Supplemental Digital Content is available in the text
Keywords: colorectal cancer, inflammation-based indexes, prognosis
Abstract
Inflammation-based indexes such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), and systemic immune-inflammation indexes (SII) have been reported to be associated with prognosis in cancer patients.
The aim of this study was to estimate the prognostic significance of inflammation-based indexes such as NLR, PLR, LMR, and SII in stage III/IV colorectal cancer (CRC) patients undertaking adjuvant chemoradiotherapy (CRT).
Two hundred twenty stage III/IV CRC patients were enrolled in this study. Inflammatory indexes were defined as follows: NLR = absolute neutrophil counts/absolute lymphocyte counts; PLR = absolute platelet counts/absolute lymphocyte counts; LMR = absolute lymphocyte counts/absolute monocyte counts; SII = absolute neutrophil counts × absolute platelet counts/absolute lymphocyte counts. The correlations between indexes and prognosis were evaluated using the Cox proportional hazard model.
The results of univariate analysis demonstrated that NLR, PLR, and SII were significantly associated with progression-free survival (PFS) and overall survival (OS). Multivariate analysis showed that SII (P = .030) was an independent predictor of PFS, and NLR (P = .047) was an independent prognostic factor of OS.
Those inflammation-based indexes could provide a convenient and secure method to predict the outcomes of stage III/IV CRC patients receiving adjuvant CRT.
1. Introduction
Over the past few decades, advances in surgical techniques, radiotherapy, and drug therapy for cancer have dramatically improved outcomes of patients with colorectal cancer (CRC).[1,2] Previous studies have reported that postoperative radiotherapy could reduce the risk of local recurrence and death from rectal cancer as compared with surgery alone.[3,4] Moreover, in contrast to either surgery or postoperative radiotherapy alone, adjuvant chemoradiotherapy (CRT) was reported to be able to significantly improve both local control and overall survival (OS).[4,5] Several parameters such as Eastern Cooperative Oncology Group (ECOG) performance status, tumor-node-metastasis (TNM) stage, serum lactic dehydrogenase (LDH) level, carbohydrate antigen 19-9 (CA 19-9), and carbohydrate antigen-125 (CA-125) have been raised to be potential prognostic indicators for CRC patients in several previous studies.[6–9] However, there are still no validated predictors for survival among CRC patients receiving adjuvant CRT.
It has been generally accepted that both tumor characteristics and host inflammatory response play an important part in tumor progression.[10,11] Furthermore, inflammation-based indexes such as neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), platelet-to-lymphocyte ratio (PLR), and systemic immune-inflammation index (SII) have been identified to be able to serve as indexes of the host immune status, and that those indexes may be of prognostic significance in patients with different types of tumor.[12,13] However, to the best of our knowledge, the prognostic power of those inflammation-based indexes among metastatic stage III/IV CRC patients receiving adjuvant CRT is still not been well established.
In order to examine the associations between pretreatment inflammation-based indexes and prognosis of CRC patients undergoing adjuvant CRT, we retrospectively reviewed 220 eligible patients treated at West China Hospital between January 2009 and December 2015. The aim of this study was to examine the prognostic value of inflammation-based indexes in stage III/IV CRC patients receiving adjuvant CRT.
2. Materials and methods
2.1. Patients
We reviewed a database of patients with CRC treated at the West China Hospital from January 2009 to December 2015. The inclusion criteria included: Patients were diagnosed as primary stage III/IV CRC; patients received adjuvant CRT but not received preoperative chemotherapy or radiotherapy. Patients were excluded if they had primary malignant tumors in other organs. Those with clinical evidence of systemic inflammation, autoimmune diseases, hematologic disease, or bone marrow disease were also excluded. Finally, 220 eligible patients were analyzed in this study. All the clinicopathological data were retrieved from medical records. The stages of CRC were based on the TNM criteria of CRC (Union for International Cancer Control, 7th edition). This study was approved by the Ethics Administration Office of West China Hospital, Sichuan University.
