Abstract
Children with fibrous dysplasia (FD) chronically suffer from pain, pathological fractures, and limb deformities. The most effective methods for managing the associated pathological fractures remain controversial. The purpose of this study was to evaluate the clinical results of the treatment of diaphyseal pathological fractures in children with monostotic fibrous dysplasia (MFD) using cortical strut allografts and internal plating.
We retrospectively analyzed outcomes in nine children (5 boys, 4 girls) with diaphyseal pathological fractures due to MFD, who were treated with cortical strut allografts and internal plating (6 femoral fractures and 3 humeral fractures) between July 2007 and November 2012. The median age of patients in our study was 10 years (range 6–14 years). The fracture healing time, pain, extremity function, refracture, graft resorption, and complications were recorded to evaluate treatment effects.
The median time of follow-up was 69 months (range 60–75 months). All patients had good postoperative fracture healing with a median healing time of 14 weeks (range 12–16 weeks). None experienced refracture, graft resorption, nerve injury, or limitation of extremity function or other complications. The fixation remained stable in all patients, with no evidence of loosening screws after surgery.
In pediatric patients, the described surgical approach is an effective and reliable treatment method for diaphyseal pathological fractures caused by MFD. Cortical strut allografts, which act as biological bone plates, can provide good mechanical support while increasing the rate of fracture union.
Keywords: cortical strut allograft, diaphyseal pathological fractures, internal plating, monostotic fibrous dysplasia
1. Introduction
Fibrous dysplasia (FD) is a skeletal disease in childhood and adolescence caused by a somatic activating mutation of the GNAS gene, which alters G-protein signaling.[1,2] It causes benign bone lesions characterized by replacement of normal bone and marrow by fibrous tissue[3] produced by poorly differentiated osteoblasts, resulting in impaired stability. The forms of FD include monostotic fibrous dysplasia (MFD) and polyostotic fibrous dysplasia (PFD).[4] The primary orthopedic problems associated with FD are pain, deformities, and increased risk of fractures.[5] Benhamou et al[6] reported that MFD represents the majority of FD cases in an overview of the epidemiology of FD in France. Moreover, Leet et al[7] reported that the peak fracture rate of patients with FD was observed between ages 5 and 10 and declined thereafter. However, the optimal surgical management of diaphyseal pathological fractures in children with MFD remains controversial. The bone of FD is characterized by widening and thinning of the cortex, which is extremely fragile and easily leads to pathological fractures. It is a great challenge for surgeons to choose the optimal treatment for a pathological fracture of the diaphysis. Enneking[8] first introduced the use of bone grafting for ablation of FD lesions, and reported achieving good outcomes. The use of cortical grafts is usually preferred over cancellous grafts, not only because of the superior physical qualities of remodeled cortical bone but also because it provides good mechanical support.[9] Thus, surgeons need to choose either bone allografts or vascularized or nonvascularized fibular autografts as bone graft material. However, there are disadvantages to using fibular autografts for the benign lesions of FD, such as the surgical morbidity of the graft harvest site and a requirement for superb surgical skill. At the same time, patients with pathological fractures have an increased risk of failure of cortical strut allografts and so should be treated with osteosynthesis.[10] Therefore, the surgical approach for pathological fractures consists of biopsy, intralesional curettage, and bone grafting with internal fixation. We hypothesize that intramedullary fixation may not be an appropriate choice for pediatric diaphyseal pathological fractures caused by MFD, as the intramedullary device may damage the growth plate and influence bone growth leading to limb length discrepancy. In addition, as the child grows, the rod becomes insufficient for the increased size of the diaphysis and the increase in body weight.[11] Thus, we decided to use cortical strut allografts after intralesional curettage and internal plating for the treatment of diaphyseal pathological fractures in children with MFD. This study aimed to evaluate the clinical results of this surgical approach.
2. Methods
2.1. Ethical statement
This study was approved by Xiamen University Ethical Review Committee (no: EC-07-012, 03/31/2007). The parents or legal guardians provided written informed consent for the publication of individual clinical details and accompanying images.
