Abstract
Several approved inactivated hepatitis A (HA) vaccines are available in Korea. These have been shown to be immunogenic and safe in European children; however, their immunogenicity and safety have not been investigated among Korean children. We aimed to compare the immunogenicity and safety of the most commonly used HA vaccines in ethnic Korean children aged 12 to 18 months.
In this open-label, randomized, prospective, multicenter study, 108 children were enrolled and randomized to receive a pediatric form of Avaxim, Epaxal, or Havrix. The 2nd dose was administered after an interval of 6 months. Anti-HA virus (HAV) immunoglobulin (Ig) G was measured to assess geometric mean concentrations (GMCs) and seropositvity rates (≥20 mIU/mL anti-HAV IgG). To assess safety, local solicited adverse events (AEs), systemic solicited AEs, unsolicited AEs, and serious AEs (SAEs) were graded.
Among the 108 participants enrolled, 37, 34, and 37 received Avaxim, Epaxal, and Havrix, respectively. After administration of 2 doses, the seropositivity rates in the Avaxim, Epaxal, and Havrix groups were all 100% (95% confidence intervals [CIs]: 99.0–100, 98.9–100, and 99.0–100, respectively; P < .001). The anti-HAV GMCs in the Avaxim, Epaxal, and Havrix groups were 5868.4 (95% CI: 4237.2–8126.6), 1962.1 (95% CI: 1298.0–2965.9), and 2232.9 mIU/mL (95% CI: 1428.4–3490.4), respectively, after administration of 2 doses (P < .001). There were no significant differences in the proportions of participants reporting local solicited AEs, systemic solicited AEs, unsolicited AEs, and SAEs among the 3 vaccine groups after the 1st and 2nd doses. All local solicited and unsolicited AEs were grade 1 or 2. Grade 3 systemic solicited AE occurred in 5.4% and 2.9% of the participants in the Havrix group after the 1st and 2nd doses, respectively. SAEs after the 1st and 2nd doses were reported in 2 participants and 1 participant, respectively, but none was assessed as being related to vaccination.
The results indicate that these vaccines were safe and immunogenic in ethnic Korean children. The results have contributed to the establishing of an HA vaccination policy in Korea and will be informative to countries that plan to initiate vaccination programs against HAV.
Keywords: child, comparison, hepatitis A vaccine, immunogenicity, safety
1. Introduction
Hepatitis A (HA) is caused by hepatitis A virus (HAV), an RNA virus and member of the Picornaviridae family. HA is mostly asymptomatic in children under 6 years of age. However, older children and adults commonly develop various symptoms, including jaundice, fever, fatigue, vomiting, and abdominal pain. Although rarely fatal, HA can cause fulminant hepatitis.[1–3] After experiencing HA, anti-HAV antibodies are induced, conferring life-long immunity.[1,2] HAV is transmitted by the fecal-oral route, and the incidence of HAV infection is strongly correlated with the socioeconomic status of the country.[1,2,4] High rates of HAV infection are found in low income countries with a lack of sanitation infrastructure and hygienic practices. Indeed, most children in these countries are exposed to HAV infection without symptoms and acquire life-long immunity. Economic growth of the country leads to major improvements in sanitation infrastructure and hygienic practices, thereby reducing the circulation of HAV. Lack of exposure to HAV during childhood has therefore yielded a large susceptible population, resulting in increased incidence of HA.[1,2]
With economic emergence, Korea has experienced large growth in the susceptible population and a progressive increase in the incidence of HA.[5,6] The seropositivity rates of HAV in individuals have decreased gradually from the 1980s to 2010s.[6,7] Outbreaks have been detected since the late 1990s, and the number of annual HA cases during the 1990s and 2000s has varied from 7000 to 54000, respectively, corresponding to approximately 0.01% to 0.03% of the total population in Korea.[5,7–9] Mostly adults have been affected during these epidemics; however, HAV infection among children has been common, and although these infections were often unrecognized, they were considered an important source of transmission within and between households.[5,7,10] Although HA does not result in chronic infection, as does hepatitis B,[11–13] HA has become a major public health concern.
Vaccinating children against HAV is the most effective strategy to reduce the occurrence of HA.[2,14] Several inactivated pediatric forms of HA vaccines, including Havrix 720 (GlaxoSmithKline, Rixensart, Belgium), Avaxim 80U Pediatrics (Sanofi Pasteur, Lyon, France), Epaxal Junior (Jassen Pharmaceutica, Beerse, Belgium), and Vaqta Paediatric (Merck & Co Inc, Kenilworth, NJ) were approved for use in Korea and became commercially available in 1997.[15] HA vaccination was included in the childhood voluntary vaccination program in 2008 and implemented in the national immunization program in 2015. Two doses are recommended, with the 1st dose given at 12 to 23 months of age and the 2nd dose given 6 months later.[15] The use of HA vaccines in the pediatric population has increased gradually from approximately 40% in 2008 to approximately 97% in 2017.[16,17] All of the approved HA vaccines have been reported to be immunogenic and safe according to studies conducted among European children and have been implemented in universal vaccination programs in several countries.[2,14,18–20] However, no studies have investigated the immunogenicity and safety of HA vaccines among Korean children. At the time these vaccines were approved, the regulations for testing of new vaccines were not strong in Korea, and conducting phase 3 clinical trials among Korean children was not mandatory. The decision regarding approval for new vaccines relied on the immunogenicity and safety profiles of developed countries and recommendations by the World Health Organization.[21] Certain ethnic groups have been shown to lack sufficient induction of immunity after hepatitis B or measles vaccination due to genetic variations[22–24]; however, this has not been reported for HA vaccination. Based on the assumption that HA vaccination is immunogenic regardless of ethnicity,[25,26] the HA vaccines were approved in Korea based on immunogenicity and safety study profiles of European children provided by manufacturers and described in the literature.[21]
Therefore, the purpose of this study was to compare 3 of the most commonly used licensed inactivated HA vaccines, that is, Avaxim, Epaxal, and Havrix, among healthy Korean children aged 12 to 18 months for immunogenicity and safety with regard to introducing these vaccines in the national immunization program. This was the 1st study to assess the immunogenicity and safety of HA vaccines among children with Korean ethnicity at vaccination recommended ages.