2.2. Inflammatory indexes and endpoints
Laboratory data of patients within 2 weeks prior to radiotherapy such as absolute neutrophil counts, lymphocyte counts, monocyte counts, and platelet counts, LDH, CA 19-9, and CA-125 were retrieved. Inflammatory indexes were defined as follows: NLR = absolute neutrophil counts/absolute lymphocyte counts; PLR = absolute platelet counts/absolute lymphocyte counts; LMR = absolute lymphocyte counts/absolute monocyte counts; SII = absolute neutrophil counts × absolute platelet counts/absolute lymphocyte counts. The end points of our research were progression-free survival (PFS) and OS. PFS was defined as the time from the radiotherapy initiation until first documented tumor progression, death or last follow-up. OS was defined as the duration between the date of radiotherapy and the date of death or last follow-up.
2.3. Statistical analysis
The cut-off values of NLR, PLR, LMR, and SII were determined using receiver operating characteristic (ROC) curve analyses. The optical cut-off values of each inflammatory index were based on the Youden index (maximum (sensitivity + specificity − 1)).[14] The associations between NLR, PLR, LMR, SII, and clincopathologic variables were analyzed by using the Student t test for continuous variables and the chi-squared test for categorical variables. Kaplan–Meier method was used to analyze survival data in this study. Univariate and multivariate analyses were conducted using Cox proportional hazard regression models. All the tests were 2-sided, and a P value < .05 was considered to be statistically significant. The statistical analysis was carried out using SPSS version 21.0 (IBM Corporation, Armonk, NY).
3. Results
3.1. Patients and follow-up
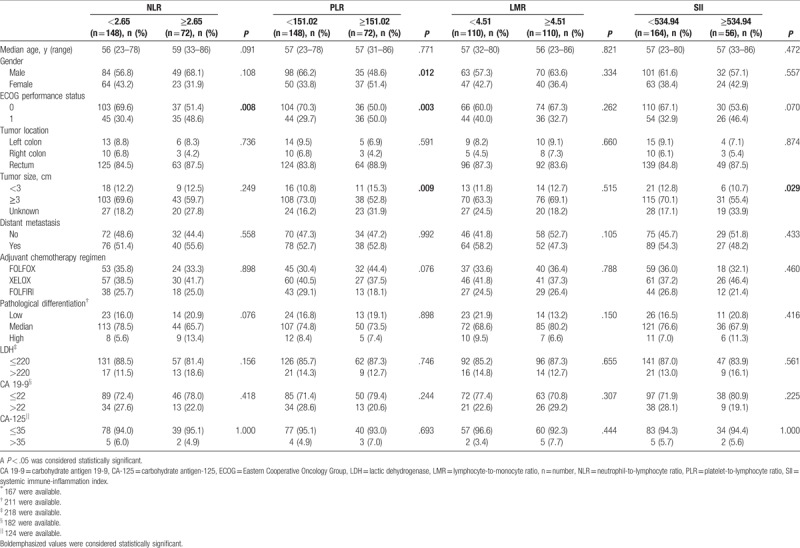
Based on the inclusion and exclusion criteria, a total 220 eligible stage III/IV CRC patients of 133 female and 87 male were enrolled in this study. The cut-off values of NLR, PLR, LMR, and SII were 2.65, 151.02, 4.51, and 534.94 according to ROC, respectively. Patients were divided into high and low groups according to the cut-off values. ECOG performance status was significant different between the high and the low NLR groups (P = .008). Gender, ECOG performance status and tumor size were significant different between the high and the low PLR groups (P = .012, P = .003, and P = .009, respectively). Furthermore, tumor size was significant different between the high and the low SII groups. The baseline characteristics of patients were shown in Table 1.
Table 1.
Baseline characteristics of the study population (n = 220).
The duration of follow-up ranged from 12 to 87 months (median, 23.90 months; mean, 27.73 months). At the time of last follow-up, 200 (90.9%) patients suffered from tumor progression, and 108 (49.1%) patients were dead. The median PFS was 16.15 months (range 1.4–62.8), and the median OS was 33.85 months (range 3.7–86.5 months).
