Supplemental Digital Content is available in the text
Keywords: colorectal adenoma, liver fibrosis, nonalcoholic fatty liver disease
Abstract
Nonalcoholic fatty liver disease (NAFLD) is associated with risks for developing colorectal adenoma. This study aimed to evaluate the association between advanced fibrosis in NAFLD and the risk for colorectal adenoma.
We retrospectively analyzed the data of 6332 adults who underwent abdominal ultrasound and 1st-time colonoscopy on the same day in a health screening program at a single center. We evaluated the presence of advanced fibrosis in NAFLD using various noninvasive score, which also analyzed the detection rate of colorectal adenoma according to the presence of advanced fibrosis in NAFLD.
The subjects with NAFLD had a higher prevalence of colorectal adenoma. In the multivariate analysis, NAFLD was an independent risk factor for colorectal adenoma (adjusted odds ratio [OR], 1.15; 95% confidence interval [CI], 1.02–1.30), advanced adenoma (adjusted OR, 1.50; 95% CI, 1.12–2.01), and multiple adenomas (adjusted OR, 1.32; 95% CI, 1.01–1.73). When NAFLD was further stratified based on the stage of fibrosis using the noninvasive score models, the subjects with NAFLD and advanced fibrosis had a significantly higher risk for colorectal adenoma, advanced adenoma, and multiple adenomas than those with NAFLD without advanced fibrosis.
NAFLD with advanced fibrosis might be risk factor for colorectal adenoma compared with NAFLD without advanced fibrosis.
1. Introduction
Colorectal cancer (CRC) is the 4th leading cause of cancer-related deaths and the 3rd most commonly diagnosed malignancy worldwide.[1] Although the incidence and mortality rates of CRC are rising in many countries, stabilizing or decreasing mortality rates tend to be seen in certain regions, such as North America, Oceania, most European countries, and Japan in Asia owing to advances in CRC management.[2] Most CRCs arise from colorectal adenoma, which is a precursor lesion of CRC based on the adenoma-carcinoma sequence.[3] Therefore, the early detection and removal of colorectal adenoma through health screening colonoscopy have been recognized as the most effective strategy for reducing the incidence, morbidity, and mortality rates of CRC.[4] The risk factors for CRC include age, family history of CRC, alcohol consumption, smoking, and increased red meat consumption.[5] Metabolic syndrome has also recently determined as a potential risk factor for the development of CRC. In several previous studies, the factors for metabolic syndrome, including obesity, dyslipidemia, impaired glucose tolerance, and insulin resistance, could be associated with the development and carcinogenesis of colorectal adenomas.[6,7]
Nonalcoholic fatty liver disease (NAFLD) is defined as the accumulation of fat within the liver and is detected on imaging or histology; its causes exclude excessive alcohol consumption, chronic viral hepatitis, steatogenic medication, and genetic disorder.[8] NAFLD is a major rising cause of liver disease, which is now regarded as a liver manifestation of metabolic syndrome.[9,10] NAFLD comprises a wide spectrum of liver damage, from simple steatosis to steatohepatitis, advanced fibrosis, and cirrhosis, which is recognized as a cause of liver failure and hepatocellular carcinoma.[11] Recently, several studies have shown that the carcinogenic effect of NAFLD was not limited to the liver, but also affected extra-hepatic neoplasm, particularly colorectal neoplasm, which was associated with metabolic risk factors, such as insulin resistance and obesity.[12] However, studies on the risk of colorectal neoplasm according to the severity of NAFLD are still lacking. Owing to the invasiveness and high costs of liver biopsy, risk stratification is difficult in patients with NAFLD. To evaluate liver fibrosis noninvasively, several scoring systems have been introduced.[13,14] In this study, we stratified the severity of NAFLD in subjects who underwent a health screening program using noninvasive score models. This study aimed to evaluate the association between colorectal adenoma and presence of NAFLD and advanced fibrosis.
2. Methods
2.1. Study subjects and design
We reviewed the data of 40,952 adults aged over 19 years who underwent a health screening program at Yeungnam University Hospital in South Korea from September 2009 to June 2017. To evaluate the association with liver fibrosis and colorectal adenoma in subjects with NAFLD, we included 9387 subjects who underwent 1st-time screening colonoscopy and abdominal ultrasound on the same day during the study period. We then excluded 3055 subjects who met any of the following exclusion criteria: missing values (n = 1321); incomplete colonoscopy owing to poor bowel preparation (n = 115); previous history of any cancer (n = 14); previous history of inflammatory bowel diseases, such as ulcerative colitis or Crohn disease (n = 4); CRC detected on colonoscopy during the study period (n = 18, Supplementary Table 1); secondary causes for steatosis (n = 1572); and liver cirrhosis on abdominal ultrasonography (n = 11). Finally, 6332 subjects were included (Supplementary Figure 1). This study was approved by the Institutional Review Board of our Hospital.