2. Methods
2.1. Study design and participants
This open-label, randomized, prospective, multicenter study was conducted from February 2012 to November 2013 in 3 hospitals affiliated with The Catholic University of Korea, Seoul, Republic of Korea (St. Vincent's Hospital, Seoul St. Mary's Hospital, and Incheon St. Mary's Hospital) and in Changwon Fatima Hospital. The study sites were secondary or tertiary hospitals. The study was advertised through a poster displayed at each study site. Healthy children at ages 12 to 18 months whose parents expressed interest in their children participating in the study and provided written informed consent were enrolled in study.
During the study period, 108 children were enrolled in the study. All study participants were indigenous Koreans, and none of the participants had parents or ancestors of foreign origins. During the 1st visit, medical history was taken, physical examinations were performed, and vaccination history was retrieved from the vaccination certificate booklet or National Immunization Registry System. Vaccinations were given only to eligible children. The following exclusion criteria were applied: had received vaccination against HA; had a history of HA; had an immunocompromised status or was on immunosuppressive therapy; had been administered blood products within 3 months; had received any investigational drug or vaccine 30 days before the study vaccine; had a history of hypersensitivity to any vaccine component, such as aluminum hydroxide; had a history of neurologic complications or thrombocytopenia after any vaccination; had acute illness 7 days prior to or at the time of visit; and had an axillary temperature of 37.5°C or more at the time of visit.
Participants received the 1st dose as a pediatric dosage of Avaxim (0.5 mL), Epaxal (0.25 mL), or Havrix (0.5 mL) randomly. Because this was an open-label study, investigators and parents or legal representatives of participants were aware of the allocation. The vaccine was administered intramuscularly in the deltoid muscle. Participants received a 2nd dose 6 months later under the same conditions.
2.2. Immunogenicity assessment
Blood samples were obtained prior to the 1st dose and at 4 to 6 weeks after the 2nd dose. The blood samples were immediately centrifuged and stored at −70°C until assayed. Antibody concentrations were quantified by electrochemiluminescence immunoassays using Elecsys Anti-HAV assays (Roche Diagnostics GmbH, Mannheim, Germany) on a Cobas 8000 e 602 analyzer (Roche Diagnostics GmbH) at Neodin Research Institute (Seoul, Korea). Anti-HAV immunoglobulin (Ig) G levels greater than or equal to 20 mIU/mL were considered seropositive.[27]
2.3. Safety assessment
All participants were monitored for any immediate adverse events (AEs) occurring within 30 minutes after each vaccination. Solicited local AEs (tenderness, erythema, and swelling) at the injection site and systemic AEs (fever) occurring within 7 days after each vaccination as well as unsolicited AEs occurring within 30 days after each vaccination were recorded by parents or legal representatives on a diary card. The intensity of solicited local AEs was assessed as follows: tenderness, grade 1 (grimace when injection site is touched), grade 2 (cries or protests when injection site is touched), grade 3 (cries when injected limb is moved or the movement of the injected limb is reduced); erythema and swelling, grade 1 (>0 to <10 mm), grade 2 (≥10 to <30 mm), and grade 3 (≥30 mm). The intensity of systemic AEs was assessed as follows: fever, grade 1 (>37.5°C to <38.5°C), grade 2 (≥38.5°C to <39.5°C), grade 3 (≥39.5°C). The intensity of unsolicited AEs was assessed as follows: grade 1 (no interference with daily activity), grade 2 (some interference with daily activity), grade 3 (significantly prevents daily activity). Serious AEs (SAEs) were defined as important medical events or events resulting in hospitalization, persistent or significant disability, life-threatening symptoms, or death. All local AEs were considered as related to the study vaccine. For systemic AEs, unsolicited AEs, and SAEs, the investigators assessed potential relationship to vaccination on an individual basis. The AEs were presented and graded accordance to the Medical Dictionary for Adverse Event published by Ministry of Korean Food and Drug Safety (MFDS), which was developed based on Medical Dictionary for Regulatory Activities (MedDRA). In this dictionary, unsolicited AEs were represented by the Systemic Organ Classes, which was directly derived from MedDRA version 13.0.[28,29] The definitions of terms are similar to those in Common Terminology Criteria for Adverse Event (CTCAE); however, unlike CTCAE's 5-point grading scale, the Medical Dictionary for Adverse Event has a 3-point grading scale.[28,30]
2.4. Statistical analysis
Statistical Package for the Social Science version 18.0 software (SPSS Inc, Chicago, IL) was used for data analysis. The sample size was determined based on funds and the assumption that 20% of patients would withdraw from the study; to achieve 80% power to rule out differences in seropositivity rates of >10% among vaccine groups using a 1-sided significance level of 0.05 for each type of vaccine, 108 participants were required. For assessment of immunogenicity, the per-protocol population was applied, and only participants who completed the study, received 2 doses of the study vaccine, and underwent blood sample collections prior to the 1st dose and at 4 to 6 weeks after the 2nd dose according to the study design were included. The seropositivity rates and anti-HAV geometric mean concentrations (GMCs) of anti-HAV IgG were calculated with 95% confidential intervals (CIs). Chi-squared tests were conducted to compare seropositivity rates among vaccine groups, and analysis of variance was conducted to compare GMCs among vaccine groups. For safety assessment, the intent-to-treat population was applied; all participants who received the respective vaccine dose and underwent safety evaluation were included. The proportions of participants reporting solicited local and systemic AEs, unsolicited AEs, and SAEs were calculated with 95% CIs. Chi-squared tests were performed to compare proportions of AEs among the vaccine groups. In this study, differences between vaccine groups were considered statistically significant if the P-value was <.05.
