Abstract
Several studies have investigated the incidence of and risk factors for acute exacerbation (AE) in patients with interstitial lung disease (ILD) after lung resection surgery. However, the incidence and risk factors for AE-ILD after non-pulmonary surgery are not known. The aim of this study was to investigate the incidence of and risk factors for AE-ILD after non-pulmonary surgery.
Eighty patients who were diagnosed with ILD on preoperative chest computed tomography (CT) imaging and underwent non-pulmonary surgery under general anesthesia at Hiroshima University Hospital between September 2011 and September 2017 were enrolled. We retrospectively compared the preoperative patient characteristics, laboratory findings, and factors associated with anesthetic management between the patients who developed AE-ILD and those who did not.
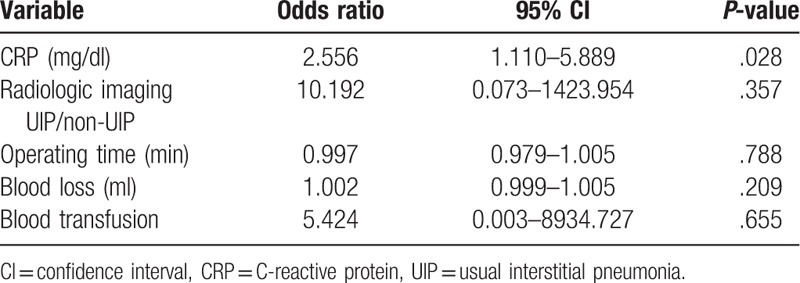
The incidence of AE-ILD after non-pulmonary surgery was 6.3% and the mortality rate was 80%. Univariate logistic analysis showed that a usual interstitial pneumonia pattern on computed tomography, a high C-reactive protein (CRP) level, a long operating time, high blood loss, and blood transfusion during surgery were significant risk factors for AE-ILD. In multivariate analysis, only a high CRP level (odds ratio 2.556, 95% confidence interval 1.110–5.889, P = .028) was identified as an independent risk factor for AE-ILD after non-pulmonary surgery.
The risk of AE-ILD should be kept in mind in patients with ILD and a high CRP level before non-pulmonary surgery. These patients should also be monitored carefully for development of AE-ILD after surgery.
Keywords: C-reactive protein, exacerbation, interstitial lung disease, non-pulmonary surgery, risk factors
1. Introduction
Interstitial lung disease (ILD) includes a group of pulmonary disorders that affect the pulmonary interstitium, that is, idiopathic interstitial pneumonia, hypersensitivity pneumonitis, sarcoidosis, drug-induced ILD, collagen vascular disease-associated ILD, and radiation pneumonitis.[1,2] Most of the ILDs progress gradually over the course of many months or years. However, 7.0%–19.1% of patients with idiopathic pulmonary fibrosis (IPF), which accounts for approximately 50% cases of ILDs, develop an acute exacerbation of ILD (AE-ILD) each year. Furthermore, 1.3%–11.1% of patients with non-IPF-ILD develop AE-ILD per year.[3] AE-ILD is a serious condition and is associated with a high mortality rate.[4–8] The overall survival rate in patients with AE-ILD was reported to be 67% at 1 month and 40% at 3 months.[8]
ILDs are associated with an increased risk of lung cancer, the incidence of which has been reported to be 4.4%–16.7%.[9] Lung resection surgery is recommended as standard treatment for patients with early-stage lung cancer. However, when patients with ILD have undergone such surgery, the rate of AE-ILD has been reported as 4.9%–9.3% and the prognosis was extremely poor, with a mortality rate of 6.5%–43.9%.[10–12] Therefore, several studies have been performed to identify the risk factors for AE-ILD.[11,13] The largest was a multicenter study in Japan that identified the following independent risk factors: surgical procedure, male sex, history of exacerbation, preoperative use of steroids, a high serum Krebs von den Lungen (KL)-6 level, usual interstitial pneumonia (UIP) pattern on computed tomography (CT), and reduced percent predicted vital capacity.[11] Some patients with ILD also developed AE-ILD after non-pulmonary surgery, such as surgery for hemoperitoneum or prostatectomy.[14] However, only one study has attempted to identify risk factors for AE-ILD after non-pulmonary surgery.[15] The only independent risk factor for AE-ILD identified in that study was use of propofol as a general anesthetic agent. However, only 21.3% of patients in that study received propofol, even though propofol is commonly used for general anesthesia worldwide. Therefore, we considered that the risk factors for AE-ILD need further investigation. In this study, we retrospectively attempted to identify patient characteristics and factors associated with anesthetic management that could increase the risk of AE-ILD in patients undergoing non-pulmonary surgery, who were anesthetized using propofol.
