Abstract
Rationale:
Sclerosing pneumocytoma accompanied with other type of tumor in one patient is very rare. Here, we report a case of a sclerosing pneumocytoma mixed with a typical carcinoid tumor in a same neoplasm.
Patient concerns:
A 55-year-old woman incidentally detected a space-occupying lesion of right lung in routine health examination. The patient was asymptomatic and there were no positive findings in routine laboratory examination, physical examination, and pulmonary function test. Computed tomography revealed a solitary round mass in the middle lobe of the right lung.
Diagnosis:
The lesion was diagnosed as a sclerosing pneumocytoma accompanied with a typical carcinoid tumor of the right lung.
Intervention:
The patient underwent thoracoscopic lobectomy in our hospital.
Outcomes:
The postoperative course was uneventful.
Lessons:
This case is rare and noteworthy for a lesion containing two different types of neoplasms, which may cause diagnostic difficulties.
Keywords: carcinoid, lung tumor, sclerosing pneumocytoma
1. Introduction
Sclerosing pneumocytoma (SP) is a benign tumor of lung, which was first reported by Liebow and Hubbell in 1956.[1] It was previously hypothesized to be vascular origin and was named “sclerosing hemangioma”. Recently, based on immunohistochemical data and clonality analysis, the tumor is considered to derive from primitive respiratory epithelium.[2–5] So, in the 2015 World Health Organization Classification of Lung Tumors, this tumor was formally renamed as SP and re-classified in the group of “adenomas”.[6] SP usually presents as peripheral solitary well-circumscribed round lesion in imaging examination, with a predilection for non-smoking middle-aged female. SP has two types of tumor cells, which can be histologically recognized: surface cuboidal cells and stroma round or polygonal cells. Most SPs have at least three of four primary histological patterns, including solid, papillary, sclerotic, and hemorrhagic regions, which vary in their proportions.[7,8] But that is not always quite accurate. Hitomi et al reported a case of multiple SP only showing a papillary pattern, and they suggested that the papillary lesion may be the very early phase of SP.[9] Most SPs are benign tumors. But, multifocal SP and SP with lymph node metastasis have been reported many times, indicating that minority of SPs might have a low malignant potential.[8,10] Here we report a rare case of SP mixed with typical carcinoid tumor in the same tumor mass of lung, which may cause diagnostic difficulties.
2. Case presentation
2.1. Ethic approval
The study was approved by the China Medical University Institutional Review Board for human studies. The ethical board approval number is LS[2018]016. Written informed consent was obtained from the patient for publication of this case report and accompanying images and the study was performed in accordance with the Helsinki II declaration.
2.2. Clinical history
A woman of 55-year-old went to our hospital in June 2018 with complaint of incidentally detecting a space-occupying lesion of right lung in routine health examination. The patient was asymptomatic and there were no positive findings in routine laboratory examination, physical examination, and pulmonary function test. Computed tomography (CT) revealed a single, solid, well-circumscribed, round mass, approximately 16 mm in the longest diameter, with CT attenuation value of 25 HU in the middle lobe of the right lung (Fig. 1). The attenuation value of contrast-enhanced CT was 54 HU. There was no enlarged lymph node. The tumor was resected using thoracoscopic lobectomy. During surgery, the tumor was sent to make frozen section and pathological evaluation. The diagnosis of intraoperative frozen section was “low malignant potential tumor, carcinoid tumor cannot be excluded”. Then, the middle lobe of the right lung was removed. The patient did not receive any postoperative adjuvant therapies. During 3 months follow up, no evidence of recurrence has been detected in this patient.
Figure 1.