3.2. Univariate and multivariate analyses
The results of univariate analysis revealed that age (P = .022), ECOG performance status (P < .001), distant metastasis (P < .001), NLR (P < .001), PLR (P = .005), SII (P < .001), LDH (P = .007), and CA 19-9 (P = .005) were significantly associated with PFS in CRC patients receiving adjuvant CRT. The results of univariate analysis also indicated that ECOG performance status (P < .001), distant metastasis (P < .001), NLR (P < .001), PLR (P = .002), SII (P < .001), LDH (P = .010), and CA 19-9 (P = .005) were significantly associated with OS in CRC patients receiving adjuvant CRT (Tables 2 and 3). However, gender, tumor location, T stages, adjuvant chemotherapy regimen, pathological differentiation, LMR, and levels of CA-125 were not shown to be associated with survival.
Table 2.
Univariate and multivariate analyses of PFS.
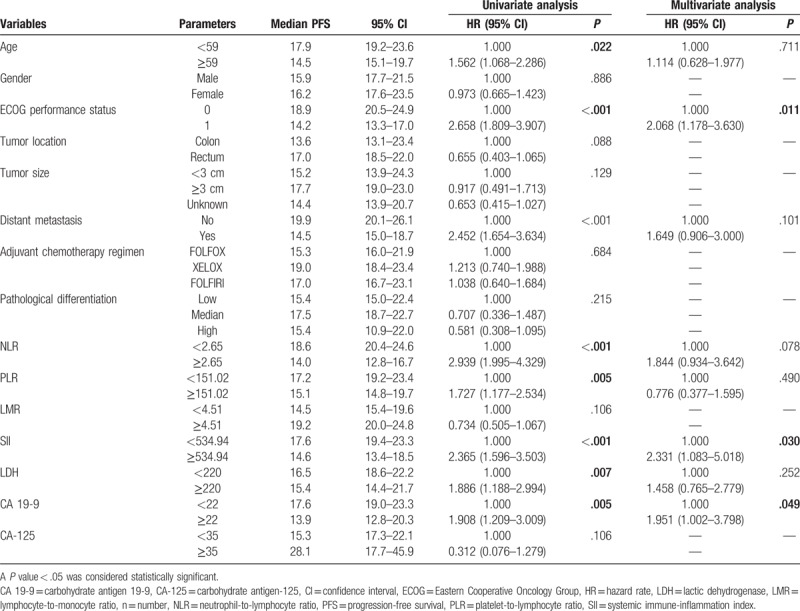
Table 3.
Univariate and multivariate analyses of OS.
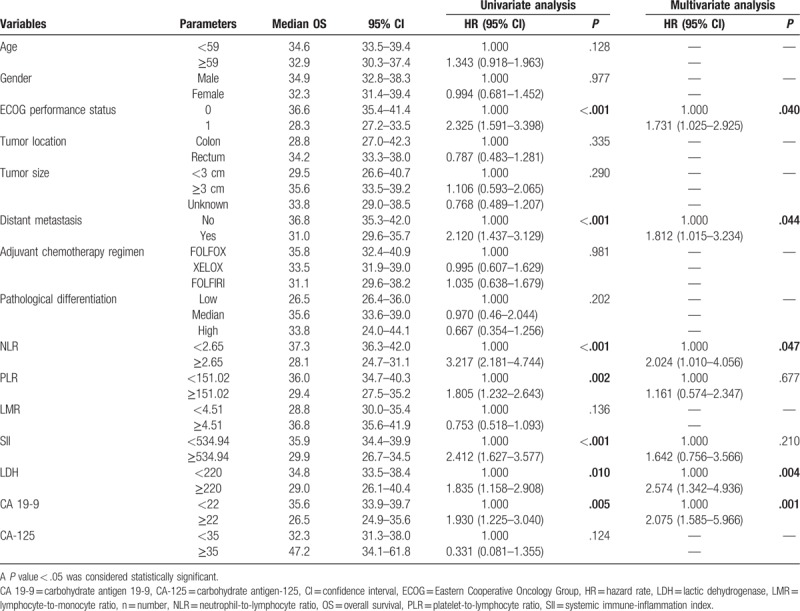
Multivariate analysis using Cox proportional hazards model showed that ECOG performance status of 1 (hazard rate [HR] 2.068, 95% confidence interval [CI] 1.178–3.630, P = .011), high SII (HR 2.331, 95% CI 1.083–5.018, P = .030) and high levels of CA 19-9 (HR 1.951, 95% CI 1.002–3.798, P = .049) were independent predictors of poor PFS in CRC patients receiving adjuvant CRT. Besides, ECOG performance status of 1 (HR 1.731, 95% CI 