Abstract
Objective:
To appropriately identify and treat noncommunicable diseases (NCDs) among persons living with HIV (PLHIV) in low-and-middle-income countries (LMICs), it is imperative to understand the burden of NCDs among PLHIV in LMICs and the current management of the diseases.
Design:
Systematic review and meta-analysis.
Methods:
We examined peer-reviewed literature published between 1 January 2010 and 31 December 2016 to assess currently available evidence regarding HIV and four selected NCDs (cardiovascular disease, cervical cancer, depression, and diabetes) in LMICs with a focus on sub-Saharan Africa. The databases, PubMed/MEDLINE, Cochrane Review, and Scopus, were searched to identify relevant literature. For conditions with adequate data available, pooled estimates for prevalence were generated using random fixed effects models.
Results:
Six thousand one hundred and forty-three abstracts were reviewed, 377 had potentially relevant prevalence data and 141 were included in the summary; 57 were selected for quantitative analysis. Pooled estimates for NCD prevalence were hyper-tension 21.2% (95% CI 16.3–27.1), hypercholesterolemia 22.2% (95% CI 14.7–32.1), elevated low-density lipoprotein 23.2% (95% CI 15.2–33.6), hypertriglyceridemia 27.2% (95% CI 20.7–34.8), low high-density lipoprotein 52.3% (95% CI 35.6–62.8), obesity 7.8% (95% CI 4.3–13.9), and depression 24.4% (95% CI 12.5–42.1). Invasive cervical cancer and diabetes prevalence were 1.3–1.7 and 1.3–18%, respectively. Few NCD-HIV integrated programs with screening and management approaches that are contextually appropriate for resource-limited settings exist.
Conclusion:
Improved data collection and surveillance of NCDs among PLHIV in LMICs are necessary to inform integrated HIV/NCD care models. Although efforts to integrate care exist, further research is needed to optimize the efficacy of these programs.
Keywords: health systems, HIV, integration, low-income and middle-income countries, noncommunicable disease
Introduction
Since 2004, HIV prevention, care, and treatment programs have been established in over 30 low-income and middle-income countries (LMICs) worldwide. These programs have enabled approximately 19.5 million people living with HIV (PLHIV) to receive antiretroviral treatment (ART)as of 2016 [1]. As the uptake of ART increases in LMICs, survival of PLHIV will improve likely to the same extent as currently noted in industrialized nations [2–4]. Once the Joint United Nations Programme on HIV/AIDS (UNAIDS) ambitious 90–90–90 goals (90% of people with HIV diagnosed, 90% of them on ART and 90% of them virally suppressed by 2020) are realized, AIDS-related opportunistic illnesses will continue to decline [5] and noncommunicable diseases (NCDs) will become increasingly prevalent [6–12].
For persons on ART, HIV becomes a chronic disease with increasing risk for chronic comorbidities, including cardiovascular disease [13], depression [14–16], cancers [17,18], and metabolic abnormalities, diabetes, and lipodystrophy [19–21]. The increased prevalence of NCDs among HIV-infected adults reflects a combination of factors, including aging, greater prevalence of traditional NCD risk factors, direct consequences of HIV infection, and exposure to specific antiretrovirals [22–32]. To appropriately treat NCDs among PLHIV in LMICs, it is imperative to understand the predominant risk factors, the consequent symptoms and complications, and the available, appropriate treatment, and preventive interventions.
Over the last decade, significant investments have been made to establish HIV/AIDS programs in LMICs [33]. If left unaddressed, NCDs may undermine the effectiveness of these programs [34]. The need to confront the emerging NCD crisis presents a unique opportunity to leverage the substantial investments made in the existing HIV health systems to deliver enhanced HIV care to achieve a sustainable reduction in preventable deaths. To do this, researchers, policymakers, public health officials, healthcare providers, and other stakeholders need to build the evidence base for NCD epidemiology, risk factors, diagnostics, prevention, and treatment among PLHIV. Four NCDs that are likely to account for the greatest comorbidity among PLHIV in LMICs are cardiovascular disease, cervical cancer, depression, and diabetes. We conducted a review to determine the burden of these four NCDs and the evidence-based approaches for their management among PLHIV in LMICs. Furthermore, we elicited the gaps in knowledge and identified a research agenda to facilitate successful HIV/NCD integrated care.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was used for this systematic review [35].
