Abstract
Rationale:
Thyroglobulin (Tg) is an accurate indicator of clinical outcome after total thyroidectomy in patients with differentiated thyroid carcinoma. Usually, Tg levels agree with whole body scan. However, in some patient, discordant results were found, often because of Tg immunoassay interference. Several reports indicated that 2-site immunoassay interference with heterophile antibodies (HAb) can lead to misinterpretation of the laboratory test result.
Patient concerns:
We report a case of a 46-year-old woman referred to our endocrine clinic for markedly increased calcitonin (CT) without the associated clinical picture. The measurement was repeated with the same patient sample on a different analytical platform and the result was an undetectable CT level. The measurement of Tg was repeated on 3 different analytical platforms using chemiluminescence and electrochemiluminescence immunoassays and the results were different on each platform. HAb blocking tubes resulted in a different level of both CT and Tg, suggesting the presence of a heterophile substance in the serum sample. Further characterization showed reactivity to several animal species antibodies and an elevated level of the rheumatoid factor (RF).
Diagnoses:
She was diagnosed as papillary thyroid carcinoma.
Interventions:
She had undergone thyroidectomy with lymph node dissection and radioactive therapy.
Outcomes:
She was found not to have recurrence despite a high serum Tg level.
Lessons:
Our report illustrates a rare case of falsely elevated tumor markers levels due to assay interference caused by RF. This finding pointed out the importance of close communication between the clinician and laboratory staff in order to bring to light discordance between laboratory test results and clinical picture and avoid unnecessary diagnostic procedures and overtreatment.
Keywords: calcitonin, heterophile antibodies, immunoassay, rheumatoid factor, thyroglobulin
1. Introduction
Anti-immunoglobulin antibodies able to interfere with immunometric assays include anti-human (Rheumatoid factor [RF]) and anti-animal (Heterophile antibodies [HAb]). HAb are found in 30% to 40% of all serum samples and may develop after exposure to animal immunoglobulins. Fortunately, these naturally occurring weak antibodies lead to immunoassay interference in less than 0.05% of the cases. However, this event may have serious clinical consequences, exposing patients to unnecessary investigations and inappropriate therapeutic options.[1] RF is a human anti-human immunoglobulin directed towards the Fc part of human immunoglobulins. The driver of the production of RF is often represented by infecting microorganisms coated with host IgG and about 70% of rheumatoid arthritis (RA) patients have increased RF levels. Of note, RF present in healthy subjects may be different from RF found in RA patients and is more reactive with self-antigens such as thyroglobulin and insulin.[2]
We describe an uncommon case of interference in immunoassays by RF in a woman with differentiated thyroid carcinoma (DTC) without RA history. Because of discordant thyroglobulin (Tg) values and clinical picture, we evaluated potential interference by HAbs.
2. Case report
A 46-years-old woman had a diagnosis of multinodular goiter in 2002 (Fig. 1). Ten years later, CT was tested with a very high result (315 pg/ml). This value was confirmed by a second measurement, so a fine-needle aspiration biopsy (FNAB) was performed. Cytology specimens showed papillary thyroid carcinoma (PTC), tall cell variant, confirmed by 2 different FNABs, and immunocytochemical analysis. A total thyroidectomy was scheduled. The histological examination evidenced multicentric papillary carcinoma, oncocytic type, with negative immunohistochemistry for CT, neuron-specific enolase and chromogranin A. Massive metastases from PTC in 2/12 isolated lymph nodes were discovered, too. The hypothesis of neuroendocrine neoplasia was, therefore, excluded and the patient underwent radioiodine treatment (dose = 163.57 mCi).
Figure 1.
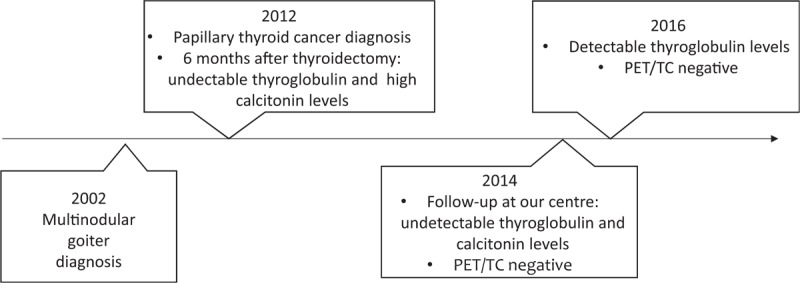
Timeline of milestones related to diagnosis and intervention.