2.5. Ethics statement
This study was consistent with the ethical standards established by the Declaration of Helsinki and conducted in accordance with the International Conference on Harmonization guidelines for Good Clinical Practice as well as national regulations enforced by MFDS. The study protocol was approved by the Central Institutional Review Board of The Catholic Medical Center (XC12MIMV0023V). In accordance with the Pediatric Regulation of Clinical Trial in Korea, written informed consent was obtained from one of the parents or legal representatives of each participant prior to enrollment in the study. This randomized trial was registered at Clinical Research Information Service of Korean Centers for Disease Control and Prevention (number NCT00483470).
3. Results
3.1. Study population
Among the 108 participants enrolled in this study, 37, 34, and 37 participants were randomized into the Avaxim, Epaxal, and Havrix groups, respectively. The baseline characteristics of each group are shown in Table 1. Children in the 3 vaccine groups showed no significant differences in age (P = .992), height (P = .086), or weight (P = .858); however, there were more girls in the Havrix group (P = .034) than in the other groups. The flow chart for participant inclusion into the study and reasons for withdrawal are shown in Figure 1.
Table 1.
Baseline characteristics of participants according to vaccine group.
Figure 1.
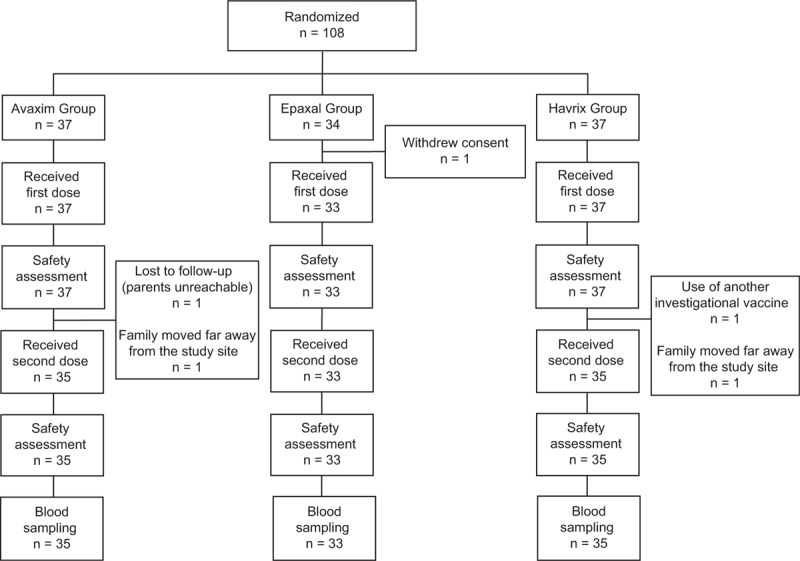
Flow chart of study participants.
3.2. Immunogenicity
The seropositivity rates and anti-HAV GMCs of the 3 vaccine groups at enrollment and at 4 to 6 weeks after the 2nd dose are summarized in Table 2. The proportions of seropositive participants at enrollment were 5.7%, 3.0% and 2.9% in the Avaxim, Epaxal, and Havrix groups, respectively. There were no significant differences in baseline seropositivity rates between the 3 vaccine groups (P = .787). After administration of 2 doses, the seropositivity rates in the Avaxim, Epaxal, and Havrix groups were all 100% (95% CIs: 99.0–100%, 98.9–100%, and 99.0–100%, respectively). Each vaccine group showed significant increases in the proportions of seropositive participants (each P < .001) after 2 doses. There were no significant differences in seropositivity rates between the 3 vaccine groups (P = .971) after 2 doses.
Table 2.
Seropositivity rates and GMCs of anti-HAV IgG at enrollment and after 2 doses.