2. Methods
2.1. Study design and patients
Patients who were diagnosed with ILD on preoperative chest CT and underwent non-pulmonary organ surgery under general anesthesia at Hiroshima University Hospital from September 2011 to September 2017 were enrolled in this study. The patient demographics, preoperative lung function, laboratory findings within 3 months of surgery, type of anesthetic management, operating time, estimated blood loss, and blood transfusion were retrieved from electronic medical records. We enrolled patients consecutively, and all patients were anesthetized using propofol.
2.2. Lung function test
The lung function tests were performed before surgery, and the parameters were expressed as a percentage of the vital capacity (%VC) and the ratio of forced expiratory volume in one second to the forced vital capacity (FEV1.0%). The %VC and FEV1.0% were measured by standard methods using the Chestac-7800 and Chestac-8900 (Chest M.I., Inc., Tokyo, Japan).
2.3. Assessment of chest CT findings
ILD was diagnosed on the basis of findings of ground-glass attenuation, reticular opacities, honeycombing, or traction bronchiectasis on CT images acquired in the 3 months before surgery. The CT findings were classified using the criteria proposed by the American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association Statement.[2] The ILD patterns seen on CT were classified as UIP pattern or non-UIP pattern. The CT images were obtained at end inspiration in the supine position and were independently evaluated by two pulmonologists who were not aware of any other clinical findings or patient outcomes. Final decisions were made by consensus between the two pulmonologists.
2.4. ASA-PS classification
The ASA physical status classification system is used to evaluate a patient's fitness for anesthesia, and includes the following six classes: I, normal healthy patient; II, patient with mild systemic disease; III, patient with severe systemic disease; IV, patient with severe systemic disease that is a constant threat to life; V, moribund patient who is not expected to survive without the operation; and VI, patient who has been declared brain dead and whose organs are being removed for donor purposes.[16] The patient ASA-PS classification was evaluated by the anesthesiologist before surgery.
2.5. Anesthetic management
Duration of anesthesia was calculated from the beginning when the patient was intubated to the time when the patient was extubated after the reversal of the anesthetic. Surgery time was calculated from anesthesia induction to patient awakening. The total bleeding value was estimated by measuring the gram of absorbed bleeding in gauze and suctioned bleeding. When we used saline for washing, we subtracted the amount of saline from this estimation. Fraction of inspired oxygen (FiO2) was calculated by the following method; the sum of the total administered oxygen dose and the total administered air dose multiplied by 0.21 was divided by the sum of the total administered oxygen and air dose.
2.6. Definition of postoperative AE-ILD
Postoperative AE-ILD was diagnosed if all of the following criteria[12] were met:
-
(1)
acute worsening or development of dyspnea, typically within 1 month after surgery;
-
(2)
CT showing new ground-glass attenuation bilaterally and/or consolidation;
-
(3)
deterioration not fully explained by cardiac failure or fluid overload;
-
(4)
development of AE-ILD within 1 month after surgery; and exclusion of pulmonary infection (i.e., pneumonia that did not improve after administration of antibacterial therapy or no bacteria in a sputum culture).