2.2. Clinical data and laboratory evaluation
During the health screening program, the height, weight, waist circumference, and sitting blood pressure (BP) were measured by well-trained nurses. Data on age, sex, smoking status, alcohol consumption, medical history, and medication use were collected using a self-administered questionnaire. The subjects’ smoking status was categorized into: never, formerly, and currently. Alcohol consumption was calculated using the number of drinks consumed multiplied by the frequency. Blood was collected following at least 10 hours of fasting and laboratory evaluation for parameters including complete blood count, blood chemistry, glucose level, and lipid profile was performed by trained staff. Body mass index (BMI) was calculated by dividing the weight (kg) by the height squared (m2), and obesity was defined as a BMI ≥25 kg/m2 according to the criteria for the Asia-Pacific region. Hypertension was defined as a systolic BP ≥140 mm Hg or a diastolic BP ≥90 mm Hg or with a history of the disease/medication use. Diabetes mellitus (DM) was defined as a fasting glucose level ≥126 mg/dL or HbA1c level ≥6.5% or with a history of the disease/medication use. Hyperlipidemia was defined as a serum triglyceride level ≥150 mg/dL or a serum cholesterol level ≥200 mg/dL or with a history of the disease/medication use. Metabolic syndrome was defined in accordance with the International Diabetes Federation criteria as central obesity (waist circumference ≥90 cm in men and ≥80 cm in women based on ethnic-specific values) plus 2 of the following 4 factors: increased triglyceride level (≥150 mg/dL) or specific treatment for this lipid abnormality; reduced high-density lipoprotein cholesterol level (<40 mg/dL in men and < 50 mg/dL in women) or on specific treatment for this lipid abnormality; systolic BP ≥130 mm Hg or diastolic BP ≥85 mm Hg or treatment of previously diagnosed hypertension; and increased fasting plasma glucose level (≥100 mg/dL) or previous diagnosis of type 2 DM.
2.3. Colonoscopy procedure and detection of colorectal adenoma
All colonoscopies were performed by well-trained board-certified gastroenterologists using a conventional colonoscope (CV-160 Evis EXTRA and CV-180 Evis EXTRA II; Olympus, Tokyo, Japan) after bowel preparation with 4 L of polyethylene glycol solutions. All subjects were sedated using midazolam and/or propofol. The degree of sedation was minimal to moderate as defined by the American Society of Anesthesiologists. A complete examination was defined in accordance with the following criteria: reached the cecum, and the appendiceal orifice could be identified; the bowel preparation was adequate with no more than adherent feces or small amount of feces or fluid not interfering with the examination; and the colonoscopy was performed for at least 6 minutes. Abnormal polypoid lesions were biopsied or resected endoscopically with records of the location, size, appearance, and numbers. All specimens of colorectal neoplasia were evaluated and classified in accordance with the World Health Organization standards by board-certified pathologists. Advanced adenoma was defined as an adenoma with a ≥10-mm diameter, high-grade dysplasia, or >25% villous component. Multiple adenomas were defined as the presence of three or more adenomas, regardless of the location.
2.4. Diagnosis of NAFLD and staging of liver fibrosis
An abdominal ultrasound was performed to assess the presence of fatty infiltration of the liver using EPIQ 5 and EPIQ 7 (Philips Healthcare, Amsterdam, Netherlands) by experienced ultrasonographists on the same day of the colonoscopy procedure. The liver of each subject was examined for size, echogenicity, contour, structure and posterior beam attenuation, and fatty infiltration, which was diagnosed in accordance with the standard criteria, including liver parenchymal brightness, liver-to-kidney contrast, and deep beam attenuation. NAFLD was diagnosed in accordance with the following criteria: evidence of fatty infiltration of the liver on abdominal ultrasound, alcohol consumption <210 g/week for men and <140 g/week for women, and absence of secondary causes of hepatic fat accumulation, such as viral hepatitis (hepatitis B virus/hepatitis C virus [HCV]), autoimmune disease, use of steatogenic medication, or hereditary disorders.[8] NAFLD was further stratified on the basis of the presence of advanced fibrosis using noninvasive methods, that is, complex score models, including the NAFLD fibrosis score (NFS) and fibrosis-4 (Fib-4) index; and simple score models, including the aspartate aminotransferase (AST)-to-platelet ratio index (APRI) and BMI, AST-to-alanine aminotransferase (ALT) ratio, Diabetes (BARD) score; each score formula is shown in Supplementary Table 2. The NFS has dual predictive cutoff values: low cutoff value of −1.455 for excluding advanced fibrosis and high cutoff value of 0.676 for diagnosing advanced fibrosis in patients with NAFLD.[15] The Fib-4 index also has dual predictive cutoff values: low cutoff value of 1.30 for excluding advanced fibrosis and high cutoff value of 2.67 for advanced fibrosis in patients with NAFLD.[16] An APRI >1 was a significant predictor of advanced fibrosis in patients with NAFLD.[17] A BARD score of 2 to 4 points was considered to indicate F3 or F4 stage of fibrosis.[18] For the subjects with NAFLD in this study, an NFS >0.676, a Fib-4 index >2.67, an APRI >1, and a BARD score ≥2 were considered to indicate advanced fibrosis in NAFLD; conversely, an NFS <−1.455, a Fib-4 index <1.30, an APRI ≤1, and a BARD score <2 were considered to indicate nonadvanced fibrosis in NAFLD. The NFS range of -1.455 to 0.676 and Fib-4 index range of 1.30 to 2.67 were considered indeterminate scores and indicate an unclear presence of advanced fibrosis in NAFLD.
