Abstract
Uveal melanoma (UM) is the major intraocular malignancy in adults, of which the molecular biology is still unknown. Therefore, this study was designed to determine the aqueous concentrations of angiogenic, inflammatory, and chemotactic cytokines in eyes with UM.
Aqueous humor samples were collected from 38 patients with UM and 22 patients undergoing cataract surgery. Interleukin 6, 8 (IL-6, IL-8, respectively), interferon-inducible protein-10 (IP-10), placental growth factor1 (PIGF1), regulated on activation, normal T Cell expressed and secreted (RANTES), monocyte chemoattractant protein-1 (MCP-1), nerve growth factor-beta (NGF-β), epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), and vascular endothelia growth factor A (VEGF-A) were assessed by multiplex bead assay.
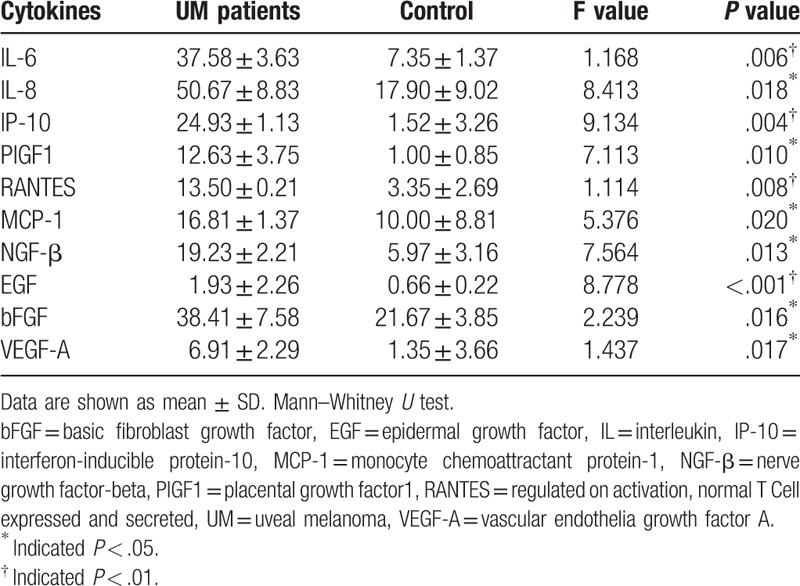
In the study group, significantly higher concentrations of IL-6 (P = .006), IL-8 (P = .018), IP-10 (P = .004), RANTES (P = .008), MCP-1 (P = .02), NGF-β (P = .013), EGF (P < .001), PIGF1 (P = .01), bFGF (P = .016), and VEGF (P = .017) were measured, when compared with the control group.
Several angiogenic, inflammatory, and chemotactic cytokines are highly expressed in the aqueous humor of the UM eyes, which provides new insights into the pathophysiology of UM and could be potential targets for treatment.
Keywords: aqueous humor, cytokines, uveal melanoma
1. Introduction
Uveal melanoma (UM) is the most common intraocular malignancy in adults with a poor prognosis because of a high incidence of metastases, of which the 5-year survival rate is about 60%.[1–4] Although many therapies have been developed to keep excellent local control, little progress has been made to alter the disease course[5] and improve the survival rate.[6,7] In metastatic UM, systemic chemotherapy is usually unsuccessful. To improve the prognosis of UM, the molecular mechanisms involved in UM progression and metastasis should be cleared.
Recently, a few studies indicated that vascular endothelia growth factor A (VEGF-A) and basic fibroblast growth factor (bFGF) were highly expressed in human eyes with UM.[8–10] As the metastasis of UM is mainly hematogenous, angiogenesis probably plays a crucial role in UM. Additionally, mounting evidence indicates that inflammatory processes may involve in the UM progression. Macrophages were detected variable in UM by several studies. A high density of tumor-associated macrophages (TAMs) probably indicates poor prognosis.[11] Meanwhile, UM cells could produce inflammatory cytokines such as IL-6 and IL-10, which induce TAMs differentiation to the M2-type. M2-type was considered to have a tumor-promoting role rather than an effective immune response. The infiltrating macrophages could also produce VEGF, which indicated macrophages probably play roles in angiogenesis and Inflammation. Therefore, in this study, we determined the aqueous concentrations of angiogenic, inflammatory, and chemotactic cytokines in UM patients, and estimated their potential implications in the pathogenesis of UM.
