Abstract
SUMO-specific Cysteine Proteases (SENPs) have involvement in the initiation and progression of human cancers. In the present study, we evaluated the association of SENPs polymorphism with susceptibility as well as clinicopathologic features and patients’ response of breast cancer (BC) in a Chinese population.
We genotyped SENP1 (rs61918808), SENP2 (rs6762208), SENP7 (rs61697963) by sequencing in a case–control study including 210 BC patients and 225 healthy volunteers. Odds ratios (ORs) and 95% confidence intervals (CIs) were used to assume the association strength.
No significant association was found between polymorphism of the 3 SENPs and BC susceptibility. However, SENP1 rs61918808 (C>T) and SENP7 rs61697963 (A>C) was associated with HER-2 expression (P < .05). SENP2 rs6762208(C>A) was correlated with increasing risk of lymph node metastases (P < .05). Among the patients who received neoadjuvant chemotherapy, T allele and TT genotype of SENP1 rs61918808 were less likely to achieve pCR (P < .05).
We first reported SENPs variants were not associated with BC risk in Chinese population, but presented specific effect on clinicopathological features of BC. Moreover, SENP1 rs61918808 may be a predictor for the clinical response in local advanced BC patients who received neoadjuvant chemotherapy.
Keywords: breast cancer, cancer susceptibility, SENPs, SUMO-specific Cysteine Proteases, variant
1. Introduction
Posttranslational modifications (PTMs) including methylation, phosphorylation, acetylation, glycosylation, ubiquitination, and SUMOylation play important roles in the normal biological function of the protein. Among these, SUMOylation is a particularly interesting PTM involved in control of various cellular processes, including cell growth, survival and apoptosis, gene expression, intracellular transport, and protein stability.[1,2]
SUMOylation requires an E1-activating enzyme, E2-conjugating enzyme (UBC9), and E3-ligating enzymes.[3] SUMO proteins are activated by the activating enzyme E1 and transferred to the conjugating enzyme UBC9. E3-ligating enzymes then promote the transfer of SUMO from E2 to the substrate, which helps the modification to be more efficient.[4] SUMOylation of target proteins results in formation of isopeptide bond between the C-terminal glycine residue of SUMO and the ε-amino group of lysine residue in the target proteins.[5] Sumoylation pathway is required to maintain the basal breast cancer (BC) subtype. Disruption of the sumoylation pathway by knockdown of sumoylation enzymes, mutation of the SUMO-target lysine of TFAP2A, or treatment with sumoylation inhibitors induced a basal to luminal transition.[5] SUMOylation is highly dynamic process that can be reversed by a family of SUMO-specific proteases (SENPS). At present, 6 SENP isoforms have been identified in humans, namely, SENP1, 2, 3, 5, 6, and 7, which have different substrate specificities and subcellular localizations.[6,7] SENP1 processes SUMO-1 in preference to SUMO-2, but shows weak activity for SUMO-3.[8,9] SENP2 processes SUMO-2 more efficiently than SUMO-1, but processes SUMO-3 as poorly as SENP1, SENP6, and SENP7 have excellent deconjugating activity for polySUMO-2/3 chains.[10] Emerging studies have demonstrated that overexpression of various SENP isoforms is involved in the tumorigenesis. SENP1, for example, is unregulated in thyroid oncocytic tumor, prostate cancer, and pancreatic ductal adenocarcinoma (PDAC).[11–14] SENP2 was observed to significantly repress estradiol-induced transcriptional activity in BC cells.[15] Moreover, SENP2 appeared to inhibit migration and invasion of bladder cancer cells by inhibiting the expression of MMP13.[16] SENP5 has been reported to be associated with differentiation of OSCC cells.[17]
BC is one of the leading causes of cancer death among women and becomes the most common cancer for women worldwide.[18] Increasing evidences suggest that single-nucleotide polymorphisms (SNPs) play important roles in carcinogenesis. Although SENPs have been reported as crucial in the carcinogenic process of many cancer types, few studies have focused on the significance of SNPs in SENPs affecting BC risk.[19] Particularly, no studies have evaluated the impact of SENPs SNPs in BC risk and patient prognosis in Chinese population. In this context, we aimed to investigate whether SENP1 rs61918808, SENP2 rs6762208, and SENP7 rs61697963 polymorphism may be associated with BC susceptibility as well as clinicopathologic features and patients prognosis in a Chinese population.