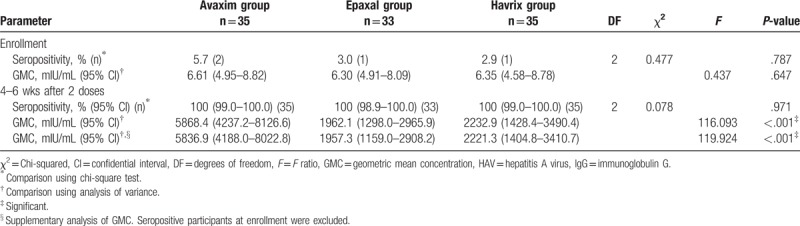
After administration of 2 doses, the GMCs the in Avaxim, Epaxal, and Havrix groups increased to 5868.4 (95% CI: 4237.2–8126.6), 1962.1 (95% CI: 1298.0–2965.9), and 2232.9 mIU/mL (95% CI: 1428.4–3490.4), respectively. Each vaccine group showed significant increases in GMCs (each P < .001). The anti-HAV GMC in the Avaxim group was significantly higher than those in the other 2 vaccine groups (P < .001).
The seropositivity at enrollment could be a confounding factor in GMC analysis after 2 doses; therefore, a supplementary analysis was performed by excluding the seropositive participants at enrollment. The GMCs were 5836.9 (95% CI: 4188.0–8022.8), 1957.3 (95% CI: 1159.0–2908.2), and 2221.3 (95% CI: 1404.8–3410.7) in the Avaxim, Epaxal, and Havrix group, respectively. The supplementary analysis showed that the anti-HAV GMC in the Avaxim group was significantly higher than those in the other 2 vaccine groups (P < .001).
3.3. Safety
There were no immediate AEs reported within 30 minutes after any vaccination. No AEs led to withdrawal from the study, and no SAEs led to persistent disability, life-threatening conditions, or death. Solicited local and systemic AEs, unsolicited AEs, and SAEs occurring after the 1st and 2nd doses are summarized in Tables 3 and 4. The proportions of participants who reported at least 1 solicited local AE after the 1st dose were 18.9%, 24.2%, and 37.8% in the Avaxim, Epaxal, and Havrix groups, respectively. The proportions of participants who reported at least 1 solicited local AE after the 2nd dose were 8.6%, 12.2%, and 17.1% in the Avaxim, Epaxal, and Havrix groups, respectively. There were no significant differences between the 3 vaccine groups after each dose (P = .129 and .535, respectively). Tenderness was the most common solicited local AE in all 3 vaccine groups after the 1st and 2nd doses. All solicited local AEs occurred within 3 days after vaccination, were grade 1 or grade 2 in intensity, and were resolved within 3 days after vaccination.
Table 3.
Proportions of participants reporting solicited local AEs, solicited systemic AEs, unsolicited AEs, and SAEs after the 1st dose in the safety analysis set.
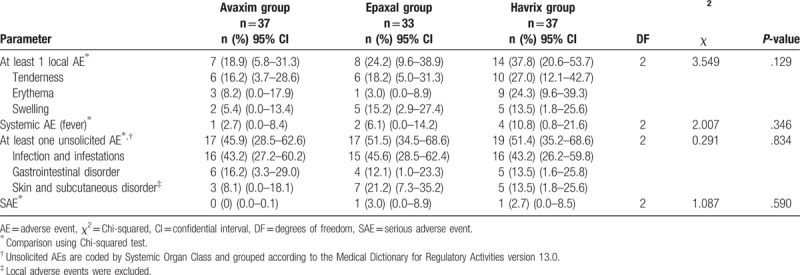
Table 4.
Proportions of participants reporting solicited local AEs, solicited systemic AEs, unsolicited AEs, and SAEs after the 2nd dose in the safety analysis set.
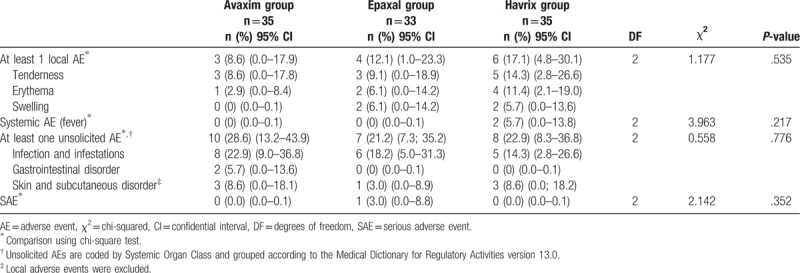
The proportions of participants reporting solicited systemic AEs after the 1st dose were 2.7%, 6.1%, and 10.8% in the Avaxim, Epaxal, and Havrix groups, respectively. The proportions of participants reporting solicited systemic AEs after the 2nd dose were 0%, 0%, and 5.7% in the Avaxim, Epaxal, and Havrix groups, respectively. There were no significant differences between the 3 vaccine groups after each dose (P = .346 and .217, respectively). Among the four participants (10.8%) who reported solicited systemic AEs in the Havrix group after the 1st dose, 2 participants (5.4%) reported AEs of grade 3 in intensity. Among the 2 participants (5.7%) who reported solicited systemic AEs in the Havrix group after the 2nd dose, one participant (2.9%) reported an AE of grade 3 in intensity. Including the cases of grade 3 AEs, all solicited systemic AEs were not related to vaccination. Moreover, all solicited systemic AEs occurred within 3 days after vaccination and resolved within 4 days after vaccination.