2.7. Statistical analysis
The results are expressed as the median (range) or mean ± standard deviation. In univariable analyses, comparisons between two groups were made using the Student t-test or Mann–Whitney nonparametric U test as appropriate for continuous variables and Fisher's exact test for categorical variables. Univariate and multivariate logistic regression analyses were used to test the effect of variables potentially influencing the development of AE-ILD and provide adjusted estimations of the odds ratio. In addition, explanatory factors with P-value < .05 in the univariate logistic regression analysis were entered into the multivariate logistic regression analysis. Continuous variables identified as risk factors in the multivariate analysis were determined by their cut-off point for prediction of AE-ILD. The optimal cut-off point was calculated by the receiver operating characteristic (ROC) analysis using the Youden index. All tests were two-sided and a P-value < .05 was considered statistically significant. Each outcome was analyzed separately. All statistical analyses were performed using IBM SPSS statistical software (version 24.0, SPSS Inc, Armonk, NY, USA).
2.8. Ethics
The study protocol was approved by the Hiroshima University Institutional Review Board (No. E-1150) and conducted in accordance with the ethical standards established by the Helsinki Declaration of 1975.
3. Results
3.1. Patient characteristics
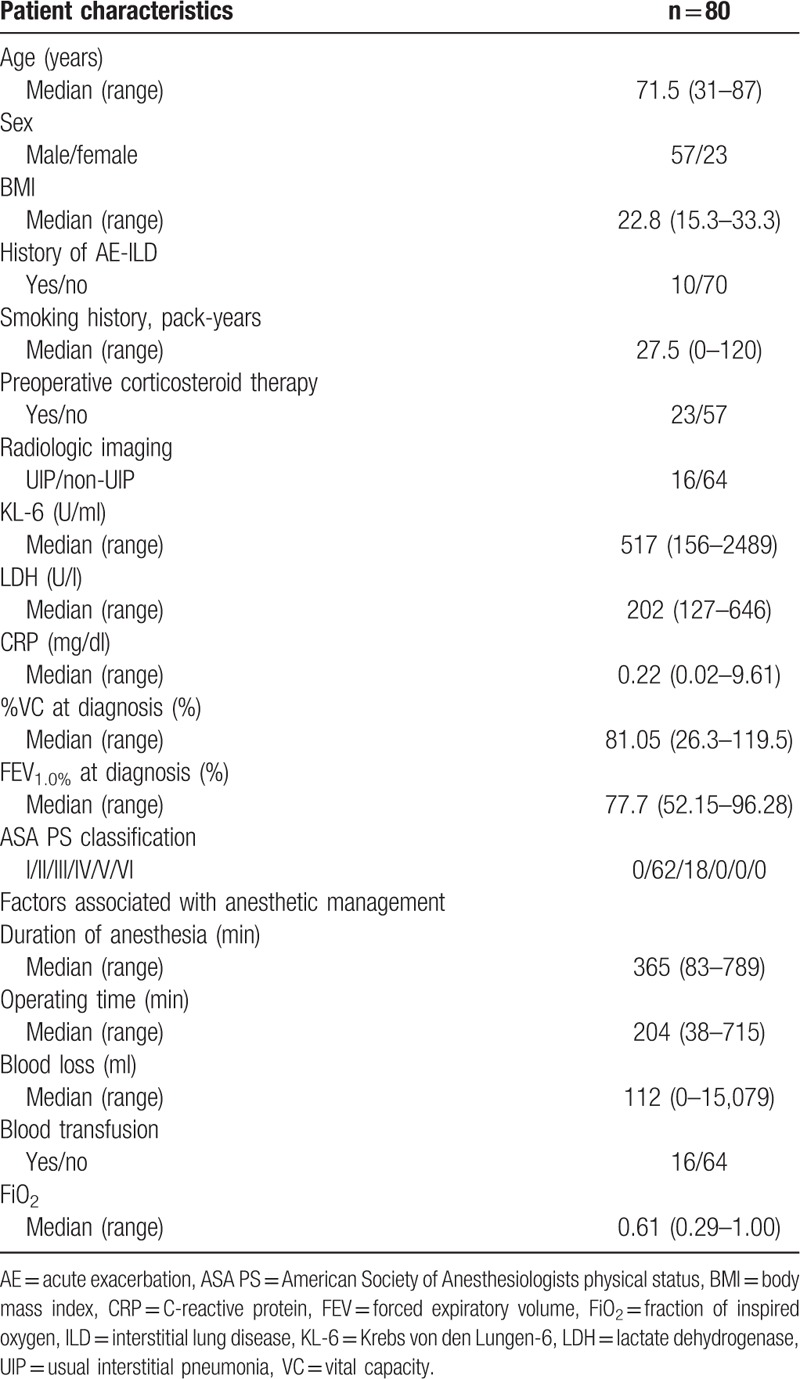
Eighty patients with ILD who underwent non-pulmonary organ surgery under general anesthesia were enrolled. The demographic and clinical characteristics of the patients are shown in Table 1. In this study, the proportion of older male smokers was large. Propofol was used as an intravenous anesthetic agent in all patients.