2. Materials and methods
2.1. Patients and study design
This case–control study was performed on 210 pathologically diagnosed BC cases from Fujian Provincial Hospital, China, and 225 healthy controls collected from December 2015 to March 2017. All participants were of Asian ethnic background. All BC patients were staged and graded according to the seventh edition of the American Joint Committee on Cancer (AJCC) tumor–node–metastasis (TNM) staging system.[20] Clinicopathological data including age, histologic type of cancer, histological grade, TNM stage, nodal status, distant metastasis, estrogen receptor (ER) status, progesterone receptor (PR) status, and human epidermal growth factor receptor 2 (HER-2/neu) status were collected.
2.2. Ethics statement
This case–control study was approved by the institutional Review Board of the Fujian Provincial Hospital. All participants were voluntary and would complete the informed consent in written before taking part in this research.
2.3. Genotyping
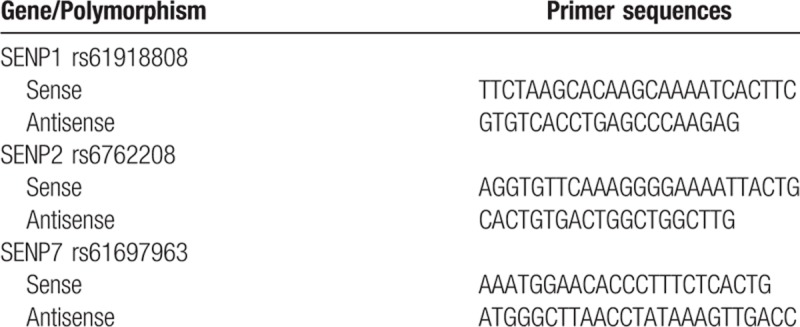
Peripheral blood samples were collected from each subject into a test tube containing EDTA as anticoagulant. Genomic DNA was isolated from the whole blood using the BioTECH Blood Genomic DNA Miniprep Kit (Beijing, China) according to the manufacturer's directions. The polymorphisms of SENP1 rs61918808, SENP2 rs6762208, and SENP7 rs61697963 were genotyped using the Sequenom MassARRAY technology platform with the complete iPLEX Gold Reagent Set (Sequenom, CA) in the conditions recommended by the manufacturer. Assay data were analyzed using Sequenom TYPER software (version 4.0). The primers were designed by ADS software 2.0 (Agena Bioscience, CA). The primer sequences used for genotyping were listed in Table 1.
Table 1.
Primer sequences of SENP1 rs61918808, SENP2 rs6762208 and SENP7 rs61697963 polymorphism.
2.4. Neoadjuvant chemotherapy and the response assessment
Sixty-four patients with local advanced BC received 4 to 8 cycles of epirubicin followed by taxanes based on neoadjuvant chemotherapy (80 mg/m2 of epirubicin, 800 mg of cyclophosphamide, and 80 mg/m2 of docetaxel on the first day, with 21 days a cycle). All these patients have been followed up carefully after the initial treatment and reassessed every 3 months. Pathologic complete response (pCR) was defined that the postoperative pathology indicating no residual invasive cancer cells in the breast or the axillary lymph node.
2.5. Statistical analysis
All statistical analyses were carried out using SPSS 21.0 for windows (IBM SPSS Statistics, IBM). Hardy–Weinberg equilibrium was evaluated by χ2 test to compare the observed genotype frequencies with the expected frequencies among BC cases and controls. Comparisons of the distributions of epidemiological variables between BC cases and controls were analyzed using Student t test for continuous variables, whereas the χ2 test was used for categorical variables. Odds ratios (ORs) and 95% confidence intervals (CIs) for assessing differences in risk were calculated by univariate and multivariate logistic regression, adjusted for age, age at menarche and menopause, menopausal status, number of pregnancies, number of abortion, breast-feeding history, and family history of BC. All tests were 2-sided and a P < .05 was considered to be statistically significant.