1.025–2.925, P = .040), distant metastasis (HR 1.812, 95% CI 1.015–3.234, P = .044), high NLR (HR 2.024, 95% CI 1.010–4.056, P = .047), high levels of LDH (HR 2.574, 95% CI 1.342–4.936, P = .004), and high levels of CA 19-9 (HR 2.075, 95% CI 1.002–3.798, P = .001) were also shown to be independent prognostic factors of poor OS in CRC patients receiving adjuvant CRT (Tables 2 and 3).
In addition, the results of Kaplan–Meier analysis and log-rank test suggested that ECOG performance status of 0 (P < .001, P < .001, respectively), without distant metastasis (P < .001, P < .001, respectively), low NLR (P < .001, P < .001, respectively), low PLR (P = .005, P = .002, respectively), low SII (P < .001, P < .001, respectively), low levels of LDH (P = .006, P = .009, respectively), and low levels of CA 19-9 (P = .005, P = .004, respectively) were indicative of better PFS and OS in CRC patients undergoing adjuvant CRT (Figs. 1 and 2; Supplementary Figs. 1–5).
Figure 1.
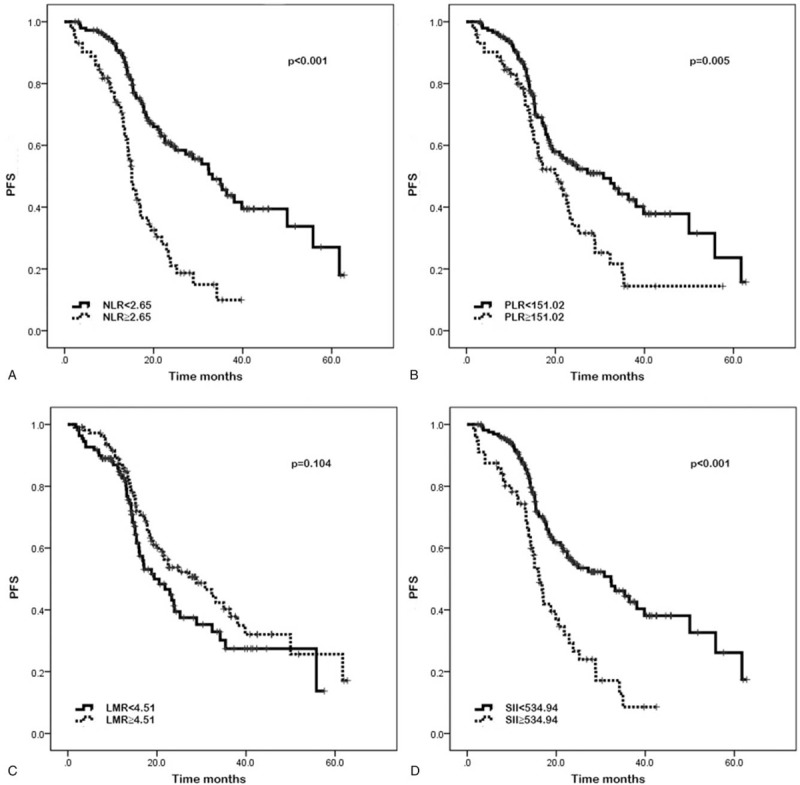
Kaplan–Meier curves of progression-free survival (PFS) based on pretreatment neutrophil-to-lymphocyte ratio (NLR) (A), platelet-to-lymphocyte ratio (PLR) (B), lymphocyte-to-monocyte ratio (LMR) (C), and systemic immune-inflammation index (SII) (D). Elevated NLR, PLR, and SII were associated with significantly poor PFS (P < .001, P = .005, and P < .001, respectively). LMR was not significantly associated with PFS (P = .104).