The proportions of participants experiencing at least one unsolicited AE after the 1st dose were 45.9%, 51.5%, and 51.4% in the Avaxim, Epaxal, and Havrix groups, respectively. The proportions of participants experiencing at least one unsolicited AE after the 2nd dose were 28.6%, 21.2%, and 22.9% in the Avaxim, Epaxal, and Havrix groups, respectively. There were no significant differences between the 3 vaccine groups after each dose (P = .834 and .776, respectively). Infection and infestations were the most frequently reported unsolicited AEs in all 3 vaccine groups after the 1st and 2nd doses. Unsolicited AEs were mainly common childhood diseases, including nasopharyngitis, herpangina, bronchiolitis, acute otitis media, acute gastroenteritis, and urticaria; none were assessed as related to vaccination by the investigator. All unsolicited AEs were grade 1 or grade 2, occurred within 3 days after vaccination, and resolved within 7 days after vaccination.
The proportions of participants reporting SAEs after the 1st dose were 0%, 3.0% (1 participant), and 2.7% (1 participant) in the Avaxim, Epaxal, and Havrix groups, respectively. The proportions of participants reporting SAEs after the 2nd dose were 0%, 3.0% (1 participant), and 0% in the Avaxim, Epaxal, and Havrix groups, respectively. There were no significant differences between the 3 vaccine groups after each dose (P = .590 and .352, respectively). Participants who exhibited SAEs in the Epaxal and Havrix groups after the 1st dose were confirmed to have viral gastroenteritis and viral bronchiolitis, respectively. The participant who exhibited an SAE in the Epaxal group after the 2nd dose experienced viral pneumonia. All SAEs were considered not related to vaccination by the investigator. There were no cases in which vaccination was associated with neurologic or viscerotropic diseases.
4. Discussion
In this study, the immunogenicity and safety of 3 inactivated HA vaccines, that is, Avaxim, Epaxal, and Havrix, were investigated among Korean children aged 12 to 18 months. No safety concerns were found in the 3 vaccine groups. After 2 doses, all seronegative participants converted to seropositive, and GMCs were sufficiently high to induce immunity against HAV infection in all 3 vaccine groups. These findings were consistent with the findings of studies conducted among European children.[18–20]
Differences in vaccine-induced immune responses in different ethnic groups were noted in previous studies, and the manufacturers or the failures of the cold chain were found to be not responsible for variations in these cases.[22–24] According to a study conducted by Hsu et al in Taiwan, the proportion of vaccine nonresponders was significantly higher in aboriginal children than in ethnic Han Chinese children following hepatitis B vaccination.[20] Additionally, in a study conducted by Voigt et al, in comparison with African-American children, Caucasian children showed significantly lower antibody titers after measles vaccination, and a higher vaccine failure rate was assumed in this group.[23,24] Differences in immune genes are known to be associated with disparities in immune responses after hepatitis B or measles vaccinations.[22–24] Although immunogenicity after HA vaccination has not been evaluated in all children worldwide, disparities in antibody responses after HA vaccination between different ethnic groups have not been reported. Therefore, HA vaccination-induced immunogenicity is considered to be consistent across races and ethnic groups.[25,26] The results of this study supported this hypothesis and demonstrated acceptable immunogenicity and safety of Avaxim, Epaxal, and Havrix among Korean children aged 12 to 18 months. Accordingly, these results were influential for initiating a national immunization program against HA in 2015.
Based on the results of this study, Avaxim, Epaxal, and Havrix, which were the most commonly used inactivated HA vaccines during the study period, were planned to be incorporated into national immunization program and fully reimbursed by government. However, distribution of Epaxal was discontinued in 2014 in Korea due to quality issue associated with the product manufacturer: traces of iron oxide particles were confirmed as being released from the manufacturing facilities.[21] Vaqta was incorporated into the national immunization program instead of Epaxal in 2015, and Avaxim, Epaxal and Vaqta are currently available.[15]
In our immunogenicity assessment after 2 doses, the GMCs were significantly higher in the group receiving Avaxim. In the supplementary analysis, the group that received Avaxim had significantly higher GMCs even after excluding participants who were seropositive at enrollment due to their possible confounding role in the comparison. These result were consistent with the results of previous studies conducted in China and Chile; Li et al and Abarca et al reported that children who received 2 doses of Avaxim exhibited significantly higher GMCs than children who received 2 doses of Havrix.[31,32] The factors influencing the generation of anti-HAV antibodies are unclear; however, Avaxim is known to exhibit faster kinetics, and higher proportions of vaccine recipients achieve seroprotective antibody titers at an earlier time after vaccination than in those who receive Epaxal and Havrix.[20]
All 3 vaccines were clinically safe and showed similar results in safety assessment. There were no significant differences in the proportions of participants exhibiting solicited local AEs, systemic AEs, unsolicited AEs, and SAEs between the 3 vaccine groups after the 1st and 2nd doses. Most of the AEs were grade 1 or grade 2, and no grade 3 AEs or SAEs were related to vaccination. Fever is a frequent systemic AE after most vaccinations, but is rare at any age following HA immunization.[33] In this study, no cases of fever were assessed as related to vaccination, and such cases may have been an indication of an underlying condition, for example, epidemic infectious diseases. The adjuvant used in the HA vaccine, aluminum hydroxide, is known to be associated with local AEs, such as tenderness, erythema, and swelling.[34] Avaxim and Havrix are aluminum-adsorbed vaccines, but have different quantities of aluminum. Avaxim contains 0.3 mg aluminum hydroxide in 0.5 mL (pediatric dose), whereas Havrix contains 0.5 mg aluminum hydroxide in 0.5 mL (pediatric dose).[15] Epaxal is an aluminum-free virosomal vaccine in which an immunopotentiating reconstituted influenza virosome was used as an alternative to aluminum hydroxide.[34] Despite differences in adjuvants, there were no significant differences in the proportions of participants reporting local AEs in the 3 vaccine groups.