3. Results
3.1. Population Characteristics
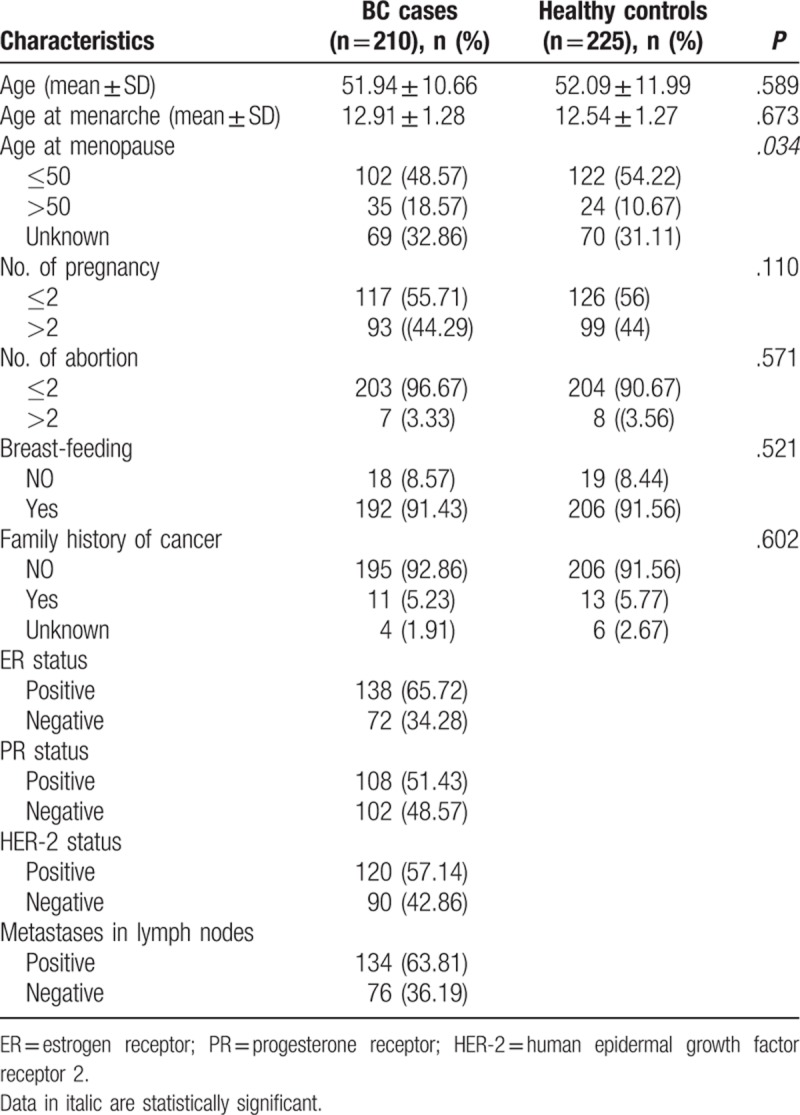
A total of 435 age-matched subjects (210 BC cases and 225 healthy controls) were genotyped to explore the association of BC susceptibility and SENP1 rs61918808, SENP2 rs6762208, and SENP7 rs61697963 polymorphisms in this study. Distributions of clinical characteristics between BC cases and healthy controls were shown in Table 2. No statistically significant difference was observed between the 2 groups in age, age at menopause, number of abortion, breast-feeding, and family history. However, the age of menopause in BC patients was older than healthy controls (P = .034).
Table 2.
Distributions of selected characteristics in breast cancer cases and healthy controls.
