Abstract
Propranolol is the mainstay of treatment for infantile hemangiomas (IHs) benefited from its low complication in the present study. However, it has an uncertainty treating period with cumbersome methods which may be related to clinical features. This study sought to considered possible influences of short-term efficacy to medication.
Retrospective analysis of 82 patients with IHs treated by propranolol was performed. The patients were grouped according to effect (excellent, good and fair/poor). ANOVA or t test was used to assess the relationships between effect and clinical features of IHs.
Twenty-seven patients were males and 55 were females. The median age of treatment initiation was 3.5 (±2.11) months. Mean follow-up time for the group was 6.2 months (1.5–16 months). There were no significant associations between short-term efficacy and gender, time points of treatment, diameter of tumor and multifocality. However, tumor thickness was associated with short-term efficacy (P = .013). Moreover, an obvious difference of short-term efficacy has been found when tumor thickness <1.2 cm.
In the present study, tumor thickness was associated with the short-term efficacy in patients with IHs. Propranolol may be gets a better outcome when tumor thickness <1.2 cm at a short time.
Keywords: infantile hemangioma, propranolol, short-term efficacy
1. Introduction
Infantile hemangiomas (IHs) has been considered as the most common benign tumor [1,2] and occurs in 5% to 10%.[3,4] Some previous investigations have revealed that more than 57% of these neoplasms settled on the maxillofacial regions .[5] These lesions are preponderant in white compared with infants in Asia. The prevalence differs among sex, since females are under a higher incidence while the incidence in male infants is somewhat lower at the rate of 3:1 to 5:1. They are characterized by a precursor lesion at birth, such as an area of telangiectasia or pallor, that then proliferate for variable periods until 8 months of age.[6,7]
Although majority of cases have been mild and regressive spontaneously, with no need for treatment, but a precious few of IHs may cause comorbidities such as facial scarring caused by skin ulceration or threat to vital structures.[1] Currently, propranolol has been indicated for the treatment of more severe IH [1,8,9]since the benefit of propranolol been discovery in 2008.[10] However, the duration of oral propranolol is assessed uncomfortably by the attending physician. Propranolol was continued for an average of 6.4 months, with a range of treatment duration from 1 week to 15 months in a systematic review.[1] Studies on correlation between efficacy and clinical characteristics are limited.[11] The main objective of our study was to help physicians assess the efficacy in further short-term according to clinical characteristics of lesion.
2. Materials and methods
2.1. Patients
In total, 82 patients with IH under 1 year of age were included between March 2016 and May 2018 at the Department of Interventional & hemangioma in the Qilu Children's Hospital of Shandong University. The group consisted of 27 boys and 55 girls, with a mean age of 3.46 months (range 1.1–11.23 months). The volume of these tumors was measured by Doppler ultrasonography. The diagnosis of hemangioma was done based on the medical history (age of onset), clinical presentations and Doppler ultrasonography. Data was gathered from patient files, including clinical characteristics, imaging examination, treatment effects and side effects. The treatment criteria for IHs were as follows: infants were diagnosed with local complications associated with IHs, such as risk of functional or aesthetic impairment or life-threatening locations. Patients with cardiovascular disorders, hypoglycemic episodes and recent outbreaks of wheezing were excluded from this study.
The baseline characteristics of patients receiving propranolol included each patient's gender, age when receive propranolol, location, treatment indication, treatment duration, and clinical outcome. In addition, details regarding propranolol treatment such as dosage, age when first started and terminated, rebound, and side effects were obtained. The ethics committee of Qilu Children's Hospital of Shandong University agreed with this study and the parents of all infants provided written informed consent for publication.
2.2. Dose and duration
All patients in this study were initially managed as inpatients, and then in an outpatient. Patients were started on oral propranolol at 1.5 to 2.0 mg/kg/day in three divided doses and monitored for any cardiovascular and metabolic side effects over 24 hours. The dosage of propranolol was adjusted to 2.0 to 3.0 mg/kg/day in 2 divided doses after 2 weeks treatment until met the requirement of stop therapy. Withdrawal was accepted under the following conditions:
-
(1)
if the lesion had completely involuted and without reappeared during the period of drug withdrawal;
-
(2)
if there was no response or mild response at 6 months after initiating treatment;
-
(3)
if the patient experienced unacceptable drug toxicity.