2.5. Statistical analysis
Continuous and categorical variables were expressed as mean ± standard deviation and n (%), respectively. Statistical analysis was performed using SPSS version 23.0 (IBM, Armonk, NY), and the level of statistical significance was set at P < .05. The characteristics of the study subjects were analyzed based on the NAFLD status using Student t test for continuous variables and the Chi-squared test for categorical variables. Logistic regression analysis was used to determine the risk factors associated with colorectal adenoma in the study subjects. The odds ratio (OR) was considered to be statistically significant if the 95% confidence interval (CI) did not include 1.0. The variables used to calculate the complex or simple score models were not included in each logistic regression analysis.
3. Results
3.1. Baseline characteristics of the study subjects
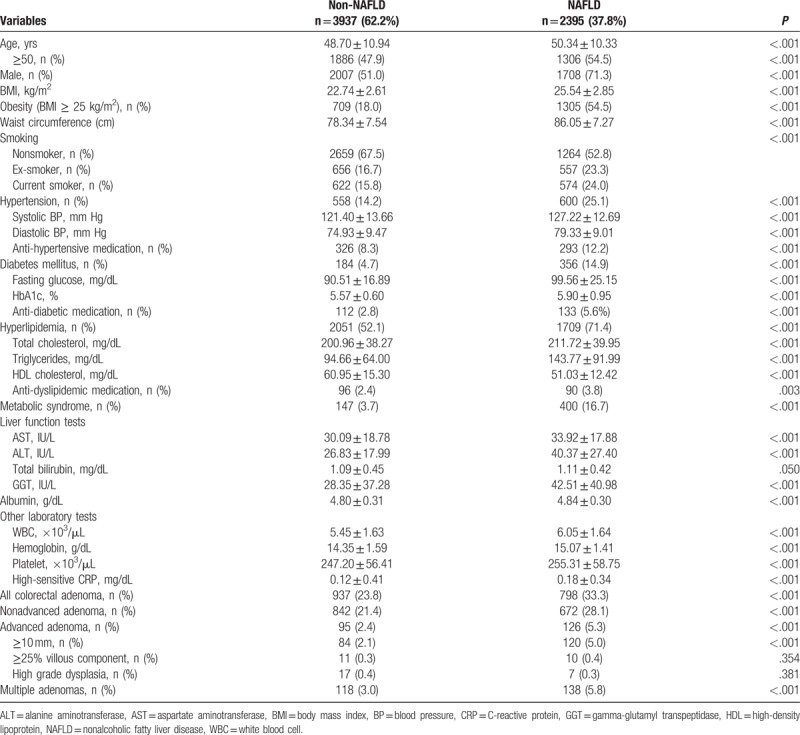
Among the 6332 subjects enrolled in this study, 2395 (37.8%) had NAFLD. The baseline characteristics are shown in Table 1. The subjects with NAFLD were older (50.34 ± 10.33 vs 48.70 ± 10.94, P < .001) and predominantly men (71.3% vs 51.0%, P < .001), had a higher BMI (25.54 ± 2.85 vs 22.74 ± 2.61; P < .001), and were predominantly current smokers (24.0% vs 15.8%, P < .001) than those without NAFLD. The prevalence of hypertension (25.1% vs 14.2%, P < .001), DM (14.9% vs 4.7%, P < .001), hyperlipidemia (71.4% vs 52.1%, P < .001), and metabolic syndrome (16.7% vs 3.7%, P < .001) was higher in the subjects with NAFLD. In the liver function test, the levels of serum AST, ALT, and gamma-glutamyl transpeptidase were also significantly higher in the subjects with NAFLD.
Table 1.
Baseline characteristics of the study subjects with and without NAFLD.