Table 1.
Patient characteristics and factors associated with anesthetic management.
3.2. Incidence of AE-ILD
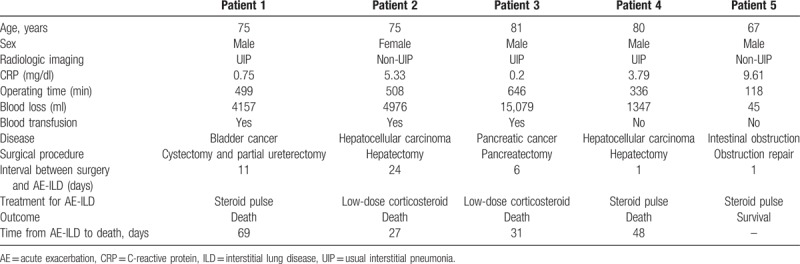
Five (6.3%) of the 80 patients developed AE-ILD postoperatively. The demographics of these 5 patients and their clinical details are shown in Table 2. AE-ILD developed within 10 days after surgery in 60% of cases. Four (80%) of the 5 patients died despite receiving steroid therapy.
Table 2.
Preoperative details of patients who developed acute exacerbation of interstitial lung disease.
3.3. Comparison of patients with and without AE-ILD
The patient characteristics and factors associated with anesthetic management are compared between patients with and without AE-ILD in Table 3. The preoperative CRP level and intraoperative blood loss were significantly higher in the patients who developed AE-ILD than in those who did not. The results of logistic regression analyses of potential risk factors for AE-ILD are shown in Tables 4 and 5. In the univariate analysis, the UIP pattern on CT images, a higher preoperative CRP level, longer operating time, high blood loss, and blood transfusion were significant risk factors for AE-ILD. In multivariate analysis, only a higher preoperative CRP level (odds ratio 2.556, 95% confidence interval 1.110–5.889, P = .028) was identified as an independent risk factor for AE-ILD after non-pulmonary surgery. In ROC analysis, CRP level ≥3.485 mg/dl was the best predictor of AE-ILD. The area under the ROC was 0.836 (95% confidence interval 0.648–1.000, P = .012). The cut-off point yielded 60.0% sensitivity and 87.0% specificity.
Table 3.
Comparison of demographics and factors associated with anesthetic management in patients with and without acute exacerbation of interstitial lung disease.