3.2. Allelic frequencies and genotype distribution of SENP1 rs61918808, SENP2 rs6762208, and SENP7 rs61697963
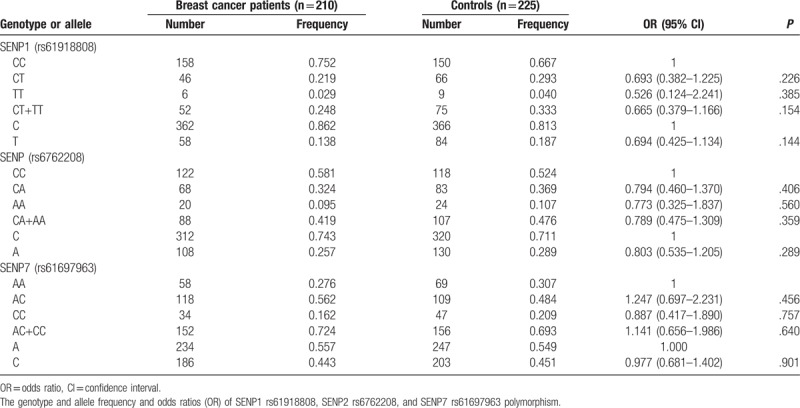
The frequency distributions of alleles and genotypes of SENP1 rs61918808, SENP2 rs6762208, and SENP7 rs61697963 for BC cases and controls were shown in Table 3. The genotype frequency distributions of the 3 studied SNPs did not deviate from Hardy–Weinberg equilibrium in the controls (P = .677 for rs61918808, P = .871 for rs6762208, and P = .837 for rs61697963, respectively). The allelic frequencies of BC patients were similar to those of the controls: SENP1 rs61918808 for patients (C: 0.862, T: 0.138) and controls (C: 0.813, T: 0.187), SENP2 rs6762208 for patients (C: 0.743, A: 0.257) and controls (G: 0.711, T: 0.289), SENP7 rs61697963 (A: 0.557, C: 0.443) and controls (A: 0.549, C: 0.451). Thus, genotypic frequencies of SENP2 rs6762208, SENP2 rs6762208 and SENP7 rs61697963 in BC patients were not significantly different from those of the controls.
Table 3.
Logistic regression analysis for associations between selected SNPs and risk of Breast Cancer.
3.3. Association between SENPs polymorphism and BC risk
To define whether there was a statistically significant increased risk of BC susceptibility in terms of the SENP1 rs61918808, SENP2 rs6762208, and SENP7 rs61697963 genotypes, we carried out logistic regression analysis. As shown in Table 3, no significant associations between these polymorphic variants and the risk for developing BC were found (for SENP1 rs61918808 T vs C: OR: 0.694, 95% CI, 0.425–1.134, P = .144; CT vs CC: OR: 0.693, 95% CI, 0.382–1.225, P = .226; TT vs CC: OR: 0.526, 95% CI, 0.124–2.241, P = .385; CT + TT vs CC: OR: 0.665, 95% CI, 0.379–1.166, P = .154. For SENP2 rs6762208 A vs C: OR: 0.803, 95% CI, 0.535–1.205, P = .289; CA vs AA: OR: 0.794, 95% CI, 0.460–1.370, P = .406; AA vs CC: OR: 0.773, 95% CI, 0.325–1.837, P = .560; CA + AA vs CC: OR: 0.789, 95% CI, 0.475–1.309, P = .359. For SENP7 rs61697963 C vs A: OR: 0.977, 95% CI, 0.681–1.402, P = .901; AC vs AA: OR: 1.247, 95% CI, 0.697–2.231, P = .456, CC vs AA: OR: 0.887, 95% CI, 0.417–1.890, P = .757; AC + CC vs AA: OR: 1.141, 95% CI, 0.656–1.986, P = .640).
3.4. Clinical Parameters of BC patients and SENP1 rs61918808, SENP2 rs6762208, and SENP7 rs61697963 polymorphism
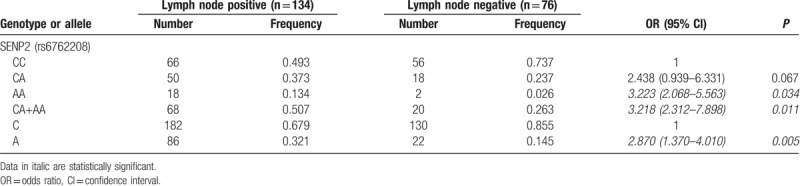
Next, we further investigated the correlations of SENP1 rs61918808, SENP2 rs6762208, and SENP7 rs61697963 polymorphism with the clinicopathological parameters in BC patients, including different hormone receptor status, HER-2 status, lymph node status, Ki-67 expression, tumor site, and differentiated grade. As shown in Tables 4 and 5, compared with CC genotype of SENP1 rs61918808, CT + TT genotypes were found to be correlated with the lack of HER-2 expression (OR: 0.383, 95% CI, 0.148–0.689, P = .037). In the case of SENP7 rs61697963 polymorphism, AC (OR: 2.830, 95%CI, 1.104–7.256, P = .030) and AC + CC (OR, 2.755, 95% CI, 1.113–6.818, P = .028) genotypes were associated with HER-2 expression. In case of SENP2 rs6762208 polymorphism, we found increased risk of lymph node metastases in patients with allele A (OR, 2.870, 95% CI, 1.370–4.010, P = .005) and the genotypes AA (OR, 3.223, 95% CI, 2.068–5.563, P = .034) and CA + AA (OR, 3.218, 95% CI, 2.312–7.898, P = .011).