2.3. Evaluation
To assess the treatment response, we get the baseline investigations before starting propranolol included longest diameter and thickness of IH according to Doppler ultrasonography, and physical appearance according to high-definition camera. Further records will be got from outpatient follow-up every month. In cases of multiple hemangiomas, each was evaluated separately, then we calculated the average of each score. In our study, patients were divided into three groups based on the above principles after 6 months, poor responders, moderate responders and good responders
Furthermore, baseline investigations before starting propranolol and further change were also recorded to evaluate drug toxicity, such as heart rate, blood pressure, coagulation parameters, blood glucose, and serum thyroid hormone level. Patients’ heart rate (HR) and blood pressure (BP) were monitored before initiating treatment, 1 hour, and 4 hours after treatment during hospitalization after the first dose of propranolol. We measured preprandial blood glucose levels at least three times a day during hospitalization and at every follow-up visit. SPSS version 19.0 for windows was utilized to conduct all statistical analyses. The t-test and X2-test were used for univariate analysis of continuous and categorical data, respectively. Statistical significance was defined by P-values <.05.
3. Results
A total of 82 IHs patients (27men and 55 women) were studied. IHs are categorized as superficial (72 cases), deep (1 case), or compound (9 cases). The most frequently involved sites were head and neck (53/82). A total of 23 patients had multifocal haemangiomas involving more than one anatomic site. The average age (SD) of the patients at propranolol initiation was 3.5 months (±2.11 months) (Table 1).
Table 1.
The clinical data of infants with infantile hemangioma.
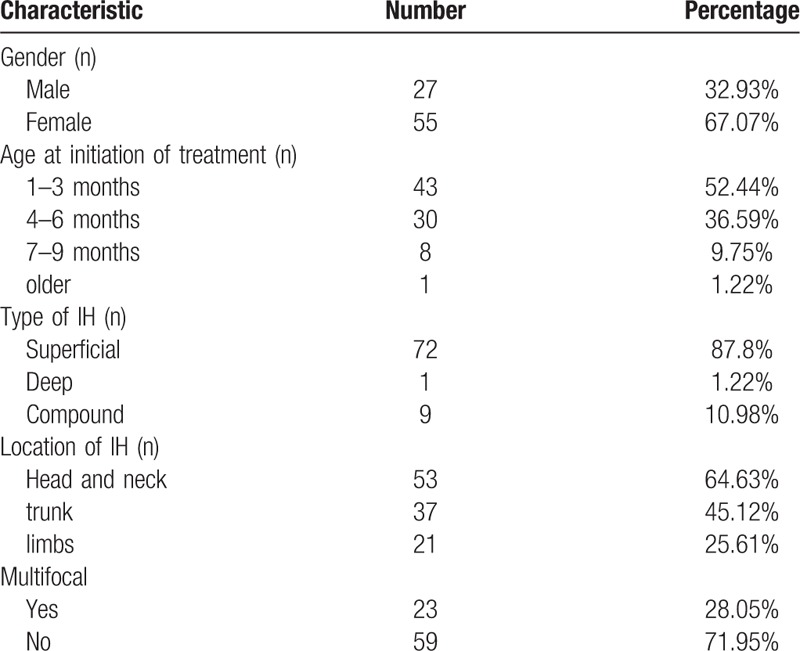
Among 82 patients, 8 were poor responders, 19 were moderate responders and 55 were good responders (Table 2). The median initiating dose was 2.0 mg/kg/day (range 1.5–3 mg/kg/day). Mean follow-up time for the group was 6.2 months (1.5–16 months). Painless diarrhoea occurred in 56 (68%) cases and was the most common complication. Only 1 case encountered hypoglycemic come due to oral propranolol on an empty stomach.
Table 2.
Associations between short-term efficacy and clinical characteristics of infantile hemangioma treated by Propranolol.
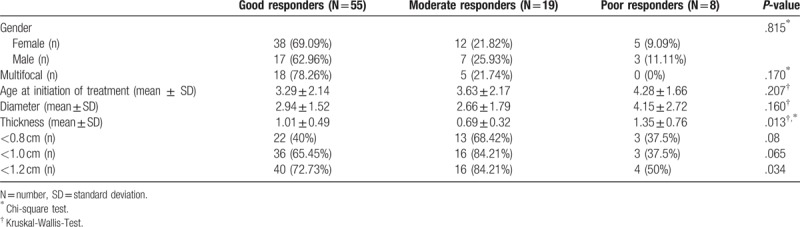
An analysis by age at treatment initiation revealed that the infants in whom had the better therapeutic responses at the short-term, the treatment was initiated earlier. This group demonstrated an average age of the patients at propranolol initiation was 3.2 months. In contrast, the group of moderate responders had an average age of 3.6 months and the group of poor responders was 4.3 months. Although the age of treatment initiation differences was not significant (P = .207).
The average diameter (SD) of the patients at propranolol initiation was 2.99 cm (±2.75 cm). A larger diameter was discovered in poor responder group than the others. It is a great pity that the evidence is poor to discover the associations between short-term efficacy and diameter (P = .160). Gender and multifocality were studied as prognostic factors for short-term efficacy. While, Chi-square test showed that gender and multifocality do not play a role in predicting the response of propranolol treatment based on the P value (P = .815 and P = .170, respectively).