2. Methods
The Ethics Committee and Institutional Review Board of Peking University People's Hospital approved this study, and written informed consent was obtained from each patient in accordance with the Declaration of Helsinki. All the patients were diagnosed at Peking University People's Hospital. Exclusion criteria included previous intraocular surgery, concomitant ocular diseases, concomitant systemic diseases such as hypertension or diabetes, prior cardio-cerebrovascular accidents within the past 6 months. Undiluted aqueous humor samples were collected from 38 eyes with UM immediately after enucleation and 22 eyes of cataract patients before cataract surgery. Aqueous samples of 0.05 mL from each eye were extracted using a standard sterilization procedure and immediately stored in a freezer at −80°C until assay. And they were tested within 6 months of collection.
Cytokines were measured via Luminex X-MAP technology using the Procarta Immunoassay kit (Panomics Inc, Fremont, CA), as described previously.[12] Analyzed cytokines in this study included interleukin 6, 8 (IL-6, IL-8, respectively), interferon-inducible protein-10 (IP-10), placental growth factor1 (PIGF1), regulated on activation, normal T cell expressed and secreted (RANTES), monocyte chemoattractant protein-1 (MCP1), nerve growth factor-beta (NGF-β), epidermal growth factor (EGF), bFGF, and VEGF-A.
All data were analyzed using SPSS 20.0 for Mac (SPSS, IBM Corp, New York). Data are presented as the mean ± standard deviation, and the normality of the distribution was assessed by the Shapiro–Wilk test. The nonparametric Mann–Whitney rank sum test and t test were performed to compare the differences between the study group and the control group. Two-tailed probability of less than.05 was considered to be a statistically significant difference.
3. Results
The study included 38 patients with UM in the study group and 22 patients with cataract in the control group. The mean age of the UM patients was 58.45 ± 12.36 years and that of the control group was 61.36 ± 7.62 years (P = .23). There was no significant difference in gender between 2 groups (P = .19). In the UM patients group, 47.7% were women, while that in the control group was 45.5% (Table 1). The tumor characteristics of UM patients were summarized in Table 2. Right eye was involved in 20 patients. 78.9% of the tumors were choroidal, while the rest were ciliochoroidal. Based on the AJCC 7th classification, tumor size categories were T3 in 29 patients (76.3%), and T4 in 9 patients (23.7%). Ten patients were in tumor stage II, while the rest were in tumor stage III. The mean largest basal tumor diameter was 15.8 mm (range, 12.5–25), and the tumor thickness was 10.2 mm (range, 8.1–17). No patient has extrascleral extension of tumor. All the patients were associated with serous retinal detachment.
Table 1.
Demographics of study population.
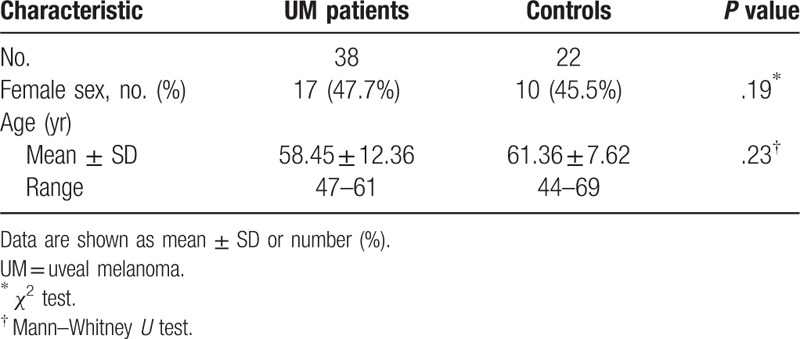
Table 2.