3.2. Association between NAFLD and risk of colorectal adenoma in the study subjects
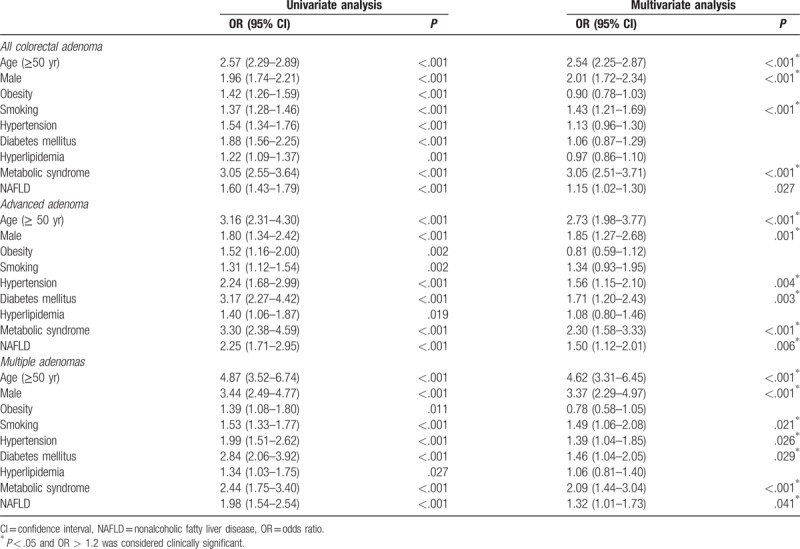
During the study period, colorectal adenoma was found in 1735 (27.4%) subjects on 1st-time colonoscopy. The subjects with NAFLD had a higher prevalence of colorectal adenoma (33.3% vs 23.8%, P < .001) than the subjects without NAFLD. Moreover, the prevalence of advanced adenoma (5.3% vs 2.4%, P < .001) and multiple adenomas (5.8% vs 3.0%, P < .001) was also higher in the subjects with NAFLD (Table 1). After adjusting for confounding factors, including age, sex, obesity, smoking, hypertension, DM, hyperlipidemia, and metabolic syndrome, the subjects with NAFLD had a significantly higher risk for colorectal adenoma (OR, 1.15; 95% CI, 1.02–1.30; P = .027). Further, NAFLD was also an independent risk factor for advanced adenoma (OR, 1.50; 95% CI, 1.12–2.01; P = .006) and multiple adenomas (OR, 1.32; 95% CI, 1.01–1.73; P = .006) (Table 2).
Table 2.
Univariate and multivariate logistic regression analyses of the risk factors of colorectal adenoma.
3.3. Association between advanced liver fibrosis and risk of colorectal adenoma in NAFLD
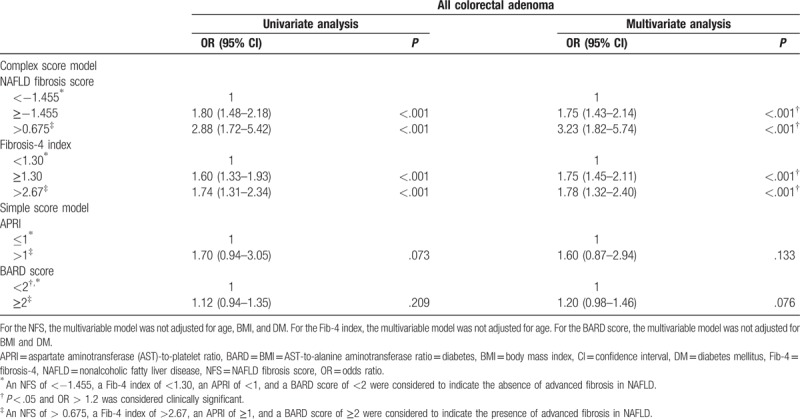
The prevalence of colorectal adenoma was significantly higher in the subjects with NAFLD with advanced fibrosis than in those with NAFLD without advanced fibrosis, which was stratified using the NFS and Fib-4 index (Supplementary Table 3). The association between advanced fibrosis in NAFLD and risk of colorectal adenoma is shown in Table 3. Advanced fibrosis, which was stratified using the NFS and Fib-4 index while adjusting for confounding factors, was associated with significantly higher risks of colorectal adenoma in the subjects with NAFLD. However, there was no association with the risk for colorectal adenoma and advanced fibrosis when stratified using the APRI and BARD score.
Table 3.
Univariate and multivariate logistic regression analyses of the risk of colorectal adenoma according to the parameters of NAFLD severity.
