Abstract
Rationale:
A craniectomy, which results in a large skull defect, is performed to decrease the intracranial pressure under conditions such as intracranial hemorrhage and ischemic stroke. When the patient's condition is stabilized, autologous cranioplasty using the bone flap previously removed in the craniectomy is performed. Bone flap infection after the autologous cranioplasty is not uncommon and is difficult to treat. After the infection is controlled, cranioplasty is needed to improve the head deformity and neurologic function. Cranioplasty with a titanium mesh can result in aesthetic improvement and a low infection rate. Using 3-dimensional computed tomography (3D-CT) and 3D printing, titanium mesh is manufactured to fit perfectly on the patient's skull defect.
Patient concerns:
Two patients with large skull defects in the right temple area due to previous craniectomy were referred to our department for reconstruction. They had histories of recurrent infections at the operation site even after removal of the autologous bone flap that had been used for the cranioplasty.
Diagnosis:
Preoperative computed tomography (CT) showed 12×16 cm and 8×8.3 cm skull defect on right temporal area, respectively.
Interventions and outcome:
The infection was controlled by well-vascularized free flap coverage. After the surgery, cranioplasty with custom-made titanium mesh was performed to improve the aesthetic and functional problems of the patients. The contour of the temporal area was symmetric. The patients were satisfied with the results.
Lessons:
Staged reconstruction of large skull defects with soft tissue infection after craniectomy using free flap followed by cranioplasty with titanium mesh on can lead to safe, aesthetic, and satisfactory result.
Keywords: 3-D images, 3D printing, craniectomy, free flap, titanium
1. Introduction
Craniectomy is an effective treatment when intracranial pressure is increased, such as in ischemic stroke and intracranial hemorrhage. Other circumstances, such as trauma, infections, and tumors, may also require a craniectomy.[1–3] Covering the large skull defect after the surgery is challenging for plastic surgeons and requires cranioplasty. This procedure leads to protection of the underlying brain, restoration of the cosmetic appearance and improvement of neurological function.[1,3,4]
Autologous cranioplasty using the previously removed bone flap from the craniectomy is the first-line treatment for covering the large skull defect.[5] However, both bacterial infections and absorption of the bone are severe problems. A long period of time after explantation of the bone flap in craniectomy can allow bacterial growth and result in low osteoblast activity.[2,3,6,7]
When scalp infection occurs after autologous cranioplasty, it tends to be recurrent, and eventually, the bone flap needs to be removed. The infection can spread to the underlying tissue when the skull is not present to act as a barrier. Epidural abscesses and encephalitis can occur. In such cases of widespread infection, many problems, such as infections of bone and soft tissue, soft tissue defects after surgical debridement of the infected wound and skull defects, are difficult to solve simultaneously and staged reconstruction should be considered. Surgical debridement and coverage of the soft tissue defect are needed.[8,9]
Cranioplasty for the skull defect should be delayed until the healing of infections occurs. Alloplastic implants can be used to cover a large defect, and choosing the proper implant material is important. The material should be sufficiently hard to protect the brain and chemically and biologically stable to lower the infection rate and decrease the deformity. Titanium fits perfectly in these conditions.
We present 2 patients with previous autologous bone flap infection histories who underwent cranioplasty with a titanium mesh after free flap coverage of the scalp due to recurrent infections at a previous operation site.
2. Case reports
2.1. Case 1
A 55-year-old male fell down after sustaining an electrical shock by a voltage transformer. Craniectomy on the right parieto-temporal area was performed to treat the subdural hematoma, and subsequent autologous cranioplasty was performed to restore the skull defect. After the surgery, the patient suffered repeated epidural abscesses at the operation site. The infection did not improve with sustained antibiotic treatment and serial surgical debridement. A latissimus dorsi (LD) muscle free flap was used to help control the infection and cover the scalp defect after surgical debridement[8,10] (Fig. 1A and B). Despite the successful treatment of the infection, atrophy of the muscle flap produced a deformity of the head (Fig. 1C and D). To correct the head deformity and cover the brain after 20 months of the surgery, cranioplasty with a custom-made titanium mesh was scheduled.
Figure 1.