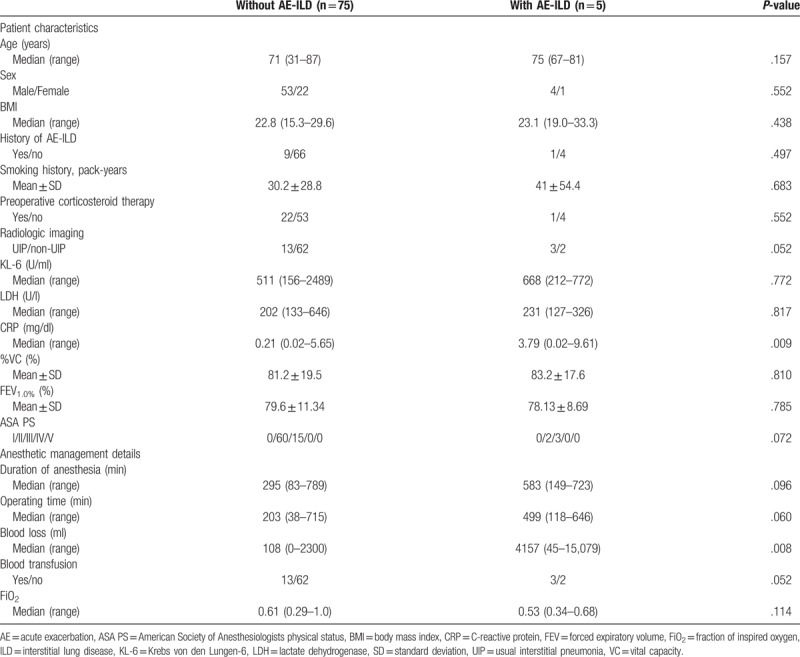
Table 4.
Univariate logistic regression analysis of potential risk factors for acute exacerbation of interstitial lung disease.
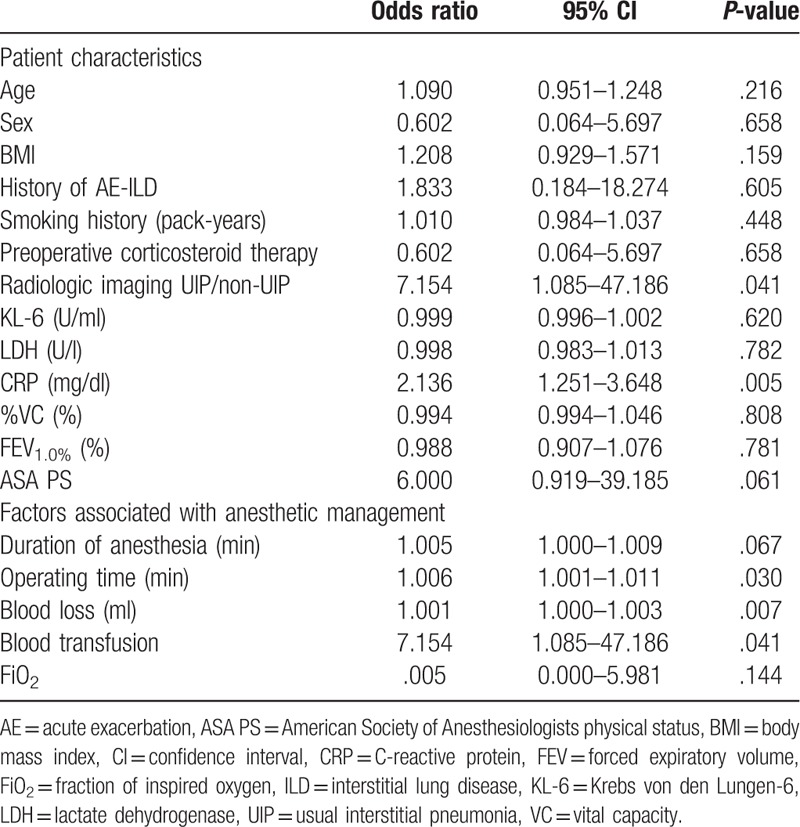
Table 5.
Multivariate logistic regression analysis of patient characteristics and factors associated with anesthetic management that are potential risk factors for acute exacerbation of interstitial lung disease.
4. Discussion
In this study, we identified a high preoperative CRP level as an independent risk factor for AE-ILD after non-pulmonary surgery. The rate of development of AE-ILD after surgery was 6.3% and the mortality rate was 80%. To the best of our knowledge, this is a first study to identify a risk factor for AE-ILD among patient characteristics in patients with ILD undergoing non-pulmonary surgery.