Figure 2.
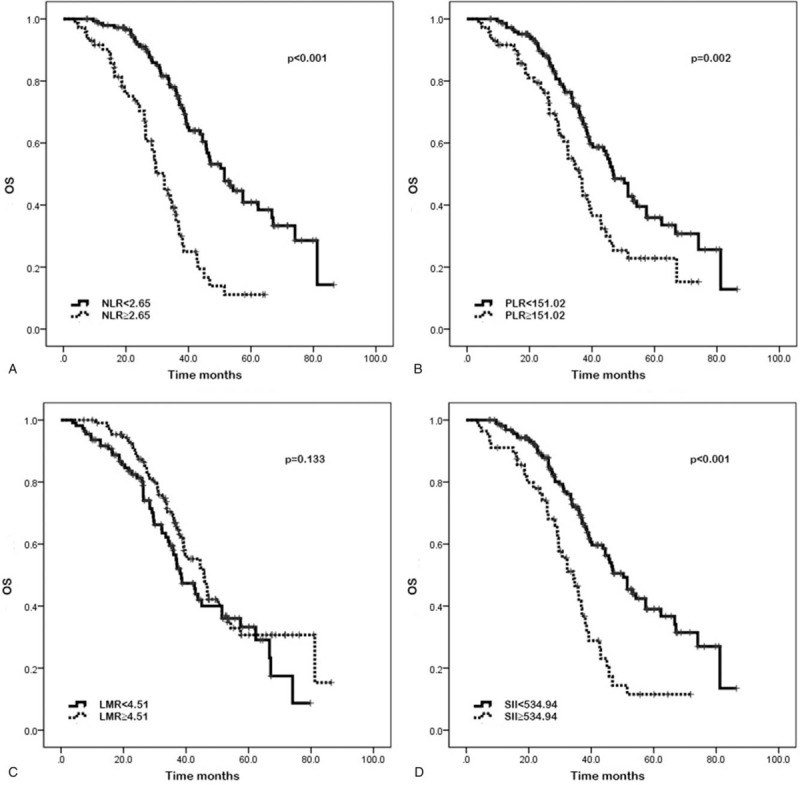
Kaplan–Meier curves of overall survival (OS) based on pretreatment neutrophil-to-lymphocyte ratio (NLR) (A), platelet-to-lymphocyte ratio (PLR) (B), lymphocyte-to-monocyte ratio (LMR) (C), and systemic immune-inflammation index (SII) (D). Elevated NLR, PLR, and SII were significantly associated with poor OS (P < .001, P = .002, and P < .001, respectively). LMR was not significantly associated with OS (P = .133).
4. Discussion
Inflammation has been recognized as a hallmark of cancer that contributes to the development and progression of malignancies.[15] In this way, NLR, PLR, LMR, and SII, which reflect systemic inflammation, are potential predictors of survival. We performed univariate and multivariate analyses to assess the associations between these inflammatory indexes and patients’ outcomes. The results of univariate analysis suggested that ECOG performance status, distant metastasis, NLR, PLR, SII, LDH, and CA 19-9 were significantly associated with PFS and OS in stage III/IV CRC patients receiving adjuvant CRT. The age of patients was also revealed to be associated with PFS but not with OS. Moreover, the results of multivariate analysis indicated that ECOG performance status, SII and high levels of CA 19-9 were significantly associated with PFS; ECOG performance status, distant metastasis, NLR, levels of LDH and levels of CA 19-9 were shown to be independent predictors of OS in stage III/IV CRC patients receiving adjuvant CRT.