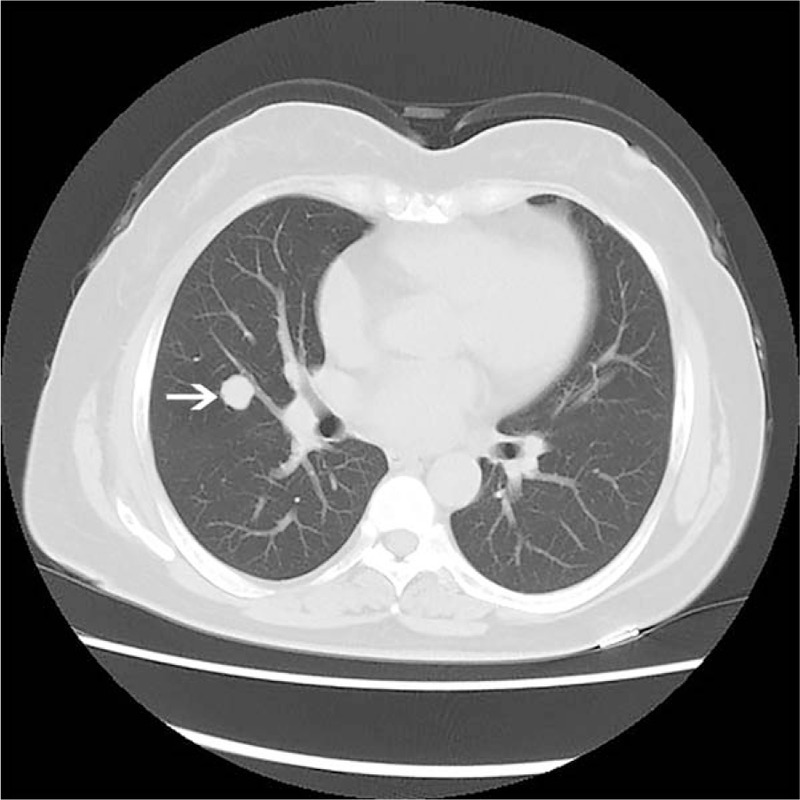
Computed tomograpgy examination of the lung. Computed tomograpgy scan displayed a solitary, well-circumscribed, round mass tumor (arrow) located at the middle lobe of the right lung.
2.3. Immunohistochemal staining
The resected tumor tissues were fixed with 10% neutral-buffered formalin, embedded into paraffin blocks, then, cut into 4-μm sections. The histological evaluation was performed on hematoxylin and eosin stained sections. The tumor tissue sections were immunostained with primary antibodies against broad-spectrum cytokeratin (CK), CK7, p63, thyroid transcription factor-1 (TTF-1), Vimentin, CD56, synaptophysin, and Ki-67. All antibodies were purchased from Maixin, Fuzhou, China. After incubation with primary antibody, the detection of antibodies was performed using the streptavidin-peroxidase method. Appropriate positive and negative controls were used to exclude the false positivity and negativity.
2.4. Morphological and immunohistochemica findings
Grossly, the tumor was located in the middle lobe of the right lung, and growth in the lung parenchyma, without involving of visceral pleura. The tumor was gray-red, solitary, round, well demarcated, with a focal light yellow-colored area, measuring 16 mm × 15 mm in size. No apparent hemorrhagic or necrotic foci were found.
Microscopically, the tumor showed a mixed multiple growth patterns, such as solid, focal sclerotic, papillary, and hemorrhagic regions (Fig. 2A). Two types of tumor cells could be discerned in most area of the tumor. One type was the cuboidal surface cells resembling type II pneumocytes, which lined on the surface of glandular and papillary structures. The other type of tumor cells was round or polygonal cells which beneath the epithelial cells, arranging as solid sheets or nests (Fig. 2B). Both the histological structures and cell features were in line with SP. Meanwhile, in the tumor tissue, we also observed that some solid cell cords or nests were near or mixed with the SP-like tissues (Fig. 2C). The area of this region was about 35% of the tumor (> 5 mm). The cells of these solid cords or nests were short fusiform with blunt nuclei and eosinophilic cytoplasm. The cells were well arrangement and showed palisading pattern at the periphery region, which were similar to the organoid pattern of carcinoid tumor, and could be distinguished from SP tissue. There was no transition between SP-like tissue and carcinoid-like tissue. The mitotic figure was rare in the whole tumor.
Figure 2.