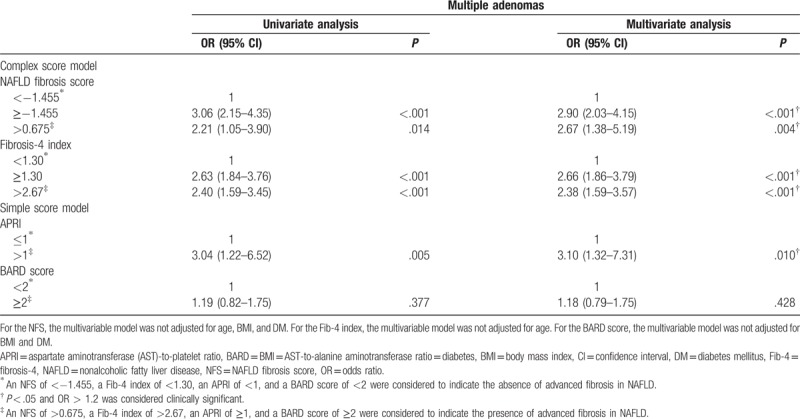
3.4. Association between advanced liver fibrosis and risk of advanced adenoma and multiple adenomas in NAFLD
The prevalence of advanced adenoma and multiple adenomas was significantly higher in the subjects with NAFLD with advanced fibrosis than in those with NAFLD without advanced fibrosis, which was stratified using the NFS, Fib-4 index, and APRI (Supplementary Table 4). After adjusting for confounding factors, NAFLD with advanced fibrosis, which was stratified using the NFS, Fib-4 index, and APRI, was an independent risk factor for advanced adenoma (Table 4) and multiple adenomas (Table 5).
Table 4.
Univariate and multivariate logistic regression analyses of the risk of advanced adenoma according to the parameters of NAFLD severity in the subjects with NAFLD.
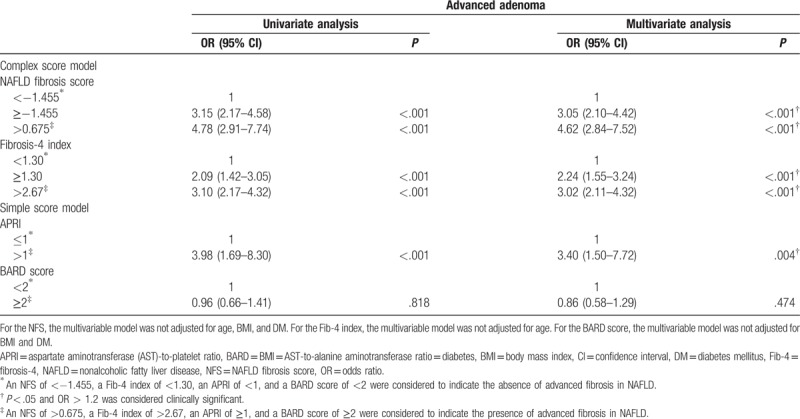
Table 5.
Univariate and multivariate logistic regression analyses of the risk of multiple adenomas according to the parameters of NAFLD severity in the subjects with NAFLD.
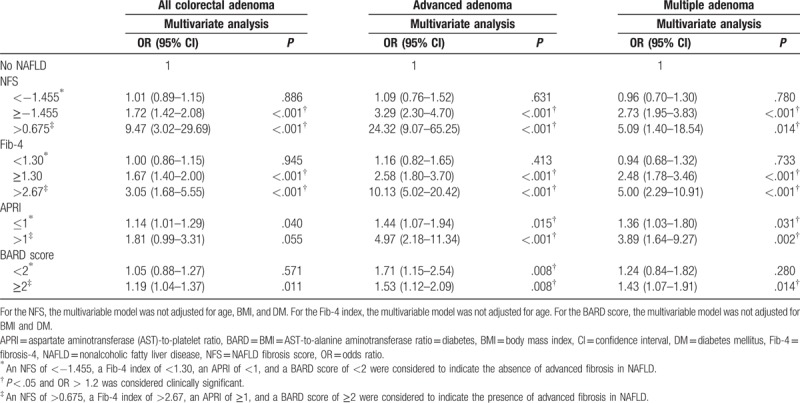
3.5. Association between advanced liver fibrosis and risk of colorectal adenoma in the study subjects
The risk of colorectal adenoma, advanced adenoma, and multiple adenomas in the subjects with NAFLD and advanced fibrosis was more robust than that in the subjects without NAFLD in all noninvasive score models (Table 6). However, the risk of colorectal adenoma, advanced adenoma, and multiple adenomas in those with NAFLD without advanced fibrosis was not significantly different from those without NAFLD.
Table 6.
Risk of colorectal adenoma according to the parameters of NAFLD severity in the study population.
4. Discussion
In this study, the presence of NAFLD was associated with the risk of colorectal adenoma, advanced adenoma, and multiple adenomas in asymptomatic adults who underwent 1st-time screening colonoscopy. After adjusting for demographic and metabolic risk factors, which are currently considered to be risk factors for colorectal neoplasm, our study showed that not only age, male sex, and metabolic syndrome but also NAFLD was a significant independent risk factor for colorectal adenoma, advanced adenoma, and multiple adenomas. Based on these results, we further investigated the risk of colorectal adenoma according to the staging of fibrosis in NAFLD, which was closely associated with a higher risk of colorectal adenoma.