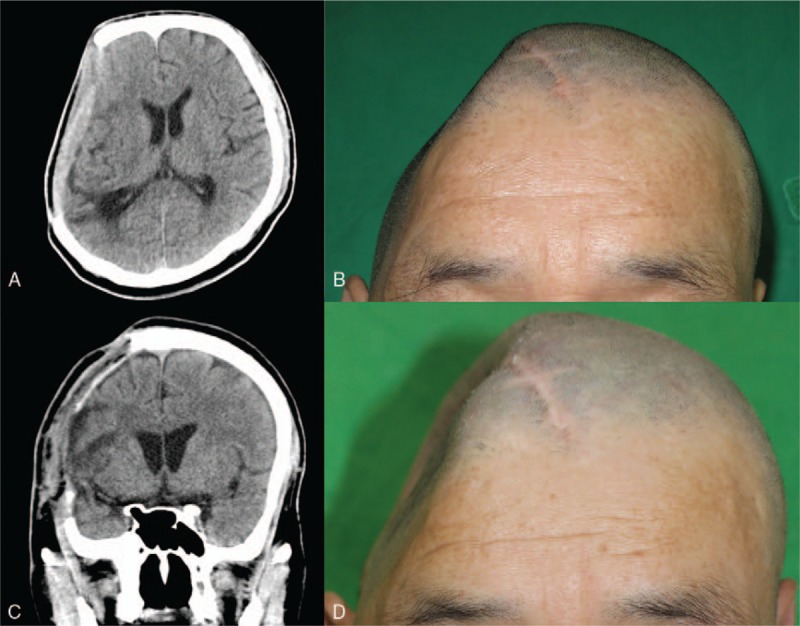
CT image and photograph before (A, B) and after (C, D) application of an LD muscle free flap. A recurrent epidural abscess with a well-enhanced lesion was observed on CT images (A). Large depression in the right parietal area (B). An immediate postoperative CT image shows the LD muscle flap from the previous craniectomy site (C). At 1 month after the LD free flap was applied, the defect was exposed due to muscle atrophy (D). CT = computed tomography, LD = latissimus dorsi.
The defect size was approximately 12 × 16 cm. Using 3-dimensional computed tomography (3D-CT) reconstruction, a plaster cast was produced. Using the plaster cast as a template, a custom-made titanium mesh was manufactured (Fig. 2A and B).
Figure 2.
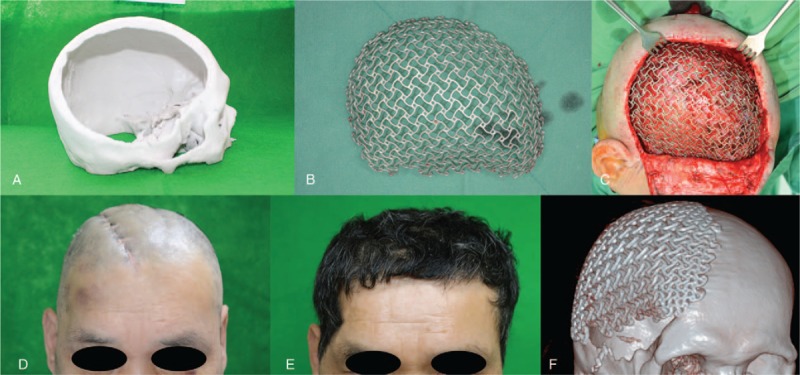
The 3D printed plaster cast (A) and the titanium mesh used in the patient (B). The titanium mesh was inserted between the LD muscle and subcutaneous layer and fixed to the adjacent bone (C). At 1 month (D) and 1 year (E) after cranioplasty with a titanium mesh. Postoperative 3D-CT reconstruction shows a well-positioned mesh (F). 3D-CT = 3-dimensional computed tomography, LD = latissimus dorsi.
In the operating room, the scalp flap was dissected over the LD muscle. The titanium mesh was inserted between the muscle and subcutaneous layer (Fig. 2C) and fixed to the adjacent bone margin with screws. After the surgery, the skull contour was restored, and no complications occurred. At 1 year after surgery, the deformity was not remarkable maintaining proper position on computed tomography (CT) (Fig. 2D, E, and F).
2.2. Case 2
A 33-year-old male had undergone craniectomy for intracranial hemorrhage due to a motorcycle accident. Subsequent autologous cranioplasty was performed. The patient had a 3 cm diameter infected soft tissue defect (Fig. 3A). We covered the defect with a contralateral temporal fascia free flap (Fig. 3B and C).