The average thickness (SD) of the patients at propranolol initiation was 0.97 cm (±0.52 cm). While the thickness of lesion in three group had a significant difference (P = .013). Then the thickness was divided into two groups separately according to critical point of 0.8 cm, 1.0 cm and 1.2 cm. Moreover, an obvious short-term efficacy has been found when tumor thickness <1.2 cm (P = .034). There was no significant difference of short-term efficacy according to critical point of 0.8 cm, 1.0 cm.
4. Discussion
Beta-blocker therapy is rapidly becoming the first-line therapy for IH. The mechanisms of action of propranolol on IH include induce hemangioma endothelial cell apoptosis via a p53-BAX mediated pathway[12] or the LIN28/let-7 pathway.[13] Currently, Glut-1 as characteristic index to distinguish IHs and all other types of vascular anomalies was confirmed to be therapeutic targets of propranolol.[14] While the real mechanisms are unclear and GLUT-1 immunohistochemical testing is inconvenient or embarrassing in clinical practice.
IHs is heterogeneous and their future efficacy treated by propranolol is not easy to evaluate before the beginning of treatment. Chong and colleagues recognized that there is no relationship between the extent of heart rate (HR) decline after propranolol administration and clinical response 6 months after propranolol therapy.[15] Furthermore, some scholars assumed that the initial response to propranolol could predict the final outcome of IHs.[8] Although the significant effect of propranolol on IHs has been conformed,[16] the number of partial responders and non-responders has also been growing. A large study of include 101 individuals showed that three were poor responders and 39 were moderate responders at 6 months after propranolol therapy.[15] A retrospective study [17] revealed that excellent response rates were achieved in 65.4% of infants mean 6.5 months after treatment initiation. In our study, the clinical good response rate was 67.07% at 6 months after treatment and 83.63% at the end of treatment. The clinical evaluation was performed 6 months after oral propranolol administration to assess the factors affecting the therapeutic effect. The different clinical response of infantile hemangiomas to propranolol is difficult to interpret.
The thickness of IHs is an important factor in the therapeutic effect of propranolol in this study. Previous studies demonstrated that superficial hemangiomas (mean thickness 0.35 cm) responded excellently and almost all patients get Grade IV response 6 months after treatment,[8] which were higher rates than those reported in other studies.[18] In our cohort, better responses were observed for hemangiomas when tumor thickness <1.2 cm.
Lesion location as a factor affects the therapeutic response of IHs to propranolol was not been evaluated in this study. Previous studies demonstrated that trunk hemangiomas responded poorly and required longer treatment durations than head and neck hemangiomas.[19] In a recent study for infantile hemangioma of the head and neck also showed the better therapeutic response of parotid, periorbital, cheek, neck and multiple lesions. While some scholars confirmed there was no difference in response to propranolol with regards to the hemangiomas’ location.[20] The response was similar in segmental versus localized hemangiomas.[21] Further studies are needed to find predictors of good clinical responses to propranolol.
Investigators have observed that children who started treatment before five months of age had a significantly better response than children who started treatment at a later age.[20] However, Dong et al[22] demonstrated that a poorer clinical response will be got in the youngest age group (1–3 months) than the other age groups. In this study, the different clinical response of infantile hemangiomas was not been observed between children who start treatment earlier and older children. We theorize that the effect of the therapeutic effect and treatment duration may be due to growth characteristics of IHs. Previous results have also demonstrated that there is no clear difference in regress rate between different ages at start of therapy.[23]
There was no significant relationship detected between short-term efficacy and clinical characteristics, such as gender, longest diameter in this study. The limitations of this study are small sample size and high patient variability. In this study, the result showed that only one case is deep lesions with poorer response and lengthier treatments. The lesion type had no significant effect on the therapeutic response rates.
Larger prospective studies should be undertaken to investigate the prognostic factors affecting the therapeutic effects of propranolol on infantile hemangiomas.
Author contributions
Conceptualization: Lei Guo.
Data curation: Liang Wang.
Formal analysis: Liang Wang.
Investigation: Jing Li.
Methodology: Jing Li.
Project administration: Changfeng Wang.
Software: Changfeng Wang.
Validation: Lei Guo.
Writing – original draft: Chang-hua Wu.
Writing – review & editing: Dan Song.
Footnotes
Abbreviations: BP = blood pressure, HR = heart rate, IHs = infantile hemangiomas.