The relationship between ultrasound-diagnosed NAFLD and colorectal adenoma has been already reported in previous population-based studies.[19–21] In these studies, NAFLD was an independent risk factor for colorectal neoplasia. Though our results are similar with ones from these studies, NAFLD was an independent risk factor for colorectal adenoma without clinical significance owing to relatively low OR (Table 2).[22] In a cross-sectional study of 181 community subjects aged 40 to 70 years and 191 subjects with biopsy-proven NAFLD, NAFLD was associated with a higher prevalence of colorectal adenoma and advanced adenoma.[20] This study showed that nonalcoholic steatohepatitis (NASH) was associated with a high prevalence of colorectal adenoma and advanced adenoma but not NAFLD without NASH among subjects with biopsy-proven NAFLD. Although liver biopsy is the gold standard for assessing the severity of NAFLD, it can be performed only in a limited number of patients with NAFLD, which also causes a potential selection bias. Moreover, liver biopsy has potential risks for sampling errors, intra- and inter-observer variabilities, and complications such as pain, bleeding, and even death.[23] Therefore, many previous studies have used various noninvasive score models, which are applicable to common patients with NAFLD, including the APRI, BARD score, NFS, Fib-4 index, Hepascore, Enhanced Liver Fibrosis test score, FibroTest score, and FibroMeter score.[13,15,18,24,25] Most of these score models stratified patients with NAFLD according to the presence of advanced fibrosis using indirect serum markers and demographic features. Among these models, we used 4 score models, including the NFS, Fib-4 index, APRI, and BARD score. The scoring system of these 4 models consists of clinical and laboratory data, including age, obesity, DM, transaminase level, platelet count, and albumin level (Supplementary Table 5), which are routinely measured and readily available through a general health screening program.
The NFS includes 6 variables, including age, hyperglycemia, BMI, platelet count, AST-to-ALT ratio, and albumin level, which are independent risk factors for advanced fibrosis. It has a dual cutoff value; a low cutoff value of −1.455 for excluding advanced fibrosis with a 93% negative predictive value and a high cutoff value of 0.676 for diagnosing advanced fibrosis with a 90% positive predictive value.[15] The Fib-4 index was originally developed for predicting advanced fibrosis in patients infected with HIV-HCV using age and routine tests, including the AST and ALT levels and platelet count.[24] The Fib-4 index has also showed a superiority to other noninvasive markers, such as the NFS, Göteborg University Cirrhosis index, AST-to-ALT ratio, APRI, and BARD score, in diagnosing advanced fibrosis in patients with NAFLD as well as in HIV-HCV coinfected patients in several studies.[16,26] It also has a dual cutoff value; the high cutoff value of 2.67 predicts the diagnosis of advanced fibrosis with an 80% positive predictive value, and the low cutoff value of 1.30 predicts the exclusion of advanced fibrosis with a 90% negative predictive value. The APRI, which was one of the simple models, consists of the AST and ALT levels and platelet count, which could predict significant fibrosis in patients with chronic hepatitis C.[25] A recent study has shown that an APRI above 1 was the most significant predictor for advanced fibrosis in NAFLD among 358 patients with biopsy-proven NAFLD.[17] The other simple model, the BARD score, consists of the weighted sum of BMI, AST-to-ALT ratio, and DM to predict significant fibrosis in patients with NAFLD.[18] Its cutoff value of 2 predicts advanced fibrosis with a 43% positive predictive value and 96% negative predictive value. However, it has limitations of a relatively high false positivity owing to the overestimation of BMI and DM and has the lowest predictive values for predicting significant fibrosis among the APRI, Fib-4 index, Hepascore, and FibroTest score.[27]
In our study, the risk of colorectal adenoma was significantly higher in the subjects with NAFLD and advanced fibrosis than in those with NAFLD without advanced fibrosis; this result was more robust when NAFLD was stratified using the complex score models, including the NFS and Fib-4 index. This result is supported by the findings of several studies that compared accuracy of predicting advanced fibrosis of NAFLD between complex score models and simple score models. In a multicenter study of 242 patients with biopsy-proven NAFLD, the complex models, including the Hepascore, FibroTest score, and Fib-4 index, were more accurate in the prediction of NAFLD with advanced fibrosis than the simple models, including the APRI and BARD score.[27] In a meta-analysis on the diagnostic accuracy of noninvasive tests for liver disease severity, the FibroTest score, Enhanced Liver Fibrosis test score, and NFS had higher diagnostic accuracies than the APRI and BARD score.[28]
There have been several observational and experimental studies conducted on the mechanisms that can support the finding that NAFLD affects the development of colorectal neoplasm. These studies have shown that chronic inflammation and insulin resistance could induce the development of colorectal neoplasm.[29] It is known that NAFLD is also related to the proinflammatory state and insulin resistance, and these affect each other.[30] Intrahepatic lipid accumulation induces hepatic cells to produce proinflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and IL-8, which play a key role in angiogenesis, promotion of cellular proliferation, and inhibition of apoptosis in the development of colorectal neoplasm.[31–33] Insulin resistance in the hepatic and adipose tissues induces hyperinsulinemia and elevated insulin-like growth factor (IGF), which may promote the development of colorectal neoplasm through proliferative and anti-apoptotic effects.[34] Adiponectin, one of the adipokines with anti-inflammatory effects, is also an important cytokine involved in the association between NAFLD and colorectal neoplasm.[34,35] Several studies have shown that adiponectin directly inhibits TNF-α and that low levels of adiponectin increase insulin and IGF-1 levels.[36,37] The expression of adiponectin was reduced in patients with NAFLD, which was associated with a high risk of colorectal adenoma.[34,38] A positive correlation was observed between the degree of liver fibrosis and level of proinflammatory cytokines, including TNF-α and IL-6. Further, NASH yields significantly lower levels of adiponectin.[38] Moreover, there was a significant association between insulin resistance and liver fibrosis.[39] These findings demonstrate the potential risk of colorectal adenoma in patients with NAFLD and advanced fibrosis.