Table 4.
The genotype and allele frequency and odds ratios (OR) of SENP1 rs61918808 and SENP7 rs61697963 polymorphism in groups of patients with breast cancer with positive and negative HER-2 status.
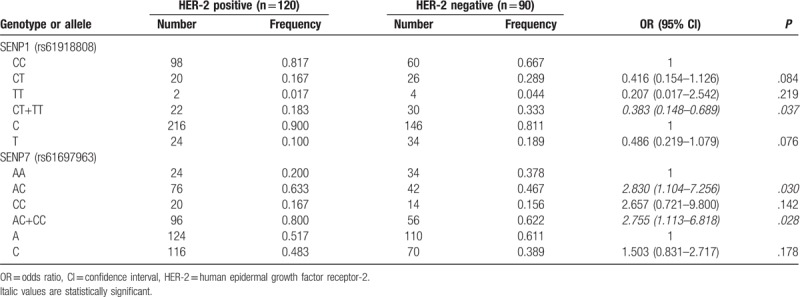
Table 5.
The genotype and allele frequency and odds ratios (OR) of SENP2 rs6762208 polymorphism in groups of patients with breast cancer with positive and negative lymph node status.
However, for all the studied SNPs, no significant association was observed according to ER status, PR status, breast tumor site, Ki-67 expression, and tumor grade (data not shown).
3.5. Associations between SENPs polymorphism and pathological complete response (pCR)
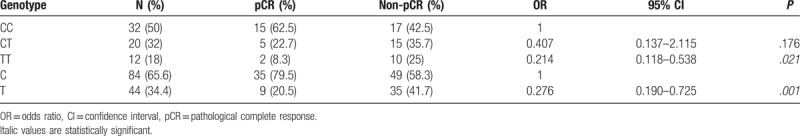
In our study, 64 BC patients received epirubicin followed by taxanes based on neoadjuvant chemotherapy (80 mg/m2 of epirubicin, 800 mg of cyclophosphamide and 80 mg/m2 of docetaxel on the first day, with 21 days a cycle). As shown in Table 6, the frequency of CC, CT, and TT genotype of SENP1 rs61918808 was 50.0%, 32.0%, and 18.0%. pCR was achieved in 24 cases (37.5%), with CC genotype 15 cases (46.9%), CT genotype 7 cases (35%), and TT allele 2 cases (16.7%). Patients with T allele and TT genotype of SENP1 rs61918808 were less likely to achieve pCR. Multivariate analysis showed the SNP rs61918808 was significant predictive factor of clinical response (T vs C, OR 0.276, 95% CI, 0.190–0.725, P = .001; CC vs TT, OR 0.214 95% CI, 0.118–0.538, P = .021).
Table 6.
Associations between patients’ response to neoadjuvant chemotherapy and SENP1 rs61918808 polymorphism.