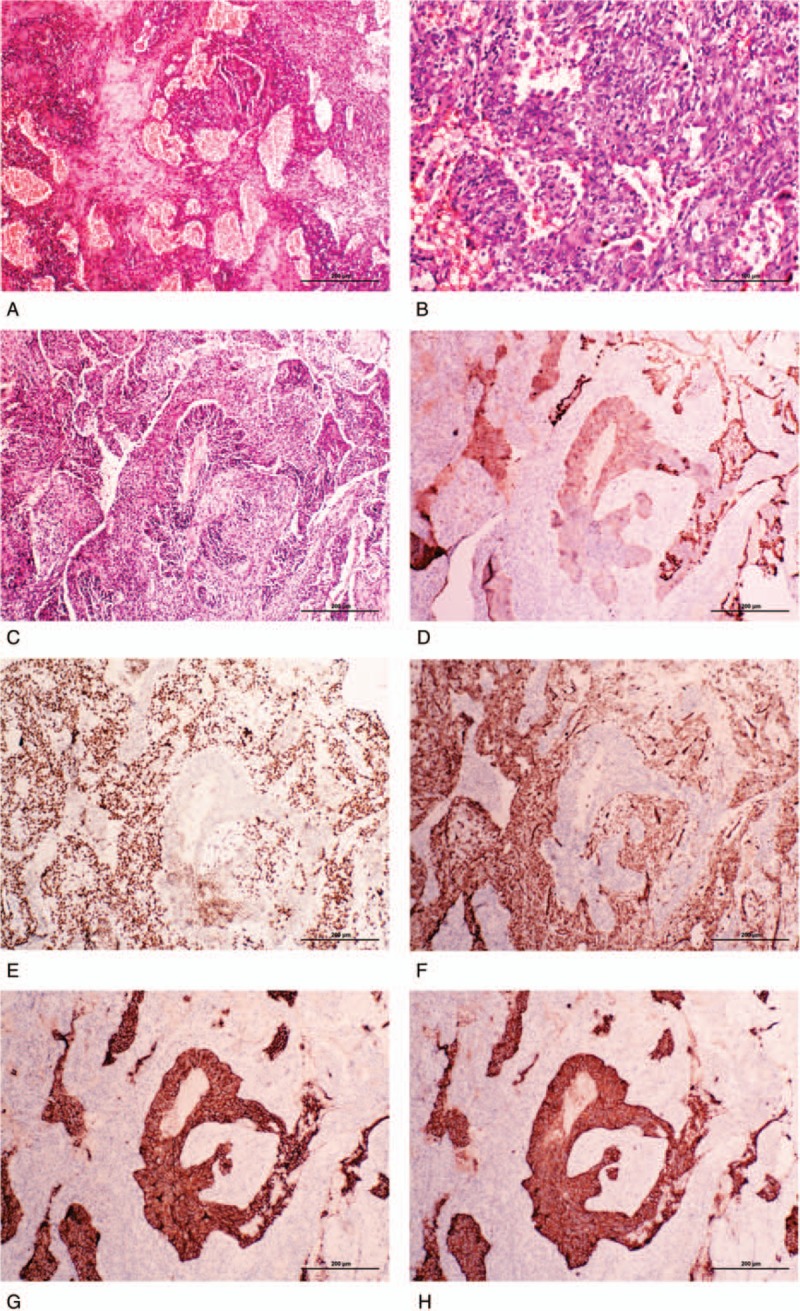
The histological and immunohistochemical features of the present sclerosing pneumocytoma accompanied with typical carcinoid tumor. (A) Typical hemorrhagic and sclerotic regions of the sclerosing pneumocytoma region of the present sclerosing pneumocytoma. (B) Two cell types were observed in the papillary and glandular region of sclerosing pneumocytoma: surface cuboidal cells and stromal round or polygonal cells. (C) A representative field of sclerosing pneumocytoma mixed with carcinoid tumor. The well-arranged cell cords in the center were carcinoid tumor, and periphery papillary and solid regions were sclerosing pneumocytoma. (D) Immunohistochemical staining of broad-spectrum cytokeratin was strongly positive in the cuboidal cells of sclerosing pneumocytoma and weakly positive in carcinoid tumor cells, but negative in the stromal polygonal cells. (E) Immunohistochemical staining of thyroid transcription factor-1 (TTF-1) was positive in both cuboidal surface cells and stromal polygonal cells, but negative in the carcinoid tumor tissue. (F) Immunohistochemical staining of vimentin was positive in the sclerosing pneumocytoma tissue, but negative in the carcinoid tumor tissue. (G) and (H) Immunohistochemical staining of CD56 and synaptophysin were both positive in the carcinoid tumor tissue, but negative in the sclerosing pneumocytoma tissue.