Literature search and review
A literature search was conducted to identify peer-reviewed articles published on the four NCDs among adult PLHIV in LMICs. The PubMed/MEDLINE, Cochrane Library, and Scopus databases were searched to identify human studies published between 1 January 2010 and 31 December 2016. Search terms used included controlled vocabulary terms (i.e. Medical Subject Headings) and keywords recommended by subject matter experts and by reviewing key articles (see Appendix, Supplemental Digital Content 1, http://links.lww.com/QAD/B294). EndNote X8 (Clarivate Analytics, Philadelphia, PA) was used to collect, de-duplicate, manage, and review citations.
Study selection
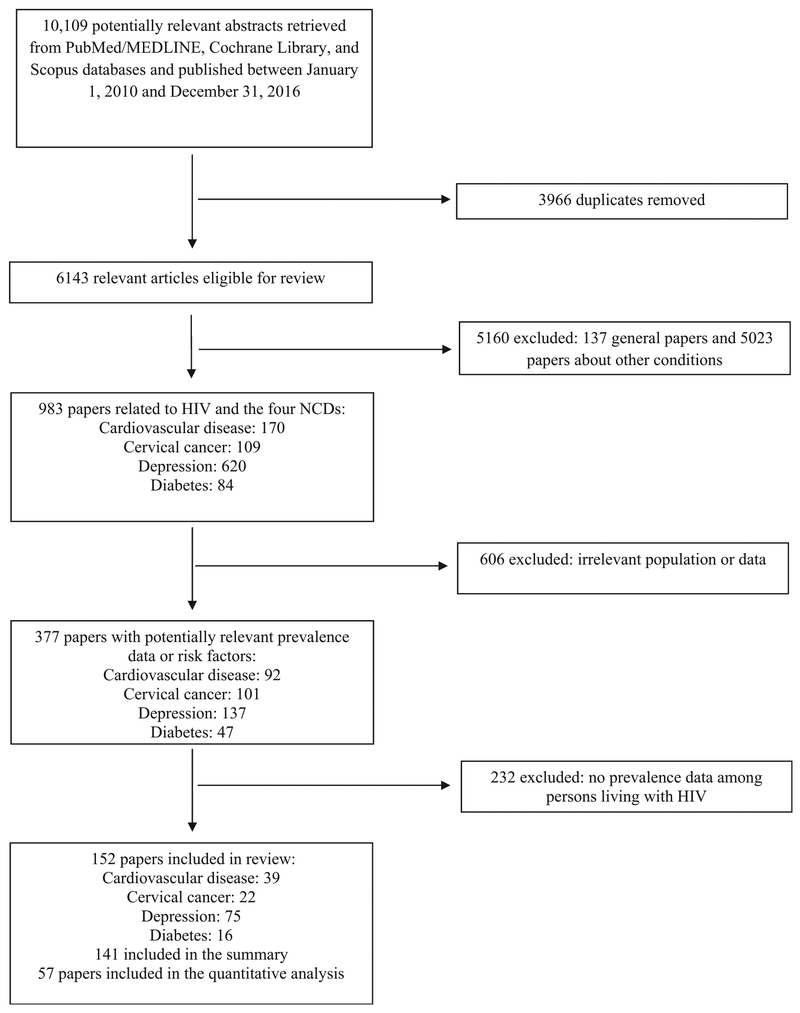
The titles and abstracts of the identified articles were screened for mention of HIV and any of the four NCDs: cardiovascular disease risk factors (i.e. hypertension, dyslipidemia, obesity), cervical cancer, depression, and diabetes. Next, the selected articles’ titles and abstracts were screened by two authors (P.P. and S.V.) to identify those reporting prevalence and management of four NCDs and their main risk factors among adult PLHIV in LMICs. For those reporting prevalence data or risk factors, the full-text was reviewed to collect pertinent data. Studies with statistically robust methods, such as standardized and unbiased data collection with adequate sample size, were included to ensure reproducibility and precision. Studies that reported prevalence among a subset of PLHIV were excluded, given the inherent bias in the estimate (e.g. prevalence of cancer among PLHIV with known high-risk HPV infection). The effects of ART were not examined as this was outside the scope of the systematic review’s objective. Lastly, the articles were categorized and coded by the four NCDs of interest. Figure 1 details the study selection procedure; 6143 abstracts were reviewed, from which 377 had potentially relevant data and 141 on prevalence were included in the summary (see Table, Supplemental Digital Content 2, http://links.lww.com/QAD/B294).