4. Discussion
As an important protein modification, SUMOylation plays a wide range role to promote the initiation and progression of human cancers.[21,22] SENPs can act as de-conjugating or maturation/processing enzymes. The roles of SENPs have been widely reported in cancer, but epidemiological studies evaluating tumor susceptibility conferred by genetic polymorphisms have not been extensively assessed.[19] Indeed, to the best of our knowledge, this is the first study to investigate the role of SENP1 rs61918808, SENP2 rs6762208, and SENP7 rs61697963 in BC susceptibility and patient prognosis. No statistically significant association emerged between the 3 SENPs polymorphism and BC risk. However, our results showed that CT + TT genotype of SENP1 rs61918808 correlated with the lack of HER-2 expression (OR 0.383, 95% CI, 0.148–0.689). SENP1 was highly expressed in triple-negative BC (TNBC) tissues.[19] The absence of SENP1 significantly suppressed the proliferation and invasion of TNBC cells.[23] Moreover, clinical data showed that SENP1 was positively associated with lymph node metastasis and TNM stage of pancreatic cancer. Silencing of SENP1 impaired pancreatic cancer cell growth, migration, and invasion. Knockdown of SENP1 downregulated the expression of MMP-9, suggesting that SENP1 played an important role in PDAC progression and metastasis.[14] Further studies showed that SENP1-silencing sensitizes lung cancer cells to radiation and enhanced IR-induced lung cancer cell cycle arrest at the G0/G1 stage. In addition, SENP1 inhibition significantly upregulated IR-induced γ-H2AX expression, which was positively associated with tumor radiosensitivity.[24,25] It has been studied a single nonconsensus nuclear localization signal was presented in N terminus of SENP1, the mutation of which results in pronounced cytoplasmic accumulation in contrast to the nuclear accumulation of the parental protein. [26] Studied by us, polymorphic site in SENP1 rs61918808 (C>T) is located in the 5-UTR region, closed to the transcription start position, and therefore may affect transcriptional activity and proteolytic activity of SENP1. All these results demonstrate that SENP1 may be a potential drug target for cancer treatment. Notably, our study found SENP1 rs61918808 may be a predictor for the clinical response in local advanced BC patients who received neoadjuvant chemotherapy.
In the case of SENP2 rs6762208 polymorphism, we did not observe association of rs6762208 with the risk of BC. Of note, increased risk of lymph node metastases was observed in patients with allele A (OR 2.870, 95% CI, 1.370–4.010) and the genotypes AA (OR 3.223, 95% CI, 2.068–5.563) and CA + AA (OR 3.218, 95% CI, 2.312–7.898). In contrast to our findings, Mirecka et al found higher risk of BC for carriers of the A allele of rs6762208 polymorphism in Poland population, yet the genotype CC and the allele C decrease a risk of BC. Moreover, they observed that the AA genotype correlated with the lack of estrogen receptor.[19] Rational explanation for the significant difference may be attributed to differences in the genetic background of studied population and genotyping techniques as well as random errors. For example, the frequency of allele mutation is related to different ethnic populations. In this study, we found that the A allele frequency of SENP2 rs6762208 polymorphism was 0.289 in our healthy Chinese women subjects. In the study conducted by Mirecka et al, the frequency of A allele was 0.39 in Polan population. Unfortunately, unlike other polymorphisms, no data related the allele frequency of SENP2 rs6762208 polymorphism recorded in international HapMap Project. In spite of this, the results of present study provide referable data of allele frequency rs6762208 for the HapMap Project and other researchers.
SENP7 are specialized chain-editing enzymes.[10,27] Multiple SENP7 isoforms have differences in cellular and histological localization and biological function. For example, SENP7S, which is located in the cytosol, is highly expressed in normal mammary epithelia, yet decreases approximately 50% in precancerous ductal carcinoma in situ (DCIS) and is lost in multiple BC subtypes and invasive carcinoma. Nevertheless, SENP7L protein is elevated in invasive carcinoma.[28] Previously, it was showed that SENP7 is required for chromatin relaxation in response to DNA damage, for homologous recombination repair and for cellular resistance to DNA-damaging agents.[29] It was also demonstrated that targeted knockdown of the SENP7 gene transcript NM_001077203.2 alters Wnt-activated β-catenin signaling in a sarcoma cell line.[30] In the present study, no association between rs61697963 polymorphism and BC susceptivity was observed. However, we found patients with genotype AC (OR 2.830, 95% CI, 1.104–7.256) and AC + CC (OR 2.755, 95% CI, 1.113–6.818) had more HER-2 expression, which is an important prognostic marker of BC.[31]
Several potential limitations of the present study are as follows: first, inherent choice and information bias might exist because it was a hospital-based case–control study, and BC cases and healthy controls were from a singer center. Therefore, it is crucial to verify results of our case–control study in population-based prospective study in the future. Second, the sample size of the present study was not large enough, which may impact on the precision and accuracy of statistical analysis, especially for statistical analyses of subgroups which are stratified by clinicopathological features. Prospective case–control studies with larger sample size should be performed to verify the association between SENP1 rs61918808, SENP2 rs6762208, and SENP7 rs61697963 polymorphism and BC risk.