We investigated whether pretreatment clinicopathological characteristics were correlated with NLR, PLR, LMR, and SII. The results indicated that NLR was significantly associated with ECOG performance status, PLR was significantly associated with gender, ECOG performance status, and tumor size, and SII was significantly associated with tumor size. However, the potential mechanisms have not been proposed in previous studies.
Most studies have reported that elevated pretreatment NLR is a significant prognostic factor for worse outcomes in patients with CRC; however, a few studies have shown opposite results that elevated NLR is associated with significantly improved OS.[9,16] The prognostic value of NLR has also been reported in patients with head and neck squamous cell carcinoma, cervical cancer, or pancreatic cancer treated by CRT.[17–19] In the present study, the results confirmed that high NLR was associated with worse OS, while no significant association was detected between NLR and PFS. The explanation for the association between NLR and prognosis is not fully clarified. However, an increasing number of studies have indicated that elevated counts of neutrophil correlate with worse prognosis.[13,20,21] Neutrophils in tumor microenvironment can produce vascular endothelial growth factor and therefore promote tumor progression and angiogenesis.[22] In addition, peripheral neutrophils are found to suppress the cytolytic effect of lymphocytes.[23] As a result, increasing neutrophils can promote tumor progression. As lymphocytes were reported to induce cytotoxic cell death and production of cytokine, elevated lymphocytes can inhibit tumor proliferation and metastasis.[24] In this study, the results also suggested that compared with ECOG performance status of 0, patients with ECOG performance status of 1 were shown to have elevated NLR.
PLR, one of the most commonly investigated systemic inflammatory indexes, consists of platelet counts and lymphocyte counts. Recent evidences demonstrated that platelets mediate tumor cell proliferation and angiogenesis.[25] Previous experimental study also concluded that blockade of key platelet receptor reduces the incidence of metastasis.[26] Reciprocally, tumor cells can induce platelet aggregation, and therefore cause cancer-associated thrombosis.[25] In general, the complex interactions between tumor cells and platelets play an important role in the growth and diffusion of cancer cell.[26] High PLR has been revealed to significantly associate with poor prognosis in CRC patients in some studies, whereas associations between PLR and survival were not observed in some studies.[27,28] In patients with pancreatic cancer or cervical esophageal squamous cell carcinoma cancer receiving preoperative CRT, elevated pretreatment PLR significantly predicted poor outcomes.[19,29] In this research, we demonstrated that PLR was associated with PFS and OS, but not an independent predictive factor of survival among our observed patients.
Monocytes involve in tumor migration and invasion.[30] Circulating monocytes develop into tumor-associated macrophages, thus suppressing adaptive immunity and promoting angiogenesis and migration of tumor.[31] Furthermore, monocytes can also directly promote the growth of cancer cells.[32] There are not as many researches on LMR as there are on NLR and PLR. As a result, the prognostic significance of LMR is still controversial. Some studies reported that high LMR is associated with favorable outcome, whereas others did not prove this.[33,34] In patients with locally advanced esophageal squamous cell carcinoma receiving CRT, elevated LMR before treatment has been reported to be associated with both good clinical tumor response and favorable prognosis.[35] In this study, patients with high LMR were shown to have a tendency of better outcome in our study, although it had no statistical significance. In addition, our results also demonstrated that SII was an independent prognostic factor of PFS in stage III/IV CRC patients receiving adjuvant CRT, which confirmed the results of previous studies.[36]
Apart from inflammation-based indexes, ECOG performance status and CA 19-9 were shown to be independent prognostic factor of PFS, while ECOG performance status, distant metastasis, LDH and CA 19-9 were proved to be useful markers to predict OS in our study.
Our study has several potential limitations. First, this is a retrospective study with a limited number of patients. Second, all the patients enrolled in this study are from single center. Therefore, prospective multicenter randomized trials are needed to confirm the results of our research. Third, the value of inflammatory indexes may change over time. However, the counts of blood cell were routinely measured before adjuvant CRT.