Figure 3.
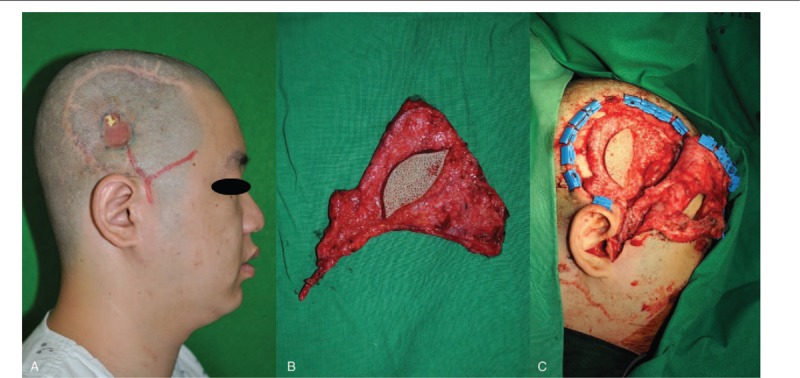
An approximately 3 cm soft tissue defect due do repeated infection (A). The defect was covered with a contralateral temporal fascia free flap (B, C).
The patient visited our department for cranioplasty 12 years after the first accident. He suffered from a head deformity and headaches. To correct these problems, we performed cranioplasty with a custom-made titanium mesh. First, we performed preoperative 3D-CT to measure the defect size (8 × 8.3 cm). A plaster cast and custom-made titanium mesh were constructed based on the image (Fig. 4A and B).
Figure 4.
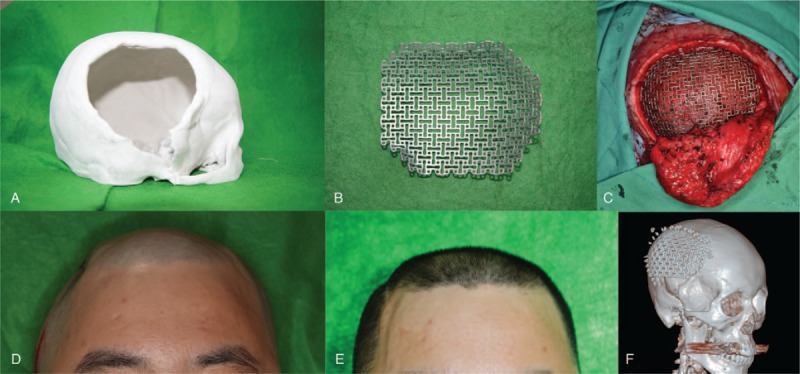
A 3D printed plaster cast (A) and titanium mesh of the patient (B) were prepared. The titanium mesh was inserted and fixed to the adjacent bone (C). Compared to the preoperative photograph that showed a depressed deformity on the right temporal area (D), after 12 days, the deformity was resolved, and both temporal areas showed symmetry (E). Postoperative 3D-CT reconstruction shows a well-positioned mesh (F). 3D-CT = 3-dimensional computed tomography.
With the patient under general anesthesia, the scalp flap was carefully dissected from the dura. After inserting the mesh between the flap and dura, we fixed the mesh with 12 screws and absorbable sutures onto the adjacent bone margin (Fig. 4C).
After the surgery, no complications, such as fluid collection, infection or wound dehiscence, occurred. The depressed external contour showed symmetry after the surgery and was aesthetically satisfying without depression or protrusion (Fig. 4D and E). The postoperative CT results indicated that the mesh was well positioned over the defect (Fig. 4F).
3. Discussion
We report 2 cases in which large skull defects were covered in two patients who had a previous autologous bone flap infection history and intractable inflammation after bone flap removal. After covering a complex scalp and skull defect with a vascularized free flap, a chemically, biologically and mechanically stable titanium mesh, which was customized to the patient using 3D-CT technology, is a good option for aesthetic and safe results.