Fig. 1.
Selection of studies regarding noncommunicable diseases among HIV-infected persons in low-income and middle-income countries.
Meta-analyses
Of the 141 articles included in the summary, 57 were selected for quantitative analysis. Among these articles, relevant data on risk factors of cardiovascular disease were reviewed. The diabetes studies demonstrated considerable heterogeneity especially with respect to screening tests used. The data were too sparse for invasive cervical cancer. Thus, both conditions were excluded from the meta-analysis. Depression was estimated using a variety of screening tools; because the majority used the Patient Health Questionnaire-9 (PHQ-9), only those were included in the summary estimate. A random-effects logistic regression model was used to estimate pooled prevalence for hypertension, hypercholesterolemia, elevated low-density lipoprotein (LDL), hypertriglyceridemia, low high-density lipoprotein (HDL), dyslipidemia, obesity, overweight, obese/overweight, and depression. The studies available for each outcome were treated as the random-effect. Data stratified by ART use, age, or sex were combined to generate an overall estimate of prevalence among all adult PLHIV. All statistical analyses were conducted using PROC NLMIXED in SAS 9.4.
Results
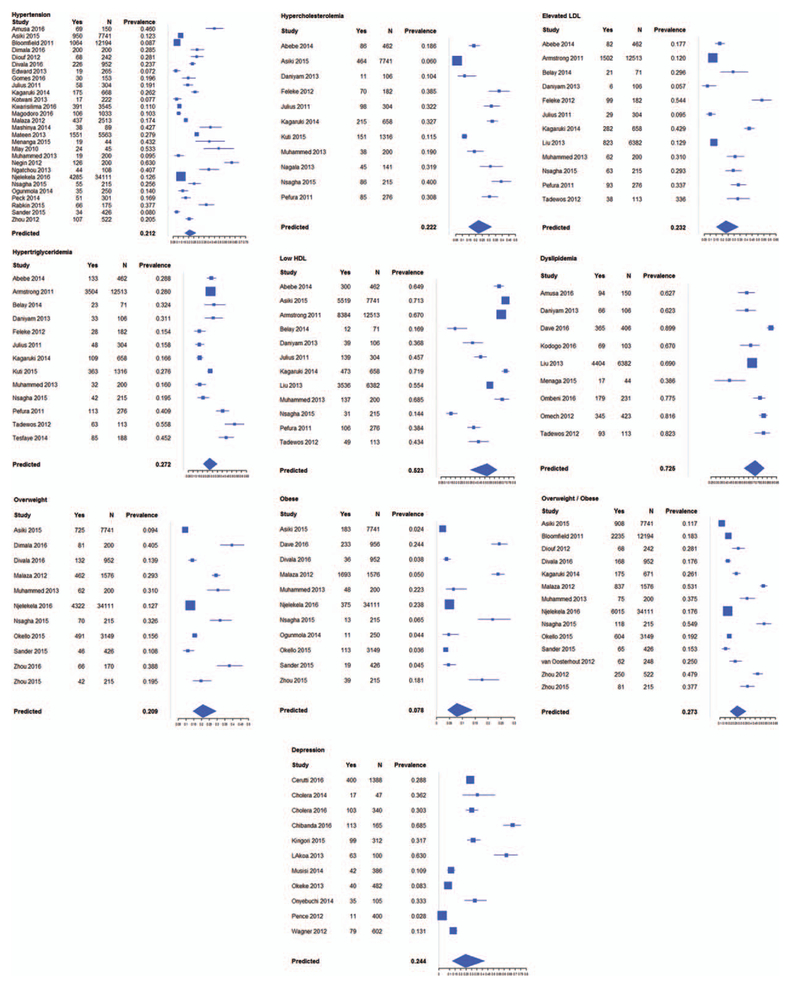
Our pooled prevalence estimates from meta-analyses of 57 articles (Fig. 2) are summarized (Table 1). We have also identified gaps that warrant attention to successfully integrate care for HIV/NCDs as well as opportunities for future research (Table 2).