Our study has some limitations. First, this study was restricted to colorectal adenoma, except for CRC, because CRC was diagnosed in only 18 subjects during the study period, and its prevalence did not show any difference according to the presence of NAFLD. We think that further large-scale studies are needed to evaluate the relationship between CRC and NAFLD with and without fibrosis. Second, this cross-sectional study was conducted during a health screening program in a single center. The subjects enrolled in this study visited the hospital for the health screening program and might not be representative of the general population with an average risk. Therefore, this study could not infer any causality between NAFLD and colorectal adenoma. Additionally, South Koreans have different colorectal adenoma characteristics, such as prevalence and location, compared with Westerners.[40] To achieve generalizability of the findings of this study, further studies involving multiple regions or ethnicities are needed. However, the findings of this study are meaningful owing to the relatively large number of subjects than in other studies. Moreover, most results from various models for the prediction of fibrosis are constant in patients with NAFLD.
Our findings demonstrated that NAFLD is independently associated with a high risk of colorectal adenoma as well as advanced adenoma and multiple adenomas. Furthermore, the presence of advanced fibrosis significantly increased the risk for colorectal adenoma, advanced adenoma, and multiple adenomas compared with the absence of advanced fibrosis. Further studies are needed to evaluate the intervals of colonoscopy surveillance after 1st-time colonoscopy in patients with NAFLD with and without fibrosis. In conclusion, when patients with NAFLD are considered for colonoscopy screening, clinicians should be aware that patients with fibrosis may have a higher risk for colorectal adenoma than those without fibrosis.
Author contributions
JGP and BIJ is guarantor of integrity of the entire study. JGP and HJL designed the study. MCK collected data, which was analyzed by MCK and WKL based on the statistical analysis plan. MCK drafted the manuscript, which was critically revised by JGP and BIJ.
Conceptualization: Jung Gil Park, Byung Ik Jang, Heon Ju Lee.
Formal analysis: Min Cheol Kim.
Investigation: Min Cheol Kim.
Methodology: Won Kee Lee.
Supervision: Jung Gil Park, Byung Ik Jang, Heon Ju Lee.
Writing – original draft: Min Cheol Kim.
Writing – review & editing: Jung Gil Park.
Supplementary Material
Footnotes
Abbreviations: ALT = alanine aminotransferase, APRI = aspartate aminotransferase-to-platelet ratio index, AST = aspartate aminotransferase, BARD = body mass index, aspartate aminotransferase-to-alanine aminotransferase ratio, diabetes, BMI = body mass index, CRC = colorectal cancer, DM = diabetes mellitus, Fib-4 = fibrosis-4, NAFLD = nonalcoholic fatty liver disease, NASH = nonalcoholic steatohepatitis, NFS = NAFLD fibrosis score.
JGP and BIJ contributed equally to this study.
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (2017R1C1B5076851).