After craniotomy or craniectomy, covering the resulting large skull defect is challenging. Autologous cranioplasty is the first-line treatment. However, infection and bone resorption are common, with rates of 11.2% and 5.35%, respectively.[11] Autologous cranioplasty using bones from other sites is another method used to cover the skull defect. The calvarial bones or the ribs are commonly used. However, if the defect size is large, locating an appropriate donor site to cover the entire defect is difficult. Donor site morbidities are also problems for a large graft. Cranioplasty with alloplastic implants is an excellent choice for patients with large skull defects. Several alloplastic implants are currently widely used, and choosing the proper implant material is of great importance.[4,7]
When infection of the autologous bone flap occurs, subsequent subdural empyemas and abscesses are uncommon but can lead to potentially lethal complications. Infections in patients with these complications are difficult to manage, even after surgical debridement and removal of the bone flap. A well-vascularized flap coverage of the infected wound provides a sufficient blood supply and fills in the dead space, which helps control infections. An intractable infected wound can be completely healed by this procedure.[8–10,12] Local flap coverage of a large scalp defect is very difficult because the extensibility of the scalp is not satisfactory. We use a free flap in such cases because it provides a short recovery time, healthy well-vascularized tissue and satisfactory aesthetic results. An LD musculocutaneous flap can be used to cover a large defect.[8–10]
However, muscle atrophy is a major complication after the use of a muscle free flap for a complex scalp and skull defect. The deformity develops after muscle shrinkage occurs.[13] Alloplastic cranioplasty is needed to correct the deformity and improve the functional problems.
Alloplastic cranioplasty should be delayed until after complete infection control is achieved. Because the alloplastic implant is vulnerable to infection, it should not be inserted in the infected area. The volumetric change of the flap after the operation should be considered. Muscle and fat atrophy, weight loss of the patient and scar contraction can lead to a change in the flap volume and a subsequent deformity of the head.[14,15] Therefore, cranioplasty should be delayed for at least 6 to 9 months, when no further volume change of the flap is anticipated.
Currently, the most widely used synthetic material is polymethylmethacrylate (PMMA) due to its malleable properties and long history of use. However, it is chemically unstable. The risk of infection is higher than that for autologous cranioplasty (approximately 5%–10% higher) because of growth of fibrous tissue around the implant, which can be a focus of bacterial growth. It is not sufficiently hard to construct a large implant to cover wide defects of the skull. Retraction and contraction are other problems that can result in deformity and bone erosion.[7]
Calcium phosphate bone cement can be used for pediatric patients, or other materials can be used in a reinforcing role.[7] However, this material is not sufficiently hard to be used for coverage of large skull defects.
Titanium is a suitable material for cranioplasty. It is chemically and biologically stable, which makes it resistant to infection, erosion, and absorption. Its long-term outcomes are more favorable than those of other materials because it does not induce infections or changes in the contour. Titanium implants have a high strength-to-weight ratio.[3,4] Titanium is sufficiently hard to construct a large implant, and it can even be used in a mesh form, which is light and malleable. Its lightweight results in increased patient comfort after the insertion of a large implant. During the operation, titanium mesh is easy to mold and is malleable. Another benefit of the mesh form is its cost-effectiveness. Compared to the plate form, a lesser quantity of titanium is needed to manufacture the titanium mesh.
The advancement of CT technology has made 3D reconstruction of the skull possible. Using 3D printing based on 3D-CT, patients’ skulls can be modeled as plaster casts.[2,3,4,7,16] This 3D-printed plaster cast can be used to manufacture a titanium mesh that is symmetrical to the contralateral side of the skull and fits perfectly to the patients’ skull bones. Patients are very satisfied with the symmetric and aesthetic results. After surgery, no protuberance or exposure of the implant is apparent, and the skull has a regular contour.
Acknowledgments
The publication of this study had obtained the patient's consent, and we thanked for the patient's cooperation during the treatment process.
Author contributions
Conceptualization: Seong Hwan Kim, In Suck Suh.
Data curation: In Suck Suh.
Formal analysis: In Suck Suh, Jun Won Lee.
Funding acquisition: Hii Sun Jeong.
Investigation: Hii Sun Jeong.
Methodology: Hii Sun Jeong, Jun Won Lee.
Project administration: Jun Won Lee.
Resources: Seong Joo Lee, Jun Won Lee.
Software: Seong Joo Lee, Jun Won Lee.
Validation: Seong Hwan Kim, In Suck Suh.
Visualization: Seong Hwan Kim.
Writing – original draft: Seong Hwan Kim, Seong Joo Lee.
Writing – review & editing: Seong Hwan Kim, In Suck Suh.
Seong Hwan Kim orcid: 0000-0001-6831-5621.