Summary data on baseline ocular and tumor variables in patients with uveal melanoma.
Compared with cataract group, eyes with UM contained higher levels of all cytokines tested: IL-6 (P = .006), IL-8 (P = .018), IP-10 (P = .004), RANTES (P = .008), MCP-1 (P = .02), NGF-β (P = .013), EGF (P < .001), PIGF1 (P = .01), bFGF (P = .016), and VEGF (P = .017) (Table 3).
Table 3.
Aqueous humor concentrations (pg/mL) (mean ± SD) of cytokines in uveal melanoma patients and subjects undergoing routine cataract surgery (control group).
4. Discussion
In the current study, several angiogenic, inflammatory, and chemotactic cytokines are detected highly expressed in the aqueous humor of the UM eyes, when compared with the control eyes.
VEGF-A is a key pro-angiogenic factor associated with angiogenesis in numerous tumors.[13] As previous studies reported,[8,9] an abnormally high intraocular concentration of VEGF-A was also detected in eyes with UM in our study, probably producing by tumor cells and the tissues around.[9] Increased serum VEGF was also detected in metastatic UM patients.[14] Anti-VEGF therapy, such as bevacizumab, is currently used for the treatment of metastatic UM.[15] We also found high levels of bFGF in aqueous of UM patients. Like VEGF-A, bFGF is also a potent pro-angiogenic cytokine, acting synergistically with VEGF-A to promote angiogenesis.[16] Furthermore, we first demonstrated that the levels of PIGF1 elevated in the aqueous of UM patients in this study. PIGF1 is another important factor during retinal vascularization, belonging to the VEGF family. PIGF1 binds to VEGFR-1 and leads to angiogenesis.[17] However, the role of PlGF in terms of tumor angiogenesis and tumor growth remains controversial. Some studies claim that PlGF is a cancer target promoting tumor angiogenesis and tumor growth, and anti-PlGF is useful for anti-cancer treatment,[18–20] although other studies indicated that overexpression of PlGF suppresses tumor neovascularization and growth. Generally speaking, elevated angiogenic cytokines were detected in eyes with UM. As the metastasis of UM is mainly hematogenous, angiogenesis plays a crucial role in UM. Although antiangiogenic therapy has not yet been tested for the treatment of primary UM, it could be a potential choice for treatment in the future.
In this study, many inflammatory cytokines were also highly expressed in the aqueous of UM eyes. Generally, elevated IL-6, IL-8, sVCAM, IP-10, and RANTES were detected in the aqueous of uveitis patients.[21] Inflammation has been proved playing important roles in tumor cells proliferation, angiogenesis, and metastasis. It disrupts the effective immune responses, and alters responses to chemotactic cytokines. IL-8 is a member of the chemokine family produced by a variety of cell types that activate and recruit polymorph nuclear leukocytes in acute and chronic inflammatory process. Previous study by Lattanzio et al[22] indicated that IL-8 signal could be activated by the UM microenvironment as an alternative pro-angiogenic pathway beside VEGF. And it has been proven to trigger angiogenesis in vivo.[23] Besides the tumor cells, normal cells around also produced cytokines like IL-6, IL-10, TGF-β, MIF, GM-CSF, and VEGF, involving immune responses.[24] Elevations in IL-6 and IP-10 correlated with increased Treg infiltration.[25] Jager et al[26] defined macrophages with a high density in UM as “inflammatory phenotype,” usually accompanied with a high microvascular density and epithelioid cells. Macrophages differentiate to M2-type induced by IL-6 and IL-10, which have a tumor-promoting role associated with poor prognosis. Although the aqueous of UM-containing eyes showed a pattern of inflammatory phenotype, no diagnostic or prognostic value of individual cytokines was reported yet.