2.2. Patients
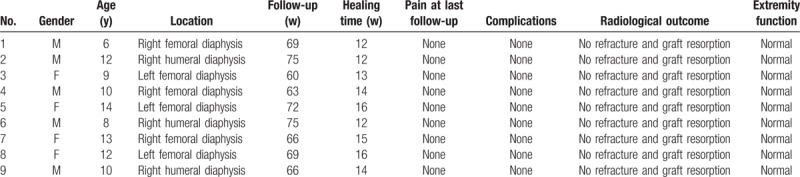
Between July 2007 and November 2012, 9 children (5 boys, 4 girls) with diaphyseal pathological fractures due to MFD were treated using this surgical approach consisting of cortical strut allografts and internal plating. There were six femoral fractures and three humeral fractures. The median age of the patients was 10 years (range 6–14 years) (Table 1).
Table 1.
Patient characteristics and outcomes.
2.3. Preoperative management
Before surgery, MRI and X-rays were performed to precisely measure the length of the bone shaft and the length of the bone lesion. Then the cortical strut allografts were cut to a length approximately 2 cm longer than the length of the bone lesions, to increase the contact between the graft and the reconstruction site.[12] The cortical strut allografts were made with freeze-dried allogeneic long bones (Xinkangchen Medicine Development, Beijing, China).
2.4. Operative procedure
All operative procedures were performed by one orthopedic surgeon.
2.4.1. Surgery for diaphyseal pathological fractures of the humerus
After a routine preanesthetic check-up and general anesthesia, the patients were placed in the supine position, the shoulder was abducted to about 60° with the elbow flexed to about 90°, and the forearm was supinated throughout the surgical procedure. Then a longitudinal incision of approximately 12 cm through the pathological fracture site was made, following the line between the coracoid and the biceps tendon of the elbow. The distal incision was terminated at 5 cm above the lateral striation of the elbow to avoid injuring the elbow joint and protect the cephalic vein. Next, the brachialis muscle was split to reach the anterior surface of the humerus; the lateral part of the split brachialis muscle, protecting the radial nerve, was retracted laterally while the biceps and underlying neurovascular bundle were retracted medially. Meanwhile, excessively strong traction was avoided to protect the nerves. During surgery, a cortical fenestration was made using an osteotomy large enough to visualize the whole lesion. Next, the lesion was removed with curettes, and the cavity was enlarged with a burr and irrigated with sterile saline. The definitive histological diagnosis was confirmed by frozen section from the removed tissue obtained at the time of operation. Then the cortical strut allograft was introduced into the medullary canal at the lesion site. A 4.5-mm locking compression plate was placed in front of the humeral shaft after the fracture was restored. Finally, we inserted screws at the proximal and distal ends of the plate, mindful of fracture alignment as well as the proximity of the radial nerve laterally and the brachial artery medially throughout this process. The quality of fracture reduction, location of the plates, and length of the screws were confirmed by fluoroscopic guidance (Fig. 1).
Figure 1.
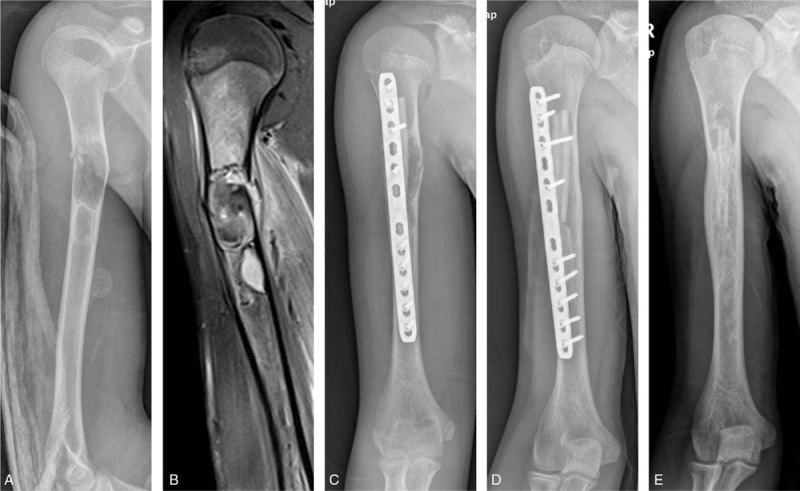
A 12-year-old male patient with monostotic fibrous dysplasia in the diaphysis of right humerus. (A) X-ray shows the fibrous dysplasia and pathological fractures occur at the diaphysis of right humerus. (B) Magnetic resonance imaging shows T1 hypointense signal at the diaphysis of right humerus, demonstrating the extent of disease. (C) X-ray anteroposterior view on the day of surgery. (D) X-ray at 12 months after surgery showing union. (E) Good healing of the fracture and bony union between the cortical strut allograft and the host bone at 5 years after surgery.