Footnotes
Abbreviations: 3D-CT = 3-dimensional computed tomography, CT = computed tomography, LD = latissimus dorsi.
Presented at the 7th Research and Reconstruction Forum, Daejeon, Korea, April 20 and 21, 2017.
The authors have no conflicts of interest to disclose.
References
- [1].Segal DH, Oppenheim JS, Murovic JA. Neurological recovery after cranioplasty. Neurosurgery 1994;34:729–31. [DOI] [PubMed] [Google Scholar]
- [2].Honeybul S, Morrison DA, Ho KM, et al. A randomized controlled trial comparing autologous cranioplasty with custom-made titanium cranioplasty. J Neurosurg 2017;126:81–90. [DOI] [PubMed] [Google Scholar]
- [3].Wiggins A, Austerberry R, Morrison D, et al. Cranioplasty with custom-made titanium plates—14 years experience. Neurosurgery 2013;72:248–56. [DOI] [PubMed] [Google Scholar]
- [4].Williams LR, Fan KF, Bentley RP. Custom-made titanium cranioplasty: early and late complications of 151 cranioplasties and review of the literature. Int J Oral Maxillofac Surg 2015;44:599–608. [DOI] [PubMed] [Google Scholar]
- [5].Lee JC, Kleiber GM, Pelletier AT, et al. Autologous immediate cranioplasty with vascularized bone in high-risk composite cranial defects. Plast Reconstr Surg 2013;132:967–75. [DOI] [PubMed] [Google Scholar]
- [6].Chan DYC, Mok YT, Lam PK, et al. Cryostored autologous skull bone for cranioplasty? A study on cranial bone flaps’ viability and microbial contamination after deep-frozen storage at -80 degrees C. J Clin Neurosci 2017;42:81–3. [DOI] [PubMed] [Google Scholar]
- [7].Park EK, Lim JY, Yun IS, et al. Cranioplasty enhanced by three-dimensional printing: custom-made three-dimensional-printed titanium implants for skull defects. J Craniofac Surg 2016;27:943–9. [DOI] [PubMed] [Google Scholar]
- [8].Mundinger GS, Latham K, Friedrich J, et al. Management of the repeatedly failed cranioplasty following large postdecompressive craniectomy: establishing the efficacy of staged free latissimus dorsi transfer/tissue expansion/custom polyetheretherketone implant reconstruction. J Craniofac Surg 2016;27:1971–7. [DOI] [PubMed] [Google Scholar]
- [9].Mikami T, Minamida Y, Sugino T, et al. Free flap transfer for the treatment of intractable postcraniotomy subdural empyemas and epidural abscesses. Neurosurgery 2007;60suppl 1:ONS83–87. [DOI] [PubMed] [Google Scholar]
- [10].Larranaga J, Rios A, Franciosi E, et al. Free flap reconstruction for complex scalp and forehead defects with associated full-thickness calvarial bone resections. Craniomaxillofac Trauma Reconstr 2012;5:205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hng D, Bhaskar I, Khan M, et al. Delayed cranioplasty: outcomes using frozen autologous bone flaps. Craniomaxillofac Trauma Reconstr 2015;8:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kiyokawa K, Tai Y, Inoue Y, et al. Surgical treatment for epidural abscess in the posterior cranial fossa using trapezius muscle or musculocutaneous flap. Skull Base Surg 2000;10:173–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lin PY, Miguel R, Chew KY, et al. The role of the anterolateral thigh flap in complex defects of the scalp and cranium. Microsurgery 2014;34:14–9. [DOI] [PubMed] [Google Scholar]
- [14].Fujioka M, Masuda K, Imamura Y. Fatty tissue atrophy of free flap used for head and neck reconstruction. Microsurgery 2011;31:32–5. [DOI] [PubMed] [Google Scholar]
- [15].Yamaguchi K, Kimata Y, Onoda S, et al. Quantitative analysis of free flap volume changes in head and neck reconstruction. Head Neck 2012;34:1403–7. [DOI] [PubMed] [Google Scholar]
- [16].Singh M, Ricci JA, Dunn IF, et al. Alloderm covering over titanium cranioplasty may minimize contour deformities in the frontal bone position. J Craniofac Surg 2016;27:1292–4. [DOI] [PubMed] [Google Scholar]