5. Conclusions
In conclusion, in the present study, NLR was proved to be closely correlated with OS in stage III/IV CRC patients receiving adjuvant CRT, while SII was shown to be significantly associated with PFS. As NLR and SII can be measured easily without invasive procedures, those indexes could provide a convenient and secure method to predict the outcomes of stage III/IV CRC patients receiving adjuvant CRT. However, further studies are needed to confirm these results.
Acknowledgments
The authors thank to Medbanks (Beijing) Network technology Co. Ltd for patients’ follow-up.
Author contributions
Conceptualization: Jing Yang, Xuelei Ma.
Data curation: Tong Wu, Kaifan Niu.
Investigation: Jing Yang, Xuelei Ma.
Methodology: Jing Yang, Xinli Guo, Xuelei Ma.
Software: Xinli Guo, Tong Wu.
Supervision: Xuelei Ma.
Validation: Jing Yang, Tong Wu, Kaifan Niu, Xuelei Ma.
Visualization: Xinli Guo.
Writing – original draft: Jing Yang.
Writing – review & editing: Jing Yang, Xinli Guo, Tong Wu, Kaifan Niu, Xuelei Ma.
Supplementary Material
Footnotes
Abbreviations: CA 19-9 = carbohydrate antigen 19-9, CA-125 = carbohydrate antigen-125, CI = confidence interval, CRC = colorectal cancer, CRT = chemoradiotherapy, ECOG = Eastern Cooperative Oncology Group, HR = hazard rate, LDH = lactic dehydrogenase, LMR = lymphocyte-to-monocyte ratio, NLR = neutrophil-to-lymphocyte ratio, OS = overall survival, PFS = progression-free survival, PLR = platelet-to-lymphocyte ratio, ROC = receiver operating characteristic, SII = systemic immune-inflammation index, TNM = tumor-node-metastasis.
JY and XM have contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Sun Z, Thacker JM. Contemporary surgical options for metastatic colorectal cancer. Curr Oncol Rep 2015;17:13. [DOI] [PubMed] [Google Scholar]
- [2].Ciombor KK, Wu C, Goldberg RM. Recent therapeutic advances in the treatment of colorectal cancer. Annu Rev Med 2015;66:83–95. [DOI] [PubMed] [Google Scholar]
- [3].Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet 2001;358:1291–304. [DOI] [PubMed] [Google Scholar]
- [4].Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. New Engl J Med 1991;324:709–15. [DOI] [PubMed] [Google Scholar]
- [5].Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically treated rectal carcinoma. New Engl J Med 1985;312:1465–72. [DOI] [PubMed] [Google Scholar]
- [6].Zlobec I, Lugli A. Prognostic and predictive factors in colorectal cancer. Postgraduate Medical Journal 2008;84:403–11. [DOI] [PubMed] [Google Scholar]
- [7].Compton CC. Colorectal carcinoma: diagnostic, prognostic, and molecular features. Mod Pathol 2003;16:376–88. [DOI] [PubMed] [Google Scholar]
- [8].Zhang D, Yu M, Xu T, et al. Predictive value of serum CEA, CA19-9 and CA125 in diagnosis of colorectal liver metastasis in Chinese population. Hepato-gastroenterology 2013;60:1297–301. [DOI] [PubMed] [Google Scholar]
- [9].Passardi A, Scarpi E, Cavanna L, et al. Inflammatory indexes as predictors of prognosis and bevacizumab efficacy in patients with metastatic colorectal cancer. Oncotarget 2016;7:33210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–45. [DOI] [PubMed] [Google Scholar]
- [12].Han Y, Wang J, Hong L, et al. Platelet-lymphocyte ratio is an independent prognostic factor in patients with ALK-positive non-small-cell lung cancer. Future Oncol 2017;13:51–61. [DOI] [PubMed] [Google Scholar]
- [13].Guo D, Han A, Jing W, et al. Preoperative to postoperative change in neutrophil-to-lymphocyte ratio predict survival in colorectal cancer patients. Future Oncol 2018;5:2017–659. [DOI] [PubMed] [Google Scholar]
- [14].Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–5. [DOI] [PubMed] [Google Scholar]
- [15].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- [16].Kubo T, Ono S, Ueno H, et al. Impact of the perioperative neutrophil-to-lymphocyte ratio on the long-term survival following an elective resection of colorectal carcinoma. Int J Colorectal Dis 2014;29:1091–9. [DOI] [PubMed] [Google Scholar]