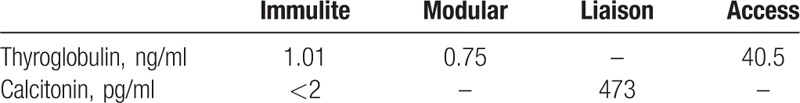
Six months later, Tg assay was negative, whereas increased values of calcitonin (CT) and CEA were detected. Subsequently, the patient was referred to our center and we tested a panel of 4 tumor markers, finding repeatedly high values (CEA ng/ml 10.1, Ca19-9 4696 U/ml, Ca125 60.7 U/ml, gastrin 472 pg/ml). Unexpectedly, basal and calcium gluconate-stimulated CT values performed on Immulite 2000 (Siemens, NJ) were undetectable in our laboratory. Two other CT measurements in external laboratories, one on Liaison (Diasorin Inc., MN) platform, showed high values. PET/TC study showed no pathological uptake. Technetium-99m-labeled octreotide acetate scintigraphy showed no abnormal uptake. A neck ultrasound was negative. At our center, basal Tg, rhTSH-stimulated Tg and TgAb measurement performed on a fully-automated Modular platform (Roche Diagnostic, Meylan, France) and, then, repeated every 6 to 12 months were negative. CT levels remained also undetectable. In May 2016, Tg measurement performed on ACCESS instrument (Beckman Coulter Inc., Brea, CA) showed a value of 40.5 ng/ml. This positive value was confirmed by 2 subsequent tests on the same platform (43 ng/ml and 56 ng/ml). A neck ultrasound was negative and rhTSH-stimulated Tg assay showed no increase in time as so as 131I whole body scan showed no abnormal uptake. Tg retested on Modular platform was 0.75 ng/ml and on Immulite 2000 system 1.01 ng/ml. The disagreement between Tg and CT level obtained by different methods (Table 1), the lack of rising in Tg level after rh-TSH stimulation, as well as the discrepancy between the clinical and the biochemical observation, led us to suspect a laboratory error. Interference by HAb may lead to false increases in Tg and CT concentrations.[3,4]
Table 1.
Results of thyroglobulin and calcitonin assays from different analytical platforms.
We measured serum CT on the Liaison (Diasorin) platform before and after (Table 2) treating serum samples in a Heterophile antibodies blocking tubes: a value of 4.5 pg/ml was found versus 506 pg/ml. Using a mix of interference-eliminating proteins, consisting of different blockers as animal-derived antibodies and an alkaline phosphatase scavenger, we detected an increased Tg concentration (240 ng/ml vs 150 ng/ml) on ACCESS platform (Beckman Coulter). The interference seems to be exacerbated by the blockers already present in the assay.
Table 2.
Results before and after incubation in heterophile antibodies blocking tubes (for calcitonin) and in presence a pool of different blockers (for thyroglobulin).

Due to the positivity in interference tests by heterophile substances, we searched for heterophilic animal antibodies by dot-blot. Reactivity to several animal species was observed for both IgG and IgM antibodies. The sample was also tested for RF by ELISA test, showing elevated RF levels (>500 IU/ml). False positive results can be caused if RF bridge the capture and detection antibodies to yield a signal even in the absence of analyte.[5–7]
3. Discussion
This report is on a rare case of a patient with a diagnosis of DTC, that showed variable Tg levels during the follow-up associated with no clinical evidence of disease relapse. RF and human anti-mouse antibodies were studied as possible causes of falsely raised Tg and CT levels. Our patient had high RF levels (>500 IU/ml), but there was no clinical picture compatible with RA or any other autoimmune disease. RF is human anti-human immunoglobulin directed towards the Fc part of human immunoglobulins.[8]
The immunoglobulin aggregation can be the result of RF (Fab-Fc reactions), or idiotypic antibody (Fab-Fab) interactions. Most of the heterophilic antibodies are specific for the Fc region and the removal of the Fc region from the antibody (resulting in Fab fragments) should eliminate the interference from Fc-specific heterophilic antibodies in a two-site immunoassay.[9]
HAb interference with tumor markers is particularly important as it may lead to a false diagnosis of malignancy and unnecessary treatment.[10]
Recently developed tests have been formulated to minimize the interference from RF and HAb. To address this aim, these tests contain blocking reagents. However, human antibodies have an unpredictable affinity and no immunoassays are free from this type of interference.