Several risk factors for AE-ILD that are unrelated to surgery, that is, poor lung function, rapid worsening of pulmonary function, and a high KL-6 level, have been reported in patients with ILD.[17] However, a high CRP level has not previously been identified as a risk factor for AE-ILD that are unrelated to surgery. One previous study showed that the CRP level was an independent risk factor for AE-ILD after thoracic surgery in patients with ILD,[18] and a high CRP level was reported to be a risk factor for AE-ILD after non-pulmonary surgery in univariate analysis in another study.[15] These observations indicate that a high CRP level could be a risk factor for AE-ILD after non-pulmonary surgery and that such patients should be monitored very carefully after surgery.
CRP is produced by hepatocytes in the liver in response to stimulation of proinflammatory cytokines. Therefore, a high preoperative CRP level would indicate inflammation due to ILD. Conversely, CRP, which is produced by alveolar macrophages, can induce secretion of several inflammatory cytokines, such as tumor necrosis factor alpha and interleukin (IL)-1 from macrophages.[19,20] IL-1 induces proliferative activity in fibroblasts, IL-6, and IL-8.[21] Given these observations, a high CRP level could induce the inflammation in ILD. Invasive surgery would cause further lung inflammation and AE-ILD in patients with ILD and a high CRP level. This mechanism may be the reason that a higher preoperative CRP level was identified as a risk factor for AE-ILD after non-pulmonary surgery in this study (Fig. 1).
Figure 1.
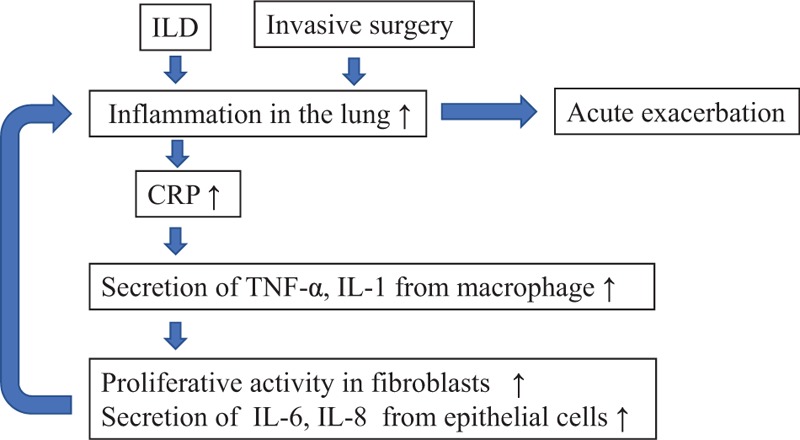
Hypothetical mechanism for AE-ILD after invasive non-pulmonary surgery in patients with high level of CRP. AE = acute exacerbation, CRP = C-reactive protein, IL = interleukin, ILD = interstitial lung disease, TNF-α = tumor necrosis factor-α.
A large-scale study by the Japan Respiratory Surgery Association/Study Group on Diffuse Pulmonary Disorders identified the following seven independent risk factors for AE-ILD after lung resection: a large pulmonary resection area, male sex, history of AE-ILD, preoperative use of steroids, a serum KL-6 level >1000 U/ml, a UIP pattern on CT imaging, and preoperative percent predicted vital capacity <80%.[11] In the present study, after exclusion of lung resection area, we examined whether the remaining six items were risk factors for AE-ILD after non-pulmonary surgery. However, none of these items were identified as independent risk factors for AE-ILD. We attribute this inconsistency in study findings to the difference of direct surgical invasion to lung between non-pulmonary and pulmonary surgery. The small number of subjects in our study could also have contributed to this discrepancy.