2.4.2. Surgery for diaphyseal pathological fractures of femur
After a routine preanesthetic check-up and epidural anesthesia, the patient was positioned supine on a radiolucent fracture table. A longitudinal incision of approximately 15 cm through the pathological fracture site was made using the standard lateral approach to the femur, and then the gap between the vastus lateralis and rectus femoris was entered to reach the surface of the femur. The vastus lateralis was retracted laterally, and a cortical fenestration was made using an osteotomy. The surgical procedure from that point was similar to that outlined above for the humerus. The plate and the cortical strut allograft were placed and stabilized with screws (Fig. 2).
Figure 2.
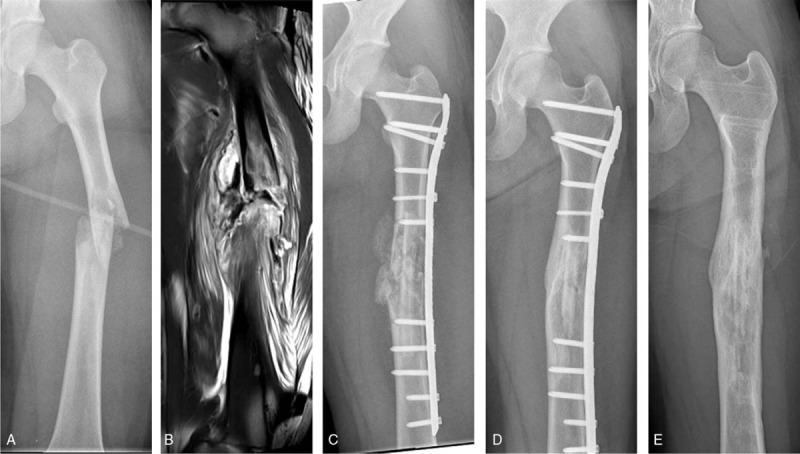
A 14-year-old female patient with monostotic fibrous dysplasia in the diaphysis of left femur. (A) X-ray shows the fibrous dysplasia and pathological fractures occur at the diaphysis of left femur. (B) Magnetic resonance imaging shows T1 hypointense signal at the diaphysis of left femur, demonstrating the extent of disease. (C) X-ray anteroposterior view on the day of surgery. (D) X-ray at 12 months after surgery showing union. (E) Good healing of the fracture and bony union between the cortical strut allograft and the host bone at 5 years after surgery.
2.5. Postoperative management
No external fixation was used after surgery. After surgery, patients were encouraged to perform gentle and protective exercises immediately as tolerated. For the patients with humeral fractures, pendulum exercises and elbow, wrist, and hand range of motion exercises were started immediately after surgery. The patients with femur fractures were encouraged to mobilize postoperatively using two crutches. The weight-bearing was gradually increased after 6 weeks if increasing consolidation of the graft was observed on plain radiographs. The patients were discharged one week after surgery, and then serial radiographs were obtained at 3, 6, and 12 months, and 5 years postoperatively.
2.6. Postoperative evaluation
Postoperatively, the patients were assessed for the clinical and radiographic outcomes at different time points as above. Radiographic outcomes included the fracture healing time, occurrence of refracture, and graft resorption. The clinical outcomes included pain and extremity function.
3. Results
All patients had a follow-up period of at least five years and the median time of follow-up was 69 months (range 60–75 months). All patients had good postoperative fracture healing with a median healing time of 14 weeks (range 12–16 weeks). None of the patients had allograft rejection, neurovascular complications, or infections. None experienced refractures or graft resorption (Table 1). The fixation remained stable in all patients, with no evidence of loosening screws after surgery. Plates were removed in all patients at a median of 12 months (range 9–15 months) after the operation. During the follow-up, we did not see any nerve injury or palsy in the affected areas of the upper and lower extremities. All the patients resumed normal activity and function. The cortical strut allografts were incorporated into the host bones one year after surgery. In addition, the medullary cavity junction was recognized between the allograft and the host bone (Figs. 1 and 2).