Korea was a low-income country until the mid 1970s and was also a highly endemic region in which more than 90% of the population became immune to HAV infection by the age of 10 years. Indeed, most people in Korea have experienced HA infection as an asymptomatic infection in their childhood, and reports of clinical disease are uncommon.[5,7] The economy of Korea exhibited dramatic growth from the late 1970s, with increased access to clean water, proper sanitation, and improved hygiene practices, resulting in a decreased incidence of HA. The decreased incidence of HA has resulted in an increase in the population of individuals who have never encountered HA infection and are thus susceptible to HA.[5–7] The seropositivity rates of HAV in individuals 10–19, 20–29, and 30–39 years of age were 87%, 96%, and 100%, respectively, in 1980, decreased to 12%, 74%, and 95%, respectively, in 1995, and decreased further to lower than 10%, lower than 20%, and lower than 70%, respectively, in 2007.[6] These changes in seroepidemiology resulted in epidemic shifting to older age groups; in the late 1990s, outbreaks occurred among individuals 20 to 29 years of age, whereas epidemics were expanded to individuals 30 to 39 years of age in the 2000s and individuals 40 to 49 years of age in the 2010s.[5,7]
To reduce the incidence of HA, HA vaccination was implemented in the childhood national immunization program in 2015: 2 doses administered between the ages of 1 and 2 years.[15] This approach was taken because although infections among children are asymptomatic, children have been reported to be an important source of transmission and to contribute to community epidemics.[10,35] Additionally, in countries in which HA vaccination has been adopted into the universal vaccination program, remarkable reductions in disease incidence have been observed, not only among vaccinated children but also among older children and adults, probably due to the herd immunity effect.[36] Catch-up vaccination for older children and adults has not been introduced,[15] and the vaccination coverage among children aged 1 and 2 years in Korea was estimated to be higher than 90% in 2016 and 2017.[17] A significant decrease in the incidence of HA has not been observed; 6806 cases were reported in 2016 and 6522 cases were reported in 2017, and mostly adults older than 30 years were affected.[8] The high proportion of susceptible population among adults and increased detection and reporting by physicians due to strengthened surveillance may account for the high incidence of reported HA. It is still too early to evaluate impact of universal HA vaccination. In this study, 5.7%, 3.0%, and 2.9% of participants were seropositive in the Avaxim, Epaxal, and Havrix groups, respectively, at enrollment. Mostly maternal antibodies decay within 6 to 12 months of age,[37] and these children could have had unrecognized HAV infection, suggesting that childhood infection still occurs in Korea. Without implementation of a universal vaccination program, subsequent transmission by these children is more likely to occur.
The HA outbreaks have been reported worldwide; however, HA is major public concern, particularly in developing nations in the Middle East and in Southeast Asia. These countries have attained middle income economic status concomitant with continued improvement in sanitation facilities and hygiene practice. Therefore, as HAV infection in childhood has decreased, the pool of susceptible adolescents and young adults has increased.[38,39] Person to person transmission still accounts for many HA cases, and outbreaks associated with foods (e.g., shellfish, frozen strawberries) are often reported due to the large number of susceptible individuals.[40,41] HA vaccination has not been introduced or promoted as universal vaccination in many of countries in the Middle East and in Southeast Asia,[42] and the results of this study will be informative for establishing strategies to reduce HA incidence in these countries.
Despite the importance of our findings, our study had several limitations. First, the number of participants was small. Second, we did not investigate the immunogenicity and safety of another inactivated HA vaccine, Vaqta, which has also been incorporated into the HA national immunization program. Third, although according to previous studies, the immunity generated by 2 doses of the inactivated vaccines persists for at least 10 years,[43] the persistence of immunity has not been investigated in Korea. Thus, further studies, including larger numbers of participants and studies comparing the immunogenicity and safety of HA vaccines including Vaqta are needed. Long-term studies following up the persistence of immunity in vaccine recipients are also needed. Furthermore, to evaluate the impact of universal vaccination on trends in the incidence of HA, HA incidence should be examined in different age groups and in vaccine recipients/nonrecipients over time.
5. Conclusion
The aim of this study was to compare the immunogenicity and safety of 3 approved inactivated HA vaccines, that is, Avaxim, Epaxal, and Havrix, among Korean children aged 12 to 18 months. These vaccines have proven safe and immunogenic in European children; however, no data have been reported among Korean children. This open-label, randomized, prospective, multicenter study was the 1st study to assess the immunogenicity and safety of these vaccines among Korean children with the aim of considering incorporation of these vaccines into the national immunization program. No safety concerns were identified, and all 3 vaccines showed similar safety profiles. Although the anti-HAV GMCs were significantly higher in participants receiving Avaxim, participants receiving all 3 vaccines induced sufficient immunity to protect against HAV infection. These results indicated that the 3 vaccines were safe and immunogenic in ethnic Korean children and suitable for including in a national immunization program. The results have contributed to establishing an HA vaccination policy in Korea and will also be informative to countries concerned with the rise of HA incidence that plan to initiate voluntary or universal vaccination programs against HAV.