This manuscript is edited by professional English editing and publication support service supported by Editage.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012: Cancer Incidence and Mortality Worldwide: IARC CancerBase no. 11. Lyon, France: International Agency for Research on Cancer; 2013. [Google Scholar]
- [2].Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683–91. [DOI] [PubMed] [Google Scholar]
- [3].Jackman R, Mayo CW. The adenoma-carcinoma sequence in cancer of the colon. Surg Gynecol Obstet 1951;93:327–30. [PubMed] [Google Scholar]
- [4].Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med 1993;329:1977–81. [DOI] [PubMed] [Google Scholar]
- [5].Tao S, Hoffmeister M, Brenner H. Development and validation of a scoring system to identify individuals at high risk for advanced colorectal neoplasms who should undergo colonoscopy screening. Clin Gastroenterol Hepatol 2014;12:478–85. [DOI] [PubMed] [Google Scholar]
- [6].Wei EK, Ma J, Pollak MN, et al. A prospective study of C-peptide, insulin-like growth factor-I, insulin-like growth factor binding protein-1, and the risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev 2005;14:850–5. [DOI] [PubMed] [Google Scholar]
- [7].Frezza EE, Wachtel MS, Chiriva-Internati M. Influence of obesity on the risk of developing colon cancer. Gut 2006;55:285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005–23. [DOI] [PubMed] [Google Scholar]
- [9].Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease. Diabetes 2001;50:1844–50. [DOI] [PubMed] [Google Scholar]
- [10].Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines: management of nonalcoholic fatty liver disease. Clin Mol Hepatol 2013;19:325–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002;346:1221–31. [DOI] [PubMed] [Google Scholar]
- [12].Sanna C, Rosso C, Marietti M, et al. Non-alcoholic fatty liver disease and extra-hepatic cancers. Int J Mol Sci 2016;17:717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Buzzetti E, Lombardi R, De Luca L, et al. Noninvasive assessment of fibrosis in patients with nonalcoholic fatty liver disease. Int J Endocrinol 2015;343828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Oh H, Jun DW, Saeed WK, et al. Non-alcoholic fatty liver diseases: update on the challenge of diagnosis and treatment. Clin Mol Hepatol 2016;22:327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846–54. [DOI] [PubMed] [Google Scholar]
- [16].Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tapper EB, Krajewski K, Lai M, et al. Simple non-invasive biomarkers of advanced fibrosis in the evaluation of non-alcoholic fatty liver disease. Gastroenterol Rep 2014;2:276–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Harrison SA, Oliver D, Arnold HL, et al. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 2008;57:1441–7. [DOI] [PubMed] [Google Scholar]
- [19].Hwang ST, Cho YK, Park JH, et al. Relationship of non-alcoholic fatty liver disease to colorectal adenomatous polyps. J Gastroenterol Hepatol 2010;25:562–7. [DOI] [PubMed] [Google Scholar]
- [20].Wong VW-S, Wong GL-H, Tsang SW-C, et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut 2011;60:829–36. [DOI] [PubMed] [Google Scholar]
- [21].Lee YI, Lim YS, Park HS. Colorectal neoplasms in relation to non-alcoholic fatty liver disease in Korean women: a retrospective cohort study. J Gastroenterol Hepatol 2012;27:91–5. [DOI] [PubMed] [Google Scholar]
- [22].Monson RR. Occupational Epidemiology. 2nd ed.Boca Raton, FL: CRC Press; 1990. [Google Scholar]
- [23].Seeff LB, Everson GT, Morgan TR, et al. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol 2010;8:877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- [25].Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518–26. [DOI] [PubMed] [Google Scholar]
- [26].Sumida Y, Yoneda M, Hyogo H, et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol 2012;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Adams LA, George J, Bugianesi E, et al. Complex non-invasive fibrosis models are more accurate than simple models in non-alcoholic fatty liver disease. J Gastroenterol Hepatol 2011;26:1536–43. [DOI] [PubMed] [Google Scholar]
- [28].Musso G, Gambino R, Cassader M, et al. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med 2011;43:617–49. [DOI] [PubMed] [Google Scholar]
- [29].Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr 2001;131:3109S–20S. [DOI] [PubMed] [Google Scholar]
- [30].Wong VWS, Hui AY, Tsang SWC, et al. Metabolic and adipokine profile of Chinese patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2006;4:1154–61. [DOI] [PubMed] [Google Scholar]
- [31].Braunersreuther V, Viviani GL, Mach F, et al. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J Gastroenterol 2012;18:727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].De Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer 2006;6:24–37. [DOI] [PubMed] [Google Scholar]
- [33].Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005;7:211–7. [DOI] [PubMed] [Google Scholar]
- [34].Hui JM, Hodge A, Farrell GC, et al. Beyond insulin resistance in NASH: TNF-α or adiponectin? Hepatology 2004;40:46–54. [DOI] [PubMed] [Google Scholar]
- [35].Ferroni P, Palmirotta R, Spila A, et al. Prognostic significance of adiponectin levels in non-metastatic colorectal cancer. Anticancer Res 2007;27:483–9. [PubMed] [Google Scholar]
- [36].Rose D, Komninou D, Stephenson G. Obesity, adipocytokines, and insulin resistance in breast cancer. Obesity Rev 2004;5:153–65. [DOI] [PubMed] [Google Scholar]
- [37].Wei EK, Giovannucci E, Fuchs CS, et al. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J Natl Cancer Inst 2005;97:1688–94. [DOI] [PubMed] [Google Scholar]
- [38].Wong VWS, Wong GLH, Tsang SWC, et al. Genetic polymorphisms of adiponectin and tumor necrosis factor-alpha and nonalcoholic fatty liver disease in Chinese people. J Gastroenterol Hepatol 2008;23:914–21. [DOI] [PubMed] [Google Scholar]
- [39].Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med 1999;107:450–5. [DOI] [PubMed] [Google Scholar]
- [40].Cha JM, Kozarek RA, La Selva D, et al. Disparities in prevalence, location, and shape characteristics of colorectal neoplasia between South Korean and US patients. Gastrointest Endosc 2015;82:1080–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