4. Discussion
FD of the bone is a type of benign bone lesion. MFD, a single skeletal site with an isolated lesion, occurs in the majority of patients.[8] Limping, pain, and pathologic fracture are the usual presentations of FD.[13] Previous surgical procedures used to treat FD lesions with pathologic fractures include intralesional curettage and bone grafting with or without internal fixation.[5,8,10,14–16]
A list of studies, which reported radiographic evaluation after the treatment of fibrous dysplasia with pathologic fractures with various types of grafts and internal fixations, are presented in Table 2. Enneking et al[8] treated 12 patients with pathologic fractures using cortical autografts without internal fixation, which achieved a good outcome. Guille et al[5] reported 22 patients with pathologic fractures treated with curettage and autogenous cancellous bone grafting; they observed complete resorption of all autogenous cancellous bone grafts radiographically at the last follow-up. Taking the above reports together, autogenous cancellous bone can be assumed to be resorbed.
Table 2.
Previous reports describing the surgical treatment of pathological fractures in patients with fibrous dysplasia.
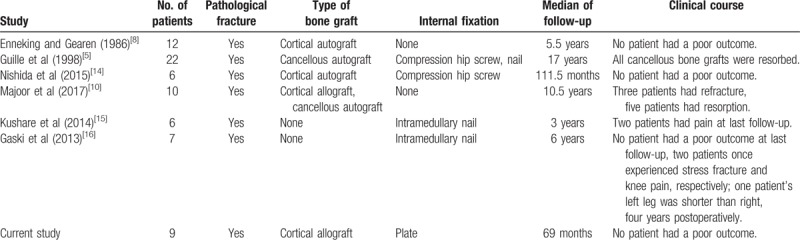
Other papers have reported the importance of internal fixation, especially in patients with pathologic fractures. Nishida et al[14] reported that six patients with pathologic fractures achieved a good outcome by using cortical autografts with compression hip screws. Majoor et al[10] reported a study of ten patients with pathologic fractures treated with either cortical allograft or cancellous autograft without internal fixation. Eight patients had poor outcomes, three patients had refractures, and five patients had resorption.
Kushare et al[15] reported 6 patients with pathologic fractures treated with intramedullary nail alone who achieved a relatively good outcome, although two patients still had pain at last follow-up. Gaski et al[16] reported that seven patients with pathologic fractures treated with intramedullary nail alone achieved good outcomes; however, two patients experienced stress fracture and knee pain during the treatment and one patient's left leg was shorter than the right at four years postoperatively. From these two articles, we see that intramedullary nails have achieved good results, but there are also some problems; these include stress fracture, knee pain, and injury of the growth plate leading to different limb lengths. Thus, we chose metal plates as the internal fixation material.
As seen in the articles listed in Table 2, the method of treating pathological fractures in patients with fibrous dysplasia is still controversial. However, there is no doubt that bone grafting is one part of the surgical strategy; bone grafting not only enhances the holding force of the screws but also increases the probability of cure. There have been numerous reports suggesting use of cortical grafts rather than cancellous grafts.[5,9,17] Reportedly, cortical grafts are much less likely, after incorporation, to be replaced by dysplastic bone than grafts of autogenous cancellous bone.[8] Thus, surgeons have tended to use cortical bone grafts, including allografts and autografts. Autografts used were mainly from the fibula, prepared by conventional nonvascularized or free vascularized techniques.[18,19] Although fibular autografts achieved good effects, those effects came at the cost of damage to the donor site and its important normal structures.[20] Use of fibular autografts simultaneously increased operative complexity and operative time, which led to increased loss of blood and risk of infection. Therefore, we chose cortical strut allografts as the material of bone grafting.