Immunohistochemically, in the SP-like region of the tumor, the cuboidal surface cells were strongly positive for CK and TTF-1, but negative for P63 and Vimentin staining. The stromal polygonal cells were strongly positive for TTF-1 and Vimentin, but negative for CK and P63 staining. Both of the 2 types of cells were negative for CD56 and synaptophysin staining. So, the immunochemical staining of this region supported it an SP. On the other hand, in the carcinoid-like region, the tumor cells were weakly positive for CK, negative for TTF-1, P63 and Vimentin, but strongly positive for CD56 and synaptophysin staining, indicating they were neuroendocrine origin (Fig. 2 D–H). The Ki-67 index of all tumor cells was less than 2%.
3. Discussion
Based on clinical information, histological features, and the immunohistochemical staining profile described above, the tumor was diagnosed as a SP accompanied with typical carcinoid tumor of the right lung.
Carcinoid tumor is a low-grade malignant neuroendocrine neoplasm which is also relatively rare in the lung. Such rare 2 different kinds of tumors mixed with each other within 1 tumor mass is extremely unusual. To the best of our knowledge, there were only 3 English literatures reported SP combined with carcinoid tumor, but the cases were not exactly the same.[11–13] The detailed information of the cases are summarized in Table 1. Kim et al reported a case of multiple carcinoid tumors associated with multiple SPs of the lung, the 2 kinds of lesions were completely separated.[12] Wang et al reported a mixture of carcinoid tumors with extensive neuroendocrine proliferation and multiple SPs.[11] In the report of Cho et al, SP coexisted with carcinoid tumor in the same neoplasm, and there was a pushing, clear border between them.[13]
Table 1.
Case summary of sclerosing pneumocytoma mixed with carcinoid tumor.
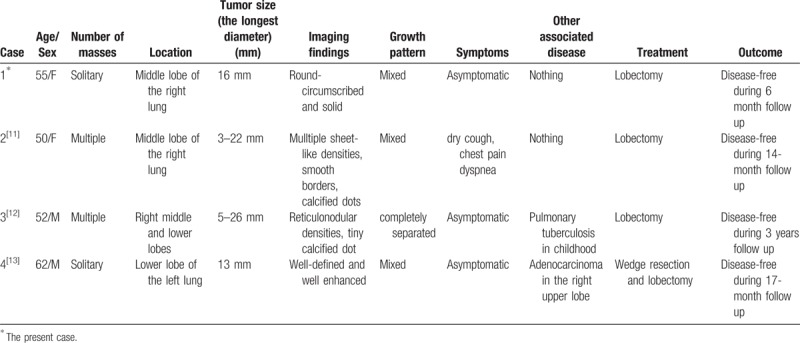
In the present case, the carcinoid tumor tissue mixed with SP so well that appeared as a single neoplasm, but there was no transition in the 2 components. The cuboidal and polygonal cells were all positive for TTF-1, but carcinoid cells were negative for TTF-1, and strong positive for CD56 and synaptophysin. So, under the help of immunohistochemical staining, the carcinoid cells could be easily distinguished from mixed SP tissue. It may be interpreted as a ‘collision tumor’, that is a SP coincidentally developing in a lung lobe with a carcinoid tumor. The pathogenesis is unclear, but there are several hypotheses about the disease: [14–17]
-
(1)
Two primary pulmonary tumor accidental happening at the same time and adjacent to each other;
-
(2)
The collision tumor originates from multipotential stem cells and showing different kinds of histologic types. For example, the multipotential tumor cells were differentiated towards SP and carcinoid tumor respectively. The most common and widely accepted example is adenosquamous carcinoma;
-
(3)
SP change the microenvironment of surrounding lung tissue and promote pulmonary neuroendocrine cells (PNCs) proliferation.