The authors report no conflicts of interest
References
- [1].Marqueling AL, Oza V, Frieden IJ, et al. Propranolol and infantile hemangiomas four years later: a systematic review. Pediatr Dermatol 2013;30:182–91. [DOI] [PubMed] [Google Scholar]
- [2].Adams DM, Ricci KW. Infantile Hemangiomas in the head and neck region. Otolaryngol Clin North Am 2018;51:77–87. [DOI] [PubMed] [Google Scholar]
- [3].Greenberger S, Bischoff J. Infantile hemangioma-mechanism (s) of drug action on a vascular tumor. Cold Spring Harb Perspect Med 2011;1:a006460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Drolet BA, Swanson EA, Frieden IJ. Infantile hemangiomas: an emerging health issue linked to an increased rate of low birth weight infants. J Pediatr 2008;153: 712-5, 15.e1. [DOI] [PubMed] [Google Scholar]
- [5].Ma X, Zhao T, Xiao Y, et al. Preliminary experience on treatment of infantile hemangioma with low-dose propranolol in China. Eur J Pediatr 2013;172:653–9. [DOI] [PubMed] [Google Scholar]
- [6].Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics 2015;136:e1060–104. [DOI] [PubMed] [Google Scholar]
- [7].Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma: executive summary. Pediatrics 2015;136:786–91. [DOI] [PubMed] [Google Scholar]
- [8].Yun YJ, Gyon YH, Yang S, et al. A prospective study to assess the efficacy and safety of oral propranolol as first-line treatment for infantile superficial hemangioma. Korean J Pediatr 2015;58:484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kaneko T, Sasaki S, Baba N, et al. Efficacy and safety of oral propranolol for infantile hemangioma in Japan. Pediatr Int 2017;59:869–77. [DOI] [PubMed] [Google Scholar]
- [10].Leaute-Labreze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med 2008;358:2649–51. [DOI] [PubMed] [Google Scholar]
- [11].Moyakine AV, Herwegen B, van der Vleuten CJM. Use of the Hemangioma Severity Scale to facilitate treatment decisions for infantile hemangiomas. J Am Acad Dermatol 2017;77:868–73. [DOI] [PubMed] [Google Scholar]
- [12].Yao TH, Pataer P, Regmi KP, et al. Propranolol induces hemangioma endothelial cell apoptosis via a p53BAX mediated pathway. Mol Med Rep 2018;18:684–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mong EF, Akat KM, Canfield J, et al. Modulation of LIN28B/Let-7 signaling by propranolol contributes to infantile hemangioma involution. Arterioscler Thromb Vasc Biol 2018;38:1321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ford JR, Gonzalez-Barlatay J, Valenzuela AA. Early orbital infantile hemangioma that emphasizes the importance of glucose-transporter-1 (GLUT-1). Can J Ophthalmol 2018;53:e58–60. [DOI] [PubMed] [Google Scholar]
- [15].Chong JH, Prey S, Mya HT, et al. Can the extent of heart rate reduction predict the clinical response of infantile haemangiomas to propranolol? Br J Dermatol 2018;178:e196–7. [DOI] [PubMed] [Google Scholar]
- [16].Padhiyar JK, Patel NH, Gajjar TP, et al. Efficacy and safety of propranolol on the proliferative phase of infantile hemangioma: a hospital-based prospective study. Ind J Paediatr Dermatol 2018;19:224. [Google Scholar]
- [17].Pandey V, Tiwari P, Gangopadhyay AN, et al. Propranolol for infantile haemangiomas: experience from a tertiary center. J Cutan Aesthet Surg 2014;7:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schiestl C, Neuhaus K, Zoller S, et al. Efficacy and safety of propranolol as first-line treatment for infantile hemangiomas. Eur J Pediatr 2011;170:493–501. [DOI] [PubMed] [Google Scholar]
- [19].Castaneda S, Garcia E, De la Cruz H, et al. Therapeutic effect of propranolol in mexican patients with infantile hemangioma. Drugs Real World Outcomes 2016;3:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Andersen IG, Rechnitzer C, Charabi B. Effectiveness of propanolol for treatment of infantile haemangioma. Dan Med J 2014;61:A4776. [PubMed] [Google Scholar]
- [21].Orozco-Covarrubias L, Lara-Mendoza L, Garrido-García LM, et al. Therapy for involuting infantile hemangioma. Propranolol Effectiveness 2017;19: [Google Scholar]
- [22].Dong JY, Ning JX, Li K, et al. Analysis of factors affecting the therapeutic effect of propranolol for infantile haemangioma of the head and neck. Sci Rep 2017;7:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schupp CJ, Kleber JB, Gunther P, et al. Propranolol therapy in 55 infants with infantile hemangioma: dosage, duration, adverse effects, and outcome. Pediatr Dermatol 2011;28:640–4. [DOI] [PubMed] [Google Scholar]