- [17].Moon H, Roh JL, Lee SW, et al. Prognostic value of nutritional and hematologic markers in head and neck squamous cell carcinoma treated by chemoradiotherapy. Radiother Oncol 2016;118:330–4. [DOI] [PubMed] [Google Scholar]
- [18].Haraga J, Nakamura K, Omichi C, et al. Pretreatment prognostic nutritional index is a significant predictor of prognosis in patients with cervical cancer treated with concurrent chemoradiotherapy. Mol Clin Oncol 2016;5:567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lee BM, Chung SY, Chang JS, et al. The neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are prognostic factors in patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Gut Liver 2018;12:342–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Trellakis S, Bruderek K, Dumitru CA, et al. Polymorphonuclear granulocytes in human head and neck cancer: enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int J Cancer 2011;129:2183–93. [DOI] [PubMed] [Google Scholar]
- [21].Schmidt H, Bastholt L, Geertsen P, et al. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. Br J Cancer 2005;93:273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shamamian P, Schwartz JD, Pocock BJ, et al. Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: a role for inflammatory cells in tumor invasion and angiogenesis. J Cell Physiol 2001;189:197–206. [DOI] [PubMed] [Google Scholar]
- [23].Petrie HT, Klassen LW, Kay HD. Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes. J Immunol (Baltimore, Md: 1950) 1985;134:230–4. [PubMed] [Google Scholar]
- [24].Lin EY, Pollard JW. Role of infiltrated leucocytes in tumour growth and spread. Br J Cancer 2004;90:2053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sharma D, Brummel-Ziedins KE, Bouchard BA, et al. Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. J Cell Physiol 2014;229:1005–15. [DOI] [PubMed] [Google Scholar]
- [26].Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost 2011;9:237–49. [DOI] [PubMed] [Google Scholar]
- [27].Park BK, Park JW, Han EC, et al. Systemic inflammatory markers as prognostic factors in stage IIA colorectal cancer. J Surg Oncol 2016;114:216–21. [DOI] [PubMed] [Google Scholar]
- [28].Wu Y, Li C, Zhao J, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios predict chemotherapy outcomes and prognosis in patients with colorectal cancer and synchronous liver metastasis. World J Surg Oncol 2016;14:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wu CC, Li SH, Lu HI, et al. Inflammation-based prognostic scores predict the prognosis of locally advanced cervical esophageal squamous cell carcinoma patients receiving curative concurrent chemoradiotherapy: a propensity score-matched analysis. PeerJ 2018;6:e5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- [31].Liu Y, Cao X. The origin and function of tumor-associated macrophages. Cell Mol Immunol 2015;12:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wilcox RA, Wada DA, Ziesmer SC, et al. Monocytes promote tumor cell survival in T-cell lymphoproliferative disorders and are impaired in their ability to differentiate into mature dendritic cells. Blood 2009;114:2936–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ozawa T, Ishihara S, Kawai K, et al. Impact of a lymphocyte to monocyte ratio in stage IV colorectal cancer. J Surg Res 2015;199:386–92. [DOI] [PubMed] [Google Scholar]
- [34].Yang J, Guo X, Wang M, et al. Pre-treatment inflammatory indexes as predictors of survival and cetuximab efficacy in metastatic colorectal cancer patients with wild-type RAS. Sci Rep 2017;7:17130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu X, Li M, Zhao F, et al. The lymphocyte-monocyte ratio predicts tumor response and survival in patients with locally advanced esophageal cancer who received definitive chemoradiotherapy. Onco Targets Ther 2017;10:871–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 2014;20:6212–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