Some pathologists think SPs devoid of normal respiratory epithelium function, inducing microenviromnental damage and local oxygen deficit. PNCs are sensitive to hypoxia and release peptide lead to further proliferation and differentiation.[12,16] Moreover, hypoxia may trigger a series of molecular change that facilitate the tumorigenesis of pulmonary carcinoids.[18]
Wang et al considered this type of tumor might be explained by the 1 tumor theory. In their case, the 2 morphologically different lesions coexistent so well that appeared as a single neoplasm, and the mixed lesions present in all of the multi-nodular lesions. Moreover, both components were positive for TTF-1.[11] They interpreted that SP and carcinoid tumor might derive from a common lineage. Song et al noted that pulmonary neuroendocrine cell (PNC) and alveolar cells may share a common lineage during lung development.[19] Some previous researches revealed neuroendocrine differentiation in some SPs, indicating the possibility of their neuroendocrine origin.[20]
Cases of SP accompanied with other tumors except of carcinoid tumor were also extremely rare. The lesions coexisted with SP including atypical alveolar hyperplasia (AAH), primary adenocarcinoma,[21,22] and glandular papilloma.[23]
The differential diagnosis of the present case included well-differentiated papillary adenocarcinoma,[24] papillary adenoma and alveolar adenoma, all of which present papillary and adenoid tubule structure, sometimes may be difficult to distinguish with SP, especially in lung biopsy specimen. However, these tumors lack of other growth patterns of SP, such as bloody background and hyalinized stroma, especially dual cell population. Immunohistochemically, the polygonal cells are positive for TTF-1 and Vimentin, and the surface lining cuboidal cells are positive for TTF-1 and CK. Histological features together with immunohistochemical markers are helpful to identify SP with other diseases. Treatment of SP mixed with a typical carcinoid tumor involves wedge resection and lobectomy and the prognosis is favorable. The tumor is rare, there is an need of longer period of follow-up and of accumulation of such cases to further clarify the biological characteristics.
4. Conclusion
In conclusion, we report a case of SP mixed with typical carcinoid tumor in a same neoplasm. The case is rare and noteworthy for a lesion containing two different types of neoplasms, which may cause diagnostic difficulties. The precise histogenesis, pathogenesis and prognosis still need further research and longer period of follow-up.
Author contributions
Funding acquisition: Di Zhang, Hong-Tao Xu.
Methodology: Zhao Wang, Mai-Qing Yang, Wen-Jing Huang.
Writing – original draft: Zhao Wang, Hong-Tao Xu.
Writing – review & editing: Zhao Wang, Di Zhang, Hong-Tao Xu.
Hong-Tao Xu orcid: 0000-0001-5062-3108.
Footnotes
Abbreviations: AAH = atypical alveolar hyperplasia, CK = cytokeratin, CT = Computed tomography, PNC = pulmonary neuroendocrine cell, SP = sclerosing pneucytoma, TTF-1 = thyroid transcription factor 1.
This study was supported by National Natural Science Foundation of China (Grant No. 81372497 to Xu HT and 81672302 to Zhang D) and Program for Liaoning Excellent Talents in University (Grant No. LR2015067 to Xu HT).
The authors have no conflicts of interest to disclose.