When Tg elevation does not agree with the clinical scenario, a positive HAb interference is possible. In such a case the simplest approach to confirm the suspicion is to repeat the assay with different methods. It is a common finding that samples showing interferences did not show the same result using assays from other manufacturers.
Of note, RF interferes with immunometric assays, but not with RIA or LC-MS/MS methods.[11,12]
In these patients, the role of imaging procedures is relevant for the treatment decision-making.
From the laboratory point of view, we suggest sera referred for Tg measurement of patients in follow-up for DTC should be treated using HBT tubes. Such an approach together with imaging procedures and agreement with the clinical picture can help to avoid unnecessary treatment and delayed identification of recurrences.
Close communication between the clinicians and laboratory staff is vital for early recognition and confirmation of suspected cases of analytical interference.
Author contributions
Conceptualization: Gelsy Arianna Lupoli.
Data curation: Roberta Lupoli, Enrico Riccio.
Formal analysis: Livia Barba, Antonietta Liotti, Evelina La Civita.
Supervision: Giuseppe Portella, Pietro Formisano, Francesco Beguinot, Daniela Terracciano.
Daniela Terracciano orcid: 0000-0003-4296-429X.
Footnotes
Abbreviations: CT = calcitonin, DTC = differentiated thyroid carcinoma, FNAB = fine-needle aspiration biopsy, HAb = heterophile antibodies, PTC = papillary thyroid carcinoma, RA = rheumathoid arthritis, RF = rheumatoid factor, Tg = thyroglobulin.
Written informed consent was obtained from the participant for publication of this case report.
The authors have no conflicts of interest to disclose.
References
- [1].Tate J, Ward G. Interferences in immunoassay. Clin Biochem Rev 2004;25:105–20. [PMC free article] [PubMed] [Google Scholar]
- [2].Nakamura M, Burastero SE, Notkins AL, et al. Human monoclonal rheumatoid factor-like antibodies from CD5 (Leu-1)+ B cells are polyreactive. J Immunol 1988;140:4180–6. [PubMed] [Google Scholar]
- [3].Preissner CM, O’Kane DJ, Singh RJ, et al. Phantoms in the assay tube: heterophile antibody interferences in serum thyroglobulin assays. J Clin Endocrinol Metab 2003;88:3069–74. [DOI] [PubMed] [Google Scholar]
- [4].Giovanella L, Suriano S. Spurious hypercalcitoninemia and heterophilic antibodies in patients with thyroid nodules. Head Neck 2011;33:95–7. [DOI] [PubMed] [Google Scholar]
- [5].Bartels EM, Falbe Watjen I, Littrup Andersen E, et al. Rheumatoid factor and its interference with cytokine measurements: problems and solutions. Arthritis 2011;2011:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ismail AA, Walker PL, Cawood ML, et al. Interference in immunoassay is an underestimated problem. Ann Clin Biochem 2002;39:366–73. [DOI] [PubMed] [Google Scholar]
- [7].Levinson SS, Miller JJ. Towards a better understanding of heterophile (and the like) antibody interference with modern immunoassays. Clin Chim Acta 2002;325:1–5. [DOI] [PubMed] [Google Scholar]
- [8].Newkirk MM. Rheumatoid factors: what do they tell us? J Rheumatol 2002;29:2034–40. [PubMed] [Google Scholar]
- [9].Preissner CM, Dodge LA, O’Kane DJ, et al. Prevalence of heterophilic antibody interference in eight automated tumor marker immunoassays. Clin Chem 2005;51:208–10. [DOI] [PubMed] [Google Scholar]
- [10].Soares DG, Millot F, Lacroix I, et al. Heterophile antibody interference led to unneeded chemotherapy in a testicular cancer patient. Urol Case Rep 2016;9:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Netzel BC, Grebe SK, Algeciras-Schimnich A. Usefulness of a thyroglobulin liquid chromatography-tandem mass spectrometry assay for evaluation of suspected heterophile interference. Clin Chem 2014;60:1016–8. [DOI] [PubMed] [Google Scholar]
- [12].Crane MS, Strachan MW, Toft AD, et al. Discordance in thyroglobulin measurements by radioimmunoassay and immunometric assay: a useful means of identifying thyroglobulin assay interference. Ann Clin Biochem 2013;50:421–32. [DOI] [PubMed] [Google Scholar]