There is no established drug that can change the bleak prognosis of postoperative AE-ILD. Several studies have investigated whether administration of sivelestat sodium, macrolide antibiotics, or inhalation of N-acetylcysteine prevents postoperative AE-ILD. However, none of these treatments significantly decreased the risk of AE-ILD. There is even controversy regarding the effects of corticosteroids, which are the agents most frequently used to prevent AE-ILD.[22,23] In our study, there was no significant difference in the proportion of patients who received preoperative corticosteroid therapy between the groups with and without AE-ILD. Furthermore, in recent years, there have been studies of the ability of pirfenidone, an antifibrotic agent, to prevent postoperative AE-ILD in patients with IPF and lung cancer.[24,25] In our study, only one patient received pirfenidone before surgery, so it was not possible to perform a statistical analysis of the association between preoperative use of pirfenidone and postoperative AE-ILD.
This study had several limitations. First, it had a retrospective single-center design and a small sample size. Therefore, a prospective multicenter cooperative study is necessary in the future. Second, we could not analyze data on pulmonary ventilation during anesthesia. It has been reported that a large tidal volume during anesthesia is a risk factor for AE-ILD after thoracotomy,[26] but we could not collect the appropriate ventilation data because the tidal volume during anesthesia varies according to measurement timing. Third, AE-ILD is a clinical diagnosis that cannot be confirmed by pathologic examination, so it was not possible to discriminate AE-ILD from conditions such as heart failure and capillary leak syndrome.
In conclusion, the incidence of AE-ILD after non-pulmonary surgery was similar to that after lung resection surgery, and the prognosis was very poor. Furthermore, we identified the preoperative CRP level as an independent risk factor for AE-ILD after non-pulmonary surgery. Therefore, the risk of AE-ILD in patients with ILD and a high preoperative CRP level should be evaluated very carefully before surgery. Such patients should be monitored closely for development of AE-ILD after surgery.
Author contributions
Conceptualization: Takeshi Masuda.
Writing – original draft: Shun Takao, Takeshi Masuda.
Writing – review & editing: Takeshi Masuda, Kakuhiro Yamaguchi, Shinjiro Sakamoto, Yasushi Horimasu, Taku Nakashima, Shintaro Miyamoto, Hiroshi Iwamoto, Kazunori Fujitaka, Hironobu Hamada, Noboru Hattori.
Takeshi Masuda orcid: 0000-0003-3557-0049.
Footnotes
Abbreviations: AE = acute exacerbation, ASA = American Society of Anesthesiologists, CRP = C-reactive protein, CT = computed tomography, IL = interleukin, ILD = interstitial lung disease, IPF = idiopathic pulmonary fibrosis, UIP = usual interstitial pneumonia.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors have no conflicts of interest to declare.
References
- [1].King TE. Clinical advances in the diagnosis and therapy of the interstitial lung diseases. Am J Respir Crit Care Med 2005;172:268–79. [DOI] [PubMed] [Google Scholar]
- [2].Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Takenaka K, Yoshimura A, Okano T, et al. Acute exacerbation of idiopathic interstitial pneumonia complicated by lung cancer, caused by treatment for lung cancer. Jpn J Lung Cancer 1999;39:955–62. [Google Scholar]
- [4].Abe M, Tsushima K, Matsumura T, et al. Efficacy of thrombomodulin for acute exacerbation of idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia: a nonrandomized prospective study. Drug Des Devel Ther 2015;9:5755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tachikawa R, Tomii K, Ueda H, et al. Clinical features and outcome of acute exacerbation of interstitial pneumonia: collagen vascular diseases-related versus idiopathic. Respiration 2012;83:20–7. [DOI] [PubMed] [Google Scholar]
- [6].Song JW, Hong SB, Lim CM, et al. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J 2011;37:356–63. [DOI] [PubMed] [Google Scholar]
- [7].Kim DS, Park JH, Park BK, et al. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J 2006;27:143–50. [DOI] [PubMed] [Google Scholar]