References
- [1].Liebow AA, Hubbell DS. Sclerosing hemangioma (histiocytoma, xanthoma) of the lung. Cancer 1956;9:53–75. [DOI] [PubMed] [Google Scholar]
- [2].Devouassoux-Shisheboran M, Hayashi T, Linnoila RI, et al. A clinicopathologic study of 100 cases of pulmonary sclerosing hemangioma with immunohistochemical studies: TTF-1 is expressed in both round and surface cells, suggesting an origin from primitive respiratory epithelium. Am J Surg Pathol 2000;24:906–16. [DOI] [PubMed] [Google Scholar]
- [3].Keylock JB, Galvin JR, Franks TJ. Sclerosing hemangioma of the lung. Arch Pathol Lab Med 2009;133:820–5. [DOI] [PubMed] [Google Scholar]
- [4].Niho S, Suzuki K, Yokose T, et al. Monoclonality of both pale cells and cuboidal cells of sclerosing hemangioma of the lung. Am J Pathol 1998;152:1065–9. [PMC free article] [PubMed] [Google Scholar]
- [5].Lin XY, Fan CF, Dong XJ, et al. Expression and significance of stem cell markers in pulmonary sclerosing haemangioma. Histopathology 2012;61:178–85. [DOI] [PubMed] [Google Scholar]
- [6].Travis WD, Brambilla E, Burke AP, et al. World health organization classification of lung tumours: impact of genetics, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015;10:1243–60. [DOI] [PubMed] [Google Scholar]
- [7].Chen B, Gao J, Chen H, et al. Pulmonary sclerosing hemangioma: a unique epithelial neoplasm of the lung (report of 26 cases). World J Surg Oncol 2013;11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sugio K, Yokoyama H, Kaneko S, et al. Sclerosing hemangioma of the lung: radiographic and pathological study. Ann Thorac Surg 1992;53:295–300. [DOI] [PubMed] [Google Scholar]
- [9].Kawai H, Takayashiki N, Otani H, et al. A case of microscopic, multiple sclerosing pneumocytoma. Pathol Int 2018;68:196–201. [DOI] [PubMed] [Google Scholar]
- [10].Pokharel S, Dhillon SS, Ylagan L, et al. Sclerosing pneumocytoma with lymph node metastasis. J Thorac Oncol 2016;11:1802–4. [DOI] [PubMed] [Google Scholar]
- [11].Wang Y, He Q, Shi W, et al. A mixture of carcinoid tumors, extensive neuroendocrine proliferation, and multiple pulmonary sclerosing hemangiomas. World J Surg Oncol 2014;12:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kim Y, Choi YD, Kim BJ, et al. Multiple peripheral typical carcinoid tumors of the lung: associated with sclerosing hemangiomas. Diagn Pathol 2013;8:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cho HJ, Lee JH, Lee GK, et al. Case of sclerosing pneumocytoma combined with a typical carcinoid and pulmonary adenocarcinoma in different lobes. Thorac Cancer 2017;8:372–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975;70:606–12. [PubMed] [Google Scholar]
- [15].Nakata S, Nagata Y, Sugaya M, et al. Primary pulmonary collision cancer consisting of large cell carcinoma and adenocarcinoma. Ann Thorac Surg 2005;80:340–2. [DOI] [PubMed] [Google Scholar]
- [16].Song H, Yao E, Lin C, et al. Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proc Natl Acad Sci USA 2012;109:17531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Spagnolo DV, Heenan PJ. Collision carcinoma at the esophagogastric junction: report of two cases. Cancer 1980;46:2702–8. [DOI] [PubMed] [Google Scholar]
- [18].Fernandez-Cuesta L, Peifer M, Lu X, et al. Frequent mutations in chromatin-remodelling genes in pulmonary carcinoids. Nat Commun 2014;5:3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Youngson C, Nurse C, Yeger H, et al. Oxygen sensing in airway chemoreceptors. Nature 1993;365:153–5. [DOI] [PubMed] [Google Scholar]
- [20].Xu H-M, Li W-H, Hou N, et al. Neuroendocrine differentiation in 32 cases of so-called sclerosing hemangioma of the lung: identified by immunohistochemical and ultrastructural study. Am J Surg Pathol 1997;21:1013–22. [DOI] [PubMed] [Google Scholar]
- [21].Liu W, Tian XY, Li Y, et al. Coexistence of pulmonary sclerosing hemangioma and primary adenocarcinoma in the same nodule of lung. Diagn Pathol 2011;6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Noguchi M, Kodama T, Morinaga S, et al. Multiple sclerosing hemangiomas of the lung. Am J Surg Pathol 1986;10:429–35. [DOI] [PubMed] [Google Scholar]
- [23].Kitawaki Y, Fujishima F, Taniuchi S, et al. Coexistence of glandular papilloma and sclerosing pneumocytoma in the bronchiole. Pathol Int 2018;68:425–30. [DOI] [PubMed] [Google Scholar]
- [24].Iyoda A, Baba M, Saitoh H, et al. Imprint cytologic features of pulmonary sclerosing hemangioma: comparison with well-differentiated papillary adenocarcinoma. Cancer 2002;96:146–9. [DOI] [PubMed] [Google Scholar]