5.1. Availability of data and materials
The data that support the findings of this study are available from the Research Management Systems of MFDS (http://rnd.mfds.go.kr/), but the webpage is only available in the Korean language.
Author contributions
Conceptualization: Jong-Hyun Kim.
Data curation: Sang Hyuk Ma, Soo Young Lee, Seung Beom Han, Jin Han Kang.
Formal analysis: Kyung-Hyo Kim.
Funding acquisition: Jong-Hyun Kim.
Resources: Kyung-Hyo Kim.
Supervision: Jong-Hyun Kim.
Writing – original draft: Seung Soo Hong, Ui Yoon Choi.
Writing – review & editing: Ui Yoon Choi.
Footnotes
Abbreviations: AE = adverse event, CI = confidential interval, CTCAE = Common Terminology Criteria for Adverse Event, GMC = geometric mean concentration, HA = hepatitis A, HAV = hepatitis A virus, Ig = immunoglobulin, MedDRA = Medical Dictionary for Regulatory Activities, MFDS = Ministry of Korean Food and Drug Safety, SAE = serious adverse event.
This study was supported by the Ministry of Korea Food and Drug Safety under grant number 12172MFDS307.
The authors have no conflicts of interest to disclose.
References
- [1].World Health Organization. WHO position paper on hepatitis A vaccines - June 2012. Wkly Epidemiol Rec 2012;87:261–76.22905367 [Google Scholar]
- [2].Nothdurft HD. Hepatitis A vaccines. Expert Rev Vaccines 2008;7:535–45. [DOI] [PubMed] [Google Scholar]
- [3].Epidemiology and Prevention of Vaccine-Preventable Diseases: Hepatitis A. Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/vaccines/pubs/pinkbook/hepa.html Accessed April 18, 2018. [Google Scholar]
- [4].Gallone MF, Desiante F, Gallone MS, et al. Serosurveillance of hepatitis A in a region which adopted the universal mass vaccination. Medicine (Baltimore) 2017;96:e5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Moon S, Han JH, Bae GR, et al. Hepatitis A in Korea from 2011 to 2013: current epidemiologic status and regional distribution. J Korean Med Sci 2016;31:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lee H, Cho HK, Kim JH, et al. Seroepidemiology of hepatitis A in Korea: changes over the past 30 years. J Korean Med Sci 2011;26:791–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Korean Centers for Disease Control and Prevention. Changing patterns of hepatitis A virus infection in Korea. Public Health Wkly Rep 2008;1:169–72. [Google Scholar]
- [8].Healthcare Bigdata Hub. Health Insurance Review and Assessment Service. Available at: http://www.hira.or.kr/main.do Accessed August 23, 2018. [Google Scholar]
- [9].Population statistics. Ministry of the Interior and Safety. Available at: http://www.mois.go.kr/frt/sub/a05/totStat/screen.do Accessed April 18, 2018. [Google Scholar]
- [10].Seo JY, Choi BY, Ki M, et al. Risk factors for acute hepatitis A infection in Korea in 2007 and 2009: a case-control study. J Korean Med Sci 2013;28:908–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lu IC, Jean MC, Lin CW, et al. Predictive factors for anti-HBs status after 1 booster dose of hepatitis B vaccine. Medicine (Baltimore) 2016;95:e5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Du X, Liu Y, Ma L, et al. Virological and serological features of acute hepatitis B in adults. Medicine (Baltimore) 2017;96:e6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Das M, Vanar V, Martin DK, et al. Seroconverting nonresponder of high-dose intramuscular HBV vaccine with intradermal HBV vaccine: a case report. Medicine (Baltimore) 2017;96:e8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Andre F, Van Damme P, Safary A, et al. Inactivated hepatitis A vaccine: immunogenicity, efficacy, safety and review of official recommendations for use. Expert Rev Vaccines 2002;1:9–23. [DOI] [PubMed] [Google Scholar]
- [15].Kim KH. The Korean Pediatric Society. Hepatitis A vaccine. Immunization Guideline 8th ed.Seoul: The Korean Pediatric Society; 2015. 216–25. [Google Scholar]
- [16].Kim JH. Recent epidemiological status and vaccination of hepatitis A in Korea. J Korean Med Assoc 2008;51:110–8. [Google Scholar]
- [17].Korean Centers for Disease Control and Prevention. National vaccination coverage among children aged 1-3 years in the Republic of Korea, 2017. Public Health Wkly Rep 2018;11:1410–6. [Google Scholar]
- [18].Van Der Wielen M, Vertruyen A, Froesner G, et al. Immunogenicity and safety of a pediatric dose of a virosome – adjuvanted hepatitis A vaccine: a controlled trial in children aged 1-16 years. Pediatr Infect Dis J 2007;26:705–10. [DOI] [PubMed] [Google Scholar]
- [19].Dagan R, Amir J, Livni G, et al. Concomitant administration of a virosome – adjuvanted hepatitis a vaccine with routine childhood vaccines at age twelve to fifteen months: a randomized controlled trial. Pediatr Infect Dis J 2007;26:787–93. [DOI] [PubMed] [Google Scholar]
