strategies. Circulation 2003;108:628–35. [DOI] [PubMed] [Google Scholar]
- [4].Tolenaar JL, Froehlich W, Jonker FH, et al. Predicting in-hospital mortality in acute type B aortic dissection: evidence from international registry of acute aortic dissection. Circulation 2014;130:S45–50. [DOI] [PubMed] [Google Scholar]
- [5].Tsai TT, Fattori R, Trimarchi S, et al. Long-term survival in patients presenting with type B acute aortic dissection: insights from the International Registry of Acute Aortic Dissection. Circulation 2006;114:2226–31. [DOI] [PubMed] [Google Scholar]
- [6].Afifi RO, Sandhu HK, Leake SS, et al. Outcomes of patients with acute type B (DeBakey III) aortic dissection: a 13-year, single-center experience. Circulation 2015;132:748–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lansman SL, McCullough JN, Nguyen KH, et al. Subtypes of acute aortic dissection. Ann Thorac Surg 1999;67:1975–8. [DOI] [PubMed] [Google Scholar]
- [8].Li D, Ye L, He Y, et al. False lumen status in patients with acute aortic dissection: a systematic review and meta-analysis. J Am Heart Assoc 2016;5:e003172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li DZ, Yu J, Du RS, et al. Thrombo-inflammatory status and prognosis of acute type A aortic dissection. Herz 2016;41:250–1. [DOI] [PubMed] [Google Scholar]
- [10].Li DZ, Li XM, Sun HP, et al. A novel simplified thrombo-inflammatory prognostic score for predicting in-hospital complications and long-term mortality in patients with type A acute aortic dissection: a prospective cohort study. Eur Heart J Suppl 2015;17:C26–33. [Google Scholar]
- [11].Li D, Ye L, Yu J, et al. Significance of the thrombo-inflammatory status-based novel prognostic score as a useful predictor for in-hospital mortality of patients with type B acute aortic dissection. Oncotarget 2017;8:79315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li DZ, Chen QJ, Sun HP, et al. Mean platelet volume to platelet count ratio predicts in-hospital complications and long-term mortality in type A acute aortic dissection. Blood Coagul Fibrinolysis 2016;27:653–9. [DOI] [PubMed] [Google Scholar]
- [13].Du R, Li D, Yu J, et al. Association of platelet to lymphocyte ratio and risk of in-hospital mortality in patients with type B acute aortic dissection. Am J Emerg Med 2017;35:368–70. [DOI] [PubMed] [Google Scholar]
- [14].Huang B, Tian L, Fan X, et al. Low admission platelet counts predicts increased risk of in-hospital mortality in patients with type A acute aortic dissection. Int J Cardiol 2014;172:e484–6. [DOI] [PubMed] [Google Scholar]
- [15].Li DZ, Yu J, Zeng R, et al. Neutrophil count is associated with risks of cardiovascular diseases. J Am Coll Cardiol 2017;70:911–2. [DOI] [PubMed] [Google Scholar]
- [16].Arques S. Human serum albumin in cardiovascular diseases. Eur J Intern Med 2018;52:8–12. [DOI] [PubMed] [Google Scholar]
- [17].Zeng R, Li D, Deng L, et al. Hypoalbuminemia predicts clinical outcome in patients with type B acute aortic dissection after endovascular therapy. Am J Emerg Med 2016;34:1369–72. [DOI] [PubMed] [Google Scholar]
- [18].Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247–54. [DOI] [PubMed] [Google Scholar]
- [19].Xia M, Zhang C, Gu J, et al. Impact of serum albumin levels on long-term all-cause, cardiovascular, and cardiac mortality in patients with first-onset acute myocardial infarction. Clin Chim Acta 2018;477:89–93. [DOI] [PubMed] [Google Scholar]
- [20].Yang LJ, Feng YX, Li T, et al. Serum albumin levels might be an adverse predictor of long term mortality in patients with acute myocardial infarction. Int J Cardiol 2016;223:647–8. [DOI] [PubMed] [Google Scholar]
- [21].Plakht Y, Gilutz H, Shiyovich A. Decreased admission serum albumin level is an independent predictor of long-term mortality in hospital survivors of acute myocardial infarction. Soroka Acute Myocardial Infarction II (SAMI-II) project. Int J Cardiol 2016;219:20–4. [DOI] [PubMed] [Google Scholar]
- [22].Nakayama H, Koyama S, Kuragaichi T, et al. Prognostic value of rising serum albumin during hospitalization in patients with acute heart failure. Am J Cardiol 2016;117:1305–9. [DOI] [PubMed] [Google Scholar]
- [23].Ogawa S, Takeuchi K, Mori T, et al. Spironolactone further reduces urinary albumin excretion and plasma B-type natriuretic peptide levels in hypertensive type II diabetes treated with angiotensin-converting enzyme inhibitor. Clin Exp Pharmacol Physiol 2006;33:477–9. [DOI] [PubMed] [Google Scholar]
- [24].Artigas A, Wernerman J, Arroyo V, et al. Role of albumin in diseases associated with severe systemic inflammation: pathophysiologic and clinical evidence in sepsis and in decompensated cirrhosis. J Crit Care 2016;33:62–70. [DOI] [PubMed] [Google Scholar]
- [25].Cifani N, Proietta M, Tritapepe L, et al. Stanford-A acute aortic dissection, inflammation, and metalloproteinases: a review. Ann Med 2015;47:441–6. [DOI] [PubMed] [Google Scholar]
- [26].Sakakura K, Kubo N, Ako J, et al. Peak C-reactive protein level predicts long-term outcomes in type B acute aortic dissection. Hypertension 2010;55:422–9. [DOI] [PubMed] [Google Scholar]
- [27].Fan X, Huang B, Lu H, et al. Impact of admission white blood cell count on short- and long-term mortality in patients with type a acute aortic dissection: an observational study. Medicine (Baltimore) 2015;94:e1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li HH, Xu J, Wasserloos KJ, et al. Cytoprotective effects of albumin, nitrosated or reduced, in cultured rat pulmonary vascular cells. Am J Physiol Lung Cell Mol Physiol 2011;300:L526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nazerian P, Mueller C, Soeiro AM, et al. Diagnostic accuracy of the aortic dissection detection risk score plus d-dimer for acute aortic syndromes: the advised prospective multicenter study. Circulation 2018;137:250–8. [DOI] [PubMed] [Google Scholar]
- [30].Huang B, Yang Y, Lu H, et al. Impact of d-dimer levels on admission on inhospital and long-term outcome in patients with type A acute aortic dissection. Am J Cardiol 2015;115:1595–600. [DOI] [PubMed] [Google Scholar]
- [31].Liu J, Sun LL, Wang J, et al. The relationship between fibrinogen and in-hospital mortality in patients with type A acute aortic dissection. Am J Emerg Med 2018;36:741–4. [DOI] [PubMed] [Google Scholar]
- [32].Liao M, Liu Z, Bao J, et al. A proteomic study of the aortic media in human thoracic aortic dissection: implication for oxidative stress. J Thorac Cardiovasc Surg 2008;136:65–72. [DOI] [PubMed] [Google Scholar]
- [33].Abe N, Kashima Y, Izawa A, et al. A 2-year follow-up of oxidative stress levels in patients with ST-segment elevation myocardial infarction: a subanalysis of the ALPS-AMI study. Angiology 2015;66:271–7. [DOI] [PubMed] [Google Scholar]