Recently, “tumor microenvironment” has attracted more attention in tumor growth and progression. A highlight in this field is the TAMs, which represent a predominant population of inflammatory cells in tumors. Chemo attractants such as MCP-1 could recruit TAMs from circulating monocytes into tissues.[27] Significantly elevated MCP-1 was detected in our study, which was proved to be correlated with TAM in UM in a recent study.[28] Besides the role of MCP-1 in chemotaxis of TAMs, it can also promote angiogenesis. And reduced angiogenesis and tumor growth were observed in a mouse model when MCP-1 was inhibited.[29] Epidermal growth factor receptor (EGFR) has been detected in many cancers and considered to be important in tumor growth. Expression of EGFR has been reported in 12.5% to 29.2% UM patients,[30,31] while the elevated vitreal EGF levels were also detected in some UM patients. And in our study, the expression of EGF in aqueous of UM patients is significantly higher than the control group. As previous study reported, the expression of EGFR and EGF were correlated with mitosis rate and scleral invasion, respectively;[31] which indicated that EGF-EGFR signal seems to be a novel therapeutic target for UM. NGF-β was another elevated cytokine in our study, which was found to modulate the tumor cells migration, inflammatory response and promote tumor progression.[32–34] Expression of NGF-R was found in primary and metastatic melanoma lesions, associated with a poor prognosis.[35]
Certainly, there are still some limitations in our study. The relations between cytokines and clinicopathological parameters of tumor could not be analyzed because of the incomplete clinical data. Previous studies reported that elevated cytokines probably correlate with tumor size and the severity of retinal detachment; however, no correlation was found in this group probably caused by the selection bias and small sample size. Meanwhile, it is unclear of the causal relationship between tumor and elevated cytokines. Thus, further studies are necessary to clarify the role of cytokines in pathogenesis of UM.
5. Conclusion
In conclusion, this study demonstrated that eyes with UM contain higher aqueous concentrations of angiogenic, inflammatory, and chemotactic cytokines, which indicate that these cytokines were closely interrelated with the disease and could be potential targets for treatment.
Author contributions
YC, JF, and XZ contributed to conception, designed the study, collected and analyzed data, as well as drafted manuscript and submission. JL contributed to protocol assessment, data interpretation, and manuscript revising. All authors read and approved the final manuscript.
Conceptualization: Jianhong Liang.
Data curation: Jing Feng.
Formal analysis: Yong Cheng.
Investigation: Xuemei Zhu.
Methodology: Yong Cheng, Jing Feng.
Project administration: Xuemei Zhu.
Supervision: Jianhong Liang.
Writing – original draft: Yong Cheng, Jing Feng.
Writing – review & editing: Xuemei Zhu.
Footnotes
Abbreviations: bFGF = basic fibroblast growth factor, EGF = epidermal growth factor, EGFR = epidermal growth factor receptor, IL = interleukin, IP-10 = interferon-inducible protein-10, MCP-1 = monocyte chemoattractant protein-1, NGF-β = nerve growth factor-beta, PIGF1 = placental growth factor1, RANTES = regulated on activation, normal T Cell expressed and secreted, sVCAM = soluble vascular cell adhesion molecule, TAMs = tumor-associated macrophages, UM = uveal melanoma, VEGF-A = vascular endothelia growth factor A.
YC, JF, and XZ are cofirst authors.
The Ethics Committee and Institutional Review Board of Peking University People's Hospital approved this study, and written informed consent was obtained from each patient in accordance with the Declaration of Helsinki.
The authors have no conflicts of interest to disclose.