In the literature, intramedullary nails for pathologic fractures have revealed more satisfactory results with decreased incidence of recurrent fracture, as well as less risk of deformity of the affected extremity postoperatively.[14–16] As FD is a benign disease and most individuals requiring intervention are below thirty years of age, the fixation needs to remain stable over a longer period of time.[9] However, intramedullary nails gradually become insufficient because of the increase in the size of the diaphysis and the increase in body weight of growing children.[11]
Reportedly, the poor physical qualities of the dysplastic bone make conventional internal fixation devices such as plates and screws less effective, unless screws can be placed outside the FD lesions, thus obtaining purchase in normal cortical bone.[17] Additionally, the age of the patients and the location and size of the lesions all influence the selection of the type of internal fixation. In this study, only the diaphyseal pathological fractures of pediatric patients with MFD were included, which means that the lesions were single and relatively small. As a result, there was enough normal cortical bone to support the internal plating. Similar to Rosario et al,[21] we chose long enough plates to bridge the lesion, which avoided mechanical stress resulting in implant fatigue. Therefore, the surgical approach of this study consisted of biopsy, intralesional curettage, high-speed burring, and reconstruction with the use of cortical strut allografts and internal plating. At the same time, intralesional curettage of the lesion leaves a cavity, which may lead to destabilization or even pathological fracture. The use of cortical strut allografts increases stability while enhancing the holding force of the screws. Moreover, the use of a high-speed burr has been shown to decrease the rate of local recurrence.[22]
No matter which surgical approach is taken, a key principle to management of these pathological fractures is that fixation should never be undertaken without a histological diagnosis; thus, in our study, a biopsy of every lesion was taken with tissue sent for frozen section to confirm the diagnosis intraoperatively.
Limitations of this study include the relatively small number of patients and the lack of a control group. In addition, the use of cortical strut allografts remains controversial, even though other problems associated with it (e.g., immune rejection and infection) have been solved.
In this study, pediatric diaphyseal pathological fractures caused by MFD were treated with internal plating and cortical strut allografts. Our surgical approach has several advantages. First, the use of cortical strut allografts is advantageous in that they are a plentiful, safe, and easily available source of bone that provides good mechanical support; in addition, they are versatile, and their use avoids any donor site morbidity.[23] Moreover, their use increases the probability of fracture union.[19] Second, compared to intramedullary fixation, internal plating fixation may avoid injury to the growth plate, which plays an important role in bone remodeling. Third, given the young age of our patients, the fixation needs to remain stable over a longer period of time; while intramedullary fixation gradually becomes insufficient for the increasing size of the diaphysis and body weight in growing children, internal plate fixation does not have this problem. Fourth, uniform contact at the allograft–host junction and stable fixation, which promote healing across gaps at the allograft–host junction, were easier to achieve with the use of a plate.[24]
Author contributions
PZ analyzed data and wrote the manuscript. QH, CX, and HY contributed to the acquisition and analysis of data for this work. LK, LW, and KL performed the surgery. LD designed this work and critically reviewed all content. All authors approved the version of the manuscript.
Data curation: Liangqi Kang, Qimiao Hu, Chenjie Xia, Huan Yu, Lei Wang, Kejian Lian, Dasheng Lin.
Formal analysis: Peng Zhang, Qimiao Hu.
Funding acquisition: Dasheng Lin.
Project administration: Liangqi Kang, Kejian Lian, Dasheng Lin.
Writing – original draft: Peng Zhang.
Writing – review & editing: Liangqi Kang, Kejian Lian, Dasheng Lin.
Footnotes
Abbreviations: FD = fibrous dysplasia, MFD = monostotic fibrous dysplasia, PFD = polyostotic fibrous dysplasia.
Funding: This study was supported by the key projects from Nanjing Military Region during the 11th Five Year Plan Period (Grant No 06MA97).
The authors declare that they have no conflicts of interest.