- [20].Vidor E, Dumas R, Porteret V, et al. Aventis Pasteur vaccines containing inactivated hepatitis A virus: a compilation of immunogenicity data. Eur J Clin Microbiol Infect Dis 2004;23:300–9. [DOI] [PubMed] [Google Scholar]
- [21].Korean Centers for Disease Control and Prevention. Introduction of hepatitis A vaccine into national immunization program in Korea. Public Health Wkly Rep 2015;8:759–60. [Google Scholar]
- [22].Hsu LC, Lin SR, Hsu HM, et al. Ethnic differences in immune response to hepatitis B vaccine. Am J Epidemiol 1996;143:718–24. [DOI] [PubMed] [Google Scholar]
- [23].Voigt EA, Ovsyannikova IG, Haralambieva IH, et al. Genetically defined race, but not sex, is associated with higher humoral and cellular immune response to measles vaccination. Vaccine 2016;34:4913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Haralambieva IH, Ovsyannikova IG, Umlauf BJ, et al. Genetic polymorphisms in host antiviral genes: associations with humoral and cellular immunity to measles vaccine. Vaccine 2011;29:8988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McMahon BJ, Williams J, Bulkow L, et al. Immunogenicity of an inactivated hepatitis A vaccine in Alaska native children and native and non-native adults. J Infect Dis 1995;171:676–9. [DOI] [PubMed] [Google Scholar]
- [26].Newcomer W, Rivin B, Reid R, et al. Immunogenicity, safety and tolerability of varying doses and regimens of inactivated hepatitis A virus vaccine in Navajo children. Pediatr Infect Dis J 1994;13:640–2. [DOI] [PubMed] [Google Scholar]
- [27].Robbins DJ, Krater J, Kiang W, et al. Detection of total antibody against hepatitis A virus by an automated microparticle enzyme immunoassay. J Virol Methods 1991;32:255–63. [DOI] [PubMed] [Google Scholar]
- [28].Ministry of Korean Food and Drug Safety. Medical Dictionary for Drug Adverse Event, 1st ed. Cheongju (KR): Ministry of Korean Food and Drug Safety; 2013. [Google Scholar]
- [29].Release 2.2 based on MedDRA version 13.0. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Available at: http://www.ich.org/search.html?id=192&q=MedDRA+version+13 Accessed November 30, 2018. [Google Scholar]
- [30].Common Terminology Criteria for Adverse Events. National Cancer Institute. Available at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm Accessed November 30, 2018. [Google Scholar]
- [31].Li RC, Li Y, Yi N, et al. An open, prospective, randomized study comparing the immunogenicity and safety of two inactivated hepatitis A pediatric vaccines in toddlers, children and adolescents in China. Pediatr Infect Dis J 2013;32:e77–81. [DOI] [PubMed] [Google Scholar]
- [32].Abarca K, Ibanez I, Perret C, et al. Immunogenicity, safety, and interchangeability of two inactivated hepatitis A vaccines in Chilean children. Int J Infect Dis 2008;12:270–7. [DOI] [PubMed] [Google Scholar]
- [33].Tapiainen T, Heininger U. Fever following immunization. Expert Rev Vaccines 2005;4:419–27. [DOI] [PubMed] [Google Scholar]
- [34].Jain H, Kumavat V, Singh T, et al. Immunogenicity and safety of a pediatric dose of a virosomal hepatitis A vaccine in healthy children in India. Hum Vaccin Immunother 2014;10:2089–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Venczel LV, Desai MM, Vertz PD, et al. The role of child care in a community – wide outbreak of hepatitis A. Pediatrics 2001;108:E78. [DOI] [PubMed] [Google Scholar]
- [36].Dagan R, Leventhal A, Anis E, et al. Incidence of hepatitis A in Israel following universal immunization of toddlers. JAMA 2005;294:202–10. [DOI] [PubMed] [Google Scholar]
- [37].Niewiesk S. Maternal antibodies: clinical significance, mechanism of interference with immune response, and possible vaccination strategies. Front Immunol 2014;5:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tufenkeji H. Hepatitis A shifting epidemiology in the Middle East and Africa. Vaccine 2000;18Suppl 1:S65–7. [DOI] [PubMed] [Google Scholar]
- [39].Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine 2010;28:6653–7. [DOI] [PubMed] [Google Scholar]
- [40].David AM. Steering Committee for Prevention and Control of Infectious Diseases. Hepatitis A outbreaks-methods of intervention in South-East Asian countries. Int J Infect Dis 2004;8:201–9. [DOI] [PubMed] [Google Scholar]
- [41].Severi E, Vennema H, Takkinen J, et al. Hepatitis A outbreaks. Lancet Infect Dis 2015;15:632–4. [DOI] [PubMed] [Google Scholar]
- [42].WHO vaccine-preventable diseases: monitoring system. 2017 global summary. World Health Organization. Available at: apps.who.int/immunization_monitoring/globalsummary Accessed April 18, 2018. [Google Scholar]
- [43].Bian GL, Ma R, Dong HJ, et al. Long-term clinical observation of the immunogenicity of inactivated hepatitis A vaccine in children. Vaccine 2010;28:4798–801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the Research Management Systems of MFDS (http://rnd.mfds.go.kr/), but the webpage is only available in the Korean language.