References
- [1].Singh AD, Topham A. Survival rates with uveal melanoma in the United States: 1973–1997. Ophthalmology 2003;110:962–5. [DOI] [PubMed] [Google Scholar]
- [2].Bergman L, Seregard S, Nilsson B, et al. Uveal melanoma survival in Sweden from 1960 to 1998. Invest Ophthalmol Vis Sci 2003;44:3282–7. [DOI] [PubMed] [Google Scholar]
- [3].Burr JM, Mitry E, Rachet B, et al. Survival from uveal melanoma in England and Wales 1986 to 2001. Ophthalmic Epidemiol 2007;14:3–8. [DOI] [PubMed] [Google Scholar]
- [4].Caminal JM, Ribes J, Cleries R, et al. Relative survival of patients with uveal melanoma managed in a single center. Melanoma Res 2012;22:271–7. [DOI] [PubMed] [Google Scholar]
- [5].Krantz BA, Dave N, Komatsubara KM, et al. Uveal melanoma: epidemiology, etiology, and treatment of primary disease. Clin Ophthalmol 2017;11:279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Buder K, Gesierich A, Gelbrich G, et al. Systemic treatment of metastatic uveal melanoma: review of literature and future perspectives. Cancer Med 2013;2:674–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch Ophthalmol 2006;124:1684–93. [DOI] [PubMed] [Google Scholar]
- [8].Boyd SR, Tan D, Bunce C, et al. Vascular endothelial growth factor is elevated in ocular fluids of eyes harbouring uveal melanoma: identification of a potential therapeutic window. Br J Ophthalmol 2002;86:448–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Missotten GS, Notting IC, Schlingemann RO, et al. Vascular endothelial growth factor a in eyes with uveal melanoma. Arch Ophthalmol 2006;124:1428–34. [DOI] [PubMed] [Google Scholar]
- [10].Boyd SR, Tan DS, de Souza L, et al. Uveal melanomas express vascular endothelial growth factor and basic fibroblast growth factor and support endothelial cell growth. Br J Ophthalmol 2002;86:440–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Makitie T, Summanen P, Tarkkanen A, et al. Tumor-infiltrating macrophages (CD68(+) cells) and prognosis in malignant uveal melanoma. Invest Ophthalmol Vis Sci 2001;42:1414–21. [PubMed] [Google Scholar]
- [12].Feng J, Zheng X, Li B, et al. Differences in aqueous concentrations of cytokines in paediatric and adult patients with Coats’ disease. Acta Ophthalmol 2017;95:608–12. [DOI] [PubMed] [Google Scholar]
- [13].Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 1990;82:4–6. [DOI] [PubMed] [Google Scholar]
- [14].Vihinen PP, Hilli J, Vuoristo MS, et al. Serum VEGF-C is associated with metastatic site in patients with malignant melanoma. Acta Oncol 2007;46:678–84. [DOI] [PubMed] [Google Scholar]
- [15].Piperno-Neumann S, Diallo A, Etienne-Grimaldi MC, et al. Phase II trial of bevacizumab in combination with temozolomide as first-line treatment in patients with metastatic uveal melanoma. Oncologist 2016;21:281–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pepper MS, Mandriota SJ, Jeltsch M, et al. Vascular endothelial growth factor (VEGF)-C synergizes with basic fibroblast growth factor and VEGF in the induction of angiogenesis in vitro and alters endothelial cell extracellular proteolytic activity. J Cell Physiol 1998;177:439–52. [DOI] [PubMed] [Google Scholar]
- [17].Akrami H, Soheili ZS, Sadeghizadeh M, et al. PlGF gene knockdown in human retinal pigment epithelial cells. Graefes Arch Clin Exp OphthalmolV 249 2011;537–46. [DOI] [PubMed] [Google Scholar]
- [18].Odorisio T, Schietroma C, Zaccaria ML, et al. Mice overexpressing placenta growth factor exhibit increased vascularization and vessel permeability. J Cell Sci 2002;115(pt 12):2559–67. [DOI] [PubMed] [Google Scholar]