- [8].Usui Y, Kaga A, Sakai F, et al. A cohort study of mortality predictors in patients with acute exacerbation of chronic fibrosing interstitial pneumonia. BMJ Open 2013;3:e002971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Raghu G, Nyberg F, Morgan G. The epidemiology of interstitial lung disease and its association with lung cancer. Br J Cancer 2004;91:S3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Omori T, Tajiri M, Baba T, et al. Pulmonary resection for lung cancer in patients with idiopathic interstitial pneumonia. Ann Thorac Surg 2015;100:954–60. [DOI] [PubMed] [Google Scholar]
- [11].Sato T, Teramukai S, Kondo H, et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg 2014;147:1604–11. [DOI] [PubMed] [Google Scholar]
- [12].Joo S, Kim DK, Sim HJ, et al. Clinical results of sublobar resection versus lobectomy or more extensive resection for lung cancer patients with idiopathic pulmonary fibrosis. J Thorac Dis 2016;8:977–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shintani Y, Ohta M, Iwasaki T, et al. Predictive factors for postoperative acute exacerbation of interstitial pneumonia combined with lung cancer. Gen Thorac Cardiovasc Surg 2010;58:182–5. [DOI] [PubMed] [Google Scholar]
- [14].Choi SM, Lee J, Park YS, et al. Postoperative pulmonary complications after surgery in patients with interstitial lung disease. Respiration 2014;87:287–93. [DOI] [PubMed] [Google Scholar]
- [15].Furuya K, Sakamoto S, Takai Y, et al. Acute exacerbation of idiopathic interstitial pneumonia after nonpulmonary surgery under general anesthesia. SVDLD 2017;34:156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].American Society of Anesthesiologists Clinical. Available from: https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system. Updated October 15, 2014 Accessed October 27, 2018. [Google Scholar]
- [17].Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An International Working Group Report. Am J Respir Crit Care Med 2016;194:265–75. [DOI] [PubMed] [Google Scholar]
- [18].Mizuno Y, Iwata H, Shirahashi K, et al. The importance of intraoperative fluid balance for the prevention of postoperative acute exacerbation of idiopathic pulmonary fibrosis after pulmonary resection for primary lung cancer. Eur J Cardiothorac Surg 2012;41:161–5. [DOI] [PubMed] [Google Scholar]
- [19].Dong Q, Wright JR. Expression of C-reactive protein by alveolar macrophages. J Immunol 1996;156:4815–20. [PubMed] [Google Scholar]
- [20].Galve-de Rochemonteix B, Wiktorowicz K, Kushner I, et al. C-reactive protein increases production of IL-1 alpha, IL-1 beta, and TNF-alpha, and expression of mRNA by human alveolar macrophages. J Leukoc Biol 1993;53:439–45. [DOI] [PubMed] [Google Scholar]
- [21].Standiford TJ, Kunkel SL, Basha MA, et al. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Invest 1990;86:1945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kondoh Y, Taniguchi H, Kawabata Y, et al. Acute exacerbation in idiopathic pulmonary fibrosis: analysis of clinical and pathologic findings in three cases. Chest 1993;103:1808–12. [DOI] [PubMed] [Google Scholar]
- [23].Chiyo M, Sekine Y, Iwata T, et al. Impact of interstitial lung disease on surgical morbidity and mortality for lung cancer: analyses of short-term and long-term outcomes. J Thorac Cardiovasc Surg 2003;126:1141–6. [DOI] [PubMed] [Google Scholar]
- [24].Iwata T, Yoshida S, Fujiwara T, et al. Effect of perioperative pirfenidone treatment in lung cancer patients with idiopathic pulmonary fibrosis. Ann Thorac Surg 2016;102:1905–10. [DOI] [PubMed] [Google Scholar]
- [25].Iwata T, Yoshino I, Yoshida S, et al. A phase II trial evaluating the efficacy and safety of perioperative pirfenidone for prevention of acute exacerbation of idiopathic pulmonary fibrosis in lung cancer patients undergoing pulmonary resection: West Japan Oncology Group 6711 L (PEOPLE Study). Respir Res 2016;17:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sakamoto S, Homma S, Mun M, et al. Acute exacerbation of idiopathic interstitial pneumonia following lung surgery in 3 of 68 consecutive patients: a retrospective study. Intern Med 2011;50:77–85. [DOI] [PubMed] [Google Scholar]