In conclusion, SENP1 rs61918808, SENP2 rs6762208, and SENP7 rs61697963 variants have not played any major role in genetic susceptibility to breast carcinogenesis within the Chinese population, but present specific effect on clinicopathological features of BC. Moreover, SENP1 rs61918808 may be a predictor for the clinical response in local advanced BC patients who received neoadjuvant chemotherapy.
Author contributions
Data curation: Jiaqin Cai, Xiaoxia Wei, Hong Sun.
Formal analysis: Guifeng Zhang.
Methodology: Xiaoxia Wei.
Resources: Yuxia Sui.
Validation: Jie Zhuang.
Writing – original draft: Jiaqin Cai.
Writing – review and editing: Zhenhua Liu, Hong Sun.
Footnotes
Abbreviations: BC = breast cancer, CI = confidence interval, ER = estrogen receptor, HER-2 = human epidermal growth factor receptor-2, HER-2 = human epidermal growth factor receptor 2, OR = odds ratio, pCR = pathological complete response, PR = progesterone receptor.
JC and XW contributed equally to this work and should be considered co-first authors.
This research was supported by grants from the National Natural Science Foundation of China (No. 81373838), Fujian Science and Technology Innovation Joint Fund Project (No. 2017Y9201), Medical Innovation Project of Fujian Provincial Health and Family Planning Commission (No. 2014-CX-5), Startup Fund for scientific research, Fujian Medical University (No. 2016QH106), Youth Scientific Research Project of Fujian Provincial Health Department (No. 2016-1-8) and the Natural Science Foundation of Fujian (No. 2019J01052019).
The authors have no conflicts of interest to disclose.
References
- [1].Psakhye I, Jentsch S. Protein group modification and synergy in the SUMO pathway as exemplified in DNA repair. Cell 2012;151:807–20. [DOI] [PubMed] [Google Scholar]
- [2].Pinder JB, Attwood KM, Dellaire G. Reading, writing, and repair: the role of ubiquitin and the ubiquitin-like proteins in DNA damage signaling and repair. Front Genet 2013;4:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Morris JR. More modifiers move on DNA damage. Cancer Res 2010;70:3861–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bologna S, Ferrari S. It takes two to tango: Ubiquitin and SUMO in the DNA damage response. Front Genet 2013;4:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bogachek MV, Chen Y, Kulak MV, et al. Sumoylation pathway is required to maintain the basal breast cancer subtype. Cancer Cell 2014;25:748–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kumar A, Zhang KY. Advances in the development of SUMO specific protease (SENP) inhibitors. Comput Struct Biotechnol J 2015;13:204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cui CP, Wong CC, Kai AK, et al. SENP1 promotes hypoxia-induced cancer stemness by HIF-1alpha deSUMOylation and SENP1/HIF-1alpha positive feedback loop. Gut 2017;66:2149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sharma P, Yamada S, Lualdi M, et al. Senp1 is essential for desumoylating Sumo1-modified proteins but dispensable for Sumo2 and Sumo3 deconjugation in the mouse embryo. Cell Rep 2013;3:1640–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shen LN, Dong C, Liu H, et al. The structure of SENP1-SUMO-2 complex suggests a structural basis for discrimination between SUMO paralogues during processing. Biochem J 2006;397:279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lima CD, Reverter D. Structure of the human SENP7 catalytic domain and poly-SUMO deconjugation activities for SENP6 and SENP7. J Biol Chem 2008;283:32045–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jacques C, Baris O, Prunier-Mirebeau D, et al. Two-step differential expression analysis reveals a new set of genes involved in thyroid oncocytic tumors. J Clin Endocrinol Metab 2005;90:2314–20. [DOI] [PubMed] [Google Scholar]