References
- [1].Weinstein LS, Shenker A, Gejman PV, et al. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med 1991;325:1688–95. [DOI] [PubMed] [Google Scholar]
- [2].Schwindinger WF, Francomano CA, Levine MA. Identification of a mutation in the gene encoding the alpha subunit of the stimulatory G protein of adenylyl cyclase in McCune-Albright syndrome. Proc Natl Acad Sci U S A 1992;89:5152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Riminucci M, Fisher LW, Shenker A, et al. Fibrous dysplasia of bone in the McCune-Albright syndrome: abnormalities in bone formation. Am J Pathol 1997;151:1587–600. [PMC free article] [PubMed] [Google Scholar]
- [4].Albright F, Butler AM, Hampton AO, et al. Syndrome characterized by osteitis fibrosa disseminata, areas of pigmentation and endocrine dysfunction, with precocious puberty in females—report of five cases. N Engl J Med 1937;216:727–46. [Google Scholar]
- [5].Guille JT, Kumar SJ, MacEwen GD. Fibrous dysplasia of the proximal part of the femur. Long-term results of curettage and bone-grafting and mechanical realignment. J Bone Joint Surg Am 1998;80:648–58. [DOI] [PubMed] [Google Scholar]
- [6].Benhamou J, Gensburger D, Messiaen C, et al. Prognostic factors from an epidemiologic evaluation of fibrous dysplasia of bone in a modern cohort: the FRANCEDYS study. J Bone Miner Res 2016;31:2167–72. [DOI] [PubMed] [Google Scholar]
- [7].Leet AI, Chebli C, Kushner H, et al. Fracture incidence in polyostotic fibrous dysplasia and the McCune-Albright syndrome. J Bone Miner Res 2004;19:571–7. [DOI] [PubMed] [Google Scholar]
- [8].Enneking WF, Gearen PF. Fibrous dysplasia of the femoral neck. Treatment by cortical bone-grafting. J Bone Joint Surg Am 1986;68:1415–22. [PubMed] [Google Scholar]
- [9].DiCaprio MR, Enneking WF. Fibrous dysplasia. Pathophysiology, evaluation, and treatment. J Bone Joint Surg Am 2005;87:1848–64. [DOI] [PubMed] [Google Scholar]
- [10].Majoor BC, Peeters-Boef MJ, van de Sande MA, et al. What is the role of allogeneic cortical strut grafts in the treatment of fibrous dysplasia of the proximal femur? Clin Orthop Relat Res 2017;475:786–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Benedetti Valentini M, Ippolito E, Catellani F, et al. Internal fixation after fracture or osteotomy of the femur in young children with polyostotic fibrous dysplasia. J Pediatr Orthop B 2015;24:291–5. [DOI] [PubMed] [Google Scholar]
- [12].Gebert C, Hillmann A, Schwappach A, et al. Free vascularized fibular grafting for reconstruction after tumor resection in the upper extremity. J Surg Oncol 2006;94:114–27. [DOI] [PubMed] [Google Scholar]
- [13].Leet AI, Collins MT. Current approach to fibrous dysplasia of bone and McCune-Albright syndrome. J Child Orthop 2007;1:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nishida Y, Tsukushi S, Hosono K, et al. Surgical treatment for fibrous dysplasia of femoral neck with mild but prolonged symptoms: a case series. J Orthop Surg Res 2015;10:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kushare IV, Colo D, Bakhshi H, et al. Fibrous dysplasia of the proximal femur: surgical management options and outcomes. J Child Orthop 2014;8:505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gaski G, Hansen D, Willis LM, et al. Intramedullary rod fixation of fibrous dysplasia without use of bisphosphonates. J Child Orthop 2013;7:277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stanton RP, Ippolito E, Springfield D, et al. The surgical management of fibrous dysplasia of bone. Orphanet J Rare Dis 2012;7Suppl 1:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Springfield DS. Massive autogenous bone grafts. Orthop Clin North Am 1987;18:249–56. [PubMed] [Google Scholar]
- [19].Manfrini M. The role of vascularized fibula in skeletal reconstruction. Chir Organi Mov 2003;88:137–42. [PubMed] [Google Scholar]
- [20].Zhang Y, Li JZ, Lu XC, et al. Intramedullary nailing combined with bone grafting for benign lesions of the proximal femur. Orthop Surg 2017;9:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rosario MS, Hayashi K, Yamamoto N, et al. Functional and radiological outcomes of a minimally invasive surgical approach to monostotic fibrous dysplasia. World J Surg Oncol 2017;15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Grzegorzewski A, Pogonowicz E, Sibinski M, et al. Treatment of benign lesions of humerus with resection and non-vascularised, autologous fibular graft. Int Orthop 2010;34:1267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Finkemeier CG. Bone grafting and bone graft substitutes. J Bone Joint Surg Am 2002;84-A:454–64. [DOI] [PubMed] [Google Scholar]
- [24].Vander Griend RA. The effect of internal fixation on the healing of large allografts. J Bone Joint Surg Am 1994;76:657–63. [DOI] [PubMed] [Google Scholar]