- [19].Fischer C, Jonckx B, Mazzone M, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 2007;131:463–75. [DOI] [PubMed] [Google Scholar]
- [20].Van de Veire S, Stalmans I, Heindryckx F, et al. Further pharmacological and genetic evidence for the efficacy of PlGF inhibition in cancer and eye disease. Cell 2010;141:178–90. [DOI] [PubMed] [Google Scholar]
- [21].van Kooij B, Rothova A, Rijkers GT, et al. Distinct cytokine and chemokine profiles in the aqueous of patients with uveitis and cystoid macular edema. Am J Ophthalmol 2006;142:192–4. [DOI] [PubMed] [Google Scholar]
- [22].Lattanzio L, Tonissi F, Torta I, et al. Role of IL-8 induced angiogenesis in uveal melanoma. Invest New Drugs 2013;31:1107–14. [DOI] [PubMed] [Google Scholar]
- [23].Giulian D, Woodward J, Young DG, et al. Interleukin-1 injected into mammalian brain stimulates astrogliosis and neovascularization. J Neurosci 1988;8:2485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Oliva M, Rullan AJ, Piulats JM. Uveal melanoma as a target for immune-therapy. Ann Transl Med 2016;4:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nagarkatti-Gude N, Bronkhorst IH, van Duinen SG, et al. Cytokines and chemokines in the vitreous fluid of eyes with uveal melanoma. Invest Ophthalmol Vis Sci 2012;53:6748–55. [DOI] [PubMed] [Google Scholar]
- [26].Jager MJ, Ly LV, El Filali M, et al. Macrophages in uveal melanoma and in experimental ocular tumor models: friends or foes? Prog Retinal Eye Res 2011;30:129–46. [DOI] [PubMed] [Google Scholar]
- [27].Lee CS, Jun IH, Kim TI, et al. Expression of 12 cytokines in aqueous humour of uveal melanoma before and after combined Ruthenium-106 brachytherapy and transpupillary thermotherapy. Acta Ophthalmol 2012;90:e314–20. [DOI] [PubMed] [Google Scholar]
- [28].Ly LV, Bronkhorst IH, van Beelen E, et al. Inflammatory cytokines in eyes with uveal melanoma and relation with macrophage infiltration. Invest Ophthalmol Vis Sci 2010;51:5445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Koga M, Kai H, Egami K, et al. Mutant MCP-1 therapy inhibits tumor angiogenesis and growth of malignant melanoma in mice. Biochem Biophys Res Commun 2008;365:279–84. [DOI] [PubMed] [Google Scholar]
- [30].Amaro A, Mirisola V, Angelini G, et al. Evidence of epidermal growth factor receptor expression in uveal melanoma: inhibition of epidermal growth factor-mediated signalling by Gefitinib and Cetuximab triggered antibody-dependent cellular cytotoxicity. Eur J Cancer 2013;49:3353–65. [DOI] [PubMed] [Google Scholar]
- [31].Topcu-Yilmaz P, Kiratli H, Saglam A, et al. Correlation of clinicopathological parameters with HGF, c-Met, EGFR, and IGF-1R expression in uveal melanoma. Melanoma Res 2010;20:126–32. [DOI] [PubMed] [Google Scholar]
- [32].Li B, Cai S, Zhao Y, et al. Nerve growth factor modulates the tumor cells migration in ovarian cancer through the WNT/beta-catenin pathway. Oncotarget 2016;7:81026–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Manni L, Lundeberg T, Fiorito S, et al. Nerve growth factor release by human synovial fibroblasts prior to and following exposure to tumor necrosis factor-alpha, interleukin-1 beta and cholecystokinin-8: the possible role of NGF in the inflammatory response. Clin Exp Rheumatol 2003;21:617–24. [PubMed] [Google Scholar]
- [34].Yue XJ, Xu LB, Zhu MS, et al. Over-expression of nerve growth factor-beta in human cholangiocarcinoma QBC939 cells promote tumor progression. PLoS One 2013;8:e62024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Brocker EB, Magiera H, Herlyn M. Nerve growth and expression of receptors for nerve growth factor in tumors of melanocyte origin. J Invest Dermatol 1991;96:662–5. [DOI] [PubMed] [Google Scholar]