- [12].Bawa-Khalfe T, Cheng J, Lin SH, et al. SENP1 induces prostatic intraepithelial neoplasia through multiple mechanisms. J Biol Chem 2010;285:25859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang C, Tao W, Ni S, et al. Tumor-suppressive microRNA-145 induces growth arrest by targeting SENP1 in human prostate cancer cells. Cancer Sci 2015;106:375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ma C, Wu B, Huang X, et al. SUMO-specific protease 1 regulates pancreatic cancer cell proliferation and invasion by targeting MMP-9. Tumour Biol 2014;35:12729–35. [DOI] [PubMed] [Google Scholar]
- [15].Nait Achour T, Sentis S, Teyssier C, et al. Transcriptional repression of estrogen receptor alpha signaling by SENP2 in breast cancer cells. Mol Endocrinol 2014;28:183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tan MY, Mu XY, Liu B, et al. SUMO-specific protease 2 suppresses cell migration and invasion through inhibiting the expression of MMP13 in bladder cancer cells. Cell Physiol Biochem 2013;32:542–8. [DOI] [PubMed] [Google Scholar]
- [17].Ding X, Sun J, Wang L, et al. Overexpression of SENP5 in oral squamous cell carcinoma and its association with differentiation. Oncol Rep 2008;20:1041–5. [PubMed] [Google Scholar]
- [18].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [19].Mirecka A, Morawiec Z, Wozniak K. Genetic polymorphism of SUMO-specific cysteine proteases—SENP1 and SENP2 in breast cancer. Pathol Oncol Res 2016;22:817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Saraswathula A, Chen M, Negoita S, et al. American Joint Committee on Cancer Eighth Edition Changes in Staging Criteria: Implications for Data Collection. Otolaryngol Head Neck Surg 2017;157:748–9. [DOI] [PubMed] [Google Scholar]
- [21].Alshareeda AT, Negm OH, Green AR, et al. SUMOylation proteins in breast cancer. Breast Cancer Res Treat 2014;144:519–30. [DOI] [PubMed] [Google Scholar]
- [22].Bettermann K, Benesch M, Weis S, et al. SUMOylation in carcinogenesis. Cancer Lett 2012;316:113–25. [DOI] [PubMed] [Google Scholar]
- [23].Wang Z, Jin J, Zhang J, et al. Depletion of SENP1 suppresses the proliferation and invasion of triple-negative breast cancer cells. Oncol Rep 2016;36:2071–8. [DOI] [PubMed] [Google Scholar]
- [24].Xu Y, Li J, Zuo Y, et al. SUMO-specific protease 1 regulates the in vitro and in vivo growth of colon cancer cells with the upregulated expression of CDK inhibitors. Cancer Lett 2011;309:78–84. [DOI] [PubMed] [Google Scholar]
- [25].Wang RT, Zhi XY, Zhang Y, et al. Inhibition of SENP1 induces radiosensitization in lung cancer cells. Exp Ther Med 2013;6:1054–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bailey D, O’Hare P. Characterization of the localization and proteolytic activity of the SUMO-specific protease, SENP1. J Biol Chem 2004;279:692–703. [DOI] [PubMed] [Google Scholar]
- [27].Shen LN, Geoffroy MC, Jaffray EG, et al. Characterization of SENP7, a SUMO-2/3-specific isopeptidase. Biochem J 2009;421:223–30. [DOI] [PubMed] [Google Scholar]
- [28].Karami S, Lin FM, Kumar S, et al. Novel SUMO-protease SENP7S regulates beta-catenin signaling and mammary epithelial cell transformation. Sci Rep 2017;7:46477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Garvin AJ, Densham RM, Blair-Reid SA, et al. The deSUMOylase SENP7 promotes chromatin relaxation for homologous recombination DNA repair. EMBO Rep 2013;14:975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Buehler E, Khan AA, Marine S, et al. siRNA off-target effects in genome-wide screens identify signaling pathway members. Sci Rep 2012;2:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gajria D, Chandarlapaty S. HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev Anticancer Ther 2011;11:263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]