Fig. 2.
Forest plots of pooled estimates generated by meta-analyses for hypertension, hypercholesterolemia, elevated low-density lipoprotein (LDL), hypertriglyceridemia, low high density-lipoprotein (HDL), dyslipidemia, obesity, overweight, depression.
Table 1.
Pooled prevalence estimates for select noncommunicable disease risk factors.
| Risk factor | Pooled prevalence estimate (%) | 95% Confidence interval |
|---|---|---|
| Hypertensiona | 21.2 | 16.3–27.1 |
| Hypercholesterolemiab | 22.2 | 14.7–32.1 |
| Elevated low-density lipoproteinc | 23.2 | 15.2–33.8 |
| Hypertriglyceridemiad | 27.2 | 20.7–34.8 |
| Low high-density lipoproteine | 52.3 | 35.6–62.8 |
| Dyslipidemiaf | 72.5 | 60.6–81.9 |
| Overweightg | 21.0 | 14.6–29.2 |
| Obeseh | 7.8 | 4.3–13.9 |
| Overweight/obesei | 27.3 | 20.2–35.9 |
| Depressionj | 24.4 | 12.5–42.1 |
SBP greater than 140 mmHg and/or DBP greater than 90 mmHg or on hypertension treatment.
Total cholesterol at least 200 mg/dl or 5.2 mmol/l.
Elevated low-density lipoprotein (LDL) at least 200 mg/dl or 3.4 mmol/l.
Hypertriglyceridemia at least 150 mg/dl or 1.7 mmol/l.
Low high-density lipoprotein less than 40 mg/dl or 1 mmol/l.
Dyslipidemia: triglycerides at least 200 mg/dl or 5.2 mmol/l or LDL-C at least 130 mg/dl or 3.4 mmol/l or triglycerides at least 150 mg/dl or 1.7 mmol/l or HDL-C less than 40 mg/dl or 1 mmol/l.
BMI 25–29.
BMI at least 30.
BMI greater than 25.
Patient Health Questionnaire-9 greater than 9.
Table 2.
Research agenda for improved integration of noncommunicable disease and HIV care delivery in low-income and middle-income countries.
| Gaps | Research questions |
|---|---|
| Noncommunicable diseases (general but also applies to all) | |
| Population-level data describing the NCD burden among general population and among PLHIV are very limited (only sub-national convenience sample estimates and/or modeling estimates are available) | What is prevalence of NCDs in PLHIV in LMICs? What is the prevalence of the NCDs’ risk factors in PLHIV in LMICs? How can we improve population-level data collection of NCDs and their risk factors? |
| Knowledge of the management of NCDs among PLHIV | Is there sufficient data to provide an accurate assessment of the prevalence of cardiovascular disease cervical cancer, depression, and diabetes among PLHIV in LMICs? What are current evidence-based approaches for NCD management among PLHIV in LMICs? |
| Cost-effectiveness of integration NCD and HIV care | What has already been done to incorporate NCD care into existing HIV care systems and programs in LMICs? How can we identify best practices for the screening, diagnosis, and management, including laboratory monitoring and treatment, of NCDs among PLHIV in LMICs? Is integration of NCD and HIV care cost-effective in LMICs? What are the factors that improve economies of scale? |
| Cardiovascular diseases | |
| Adequate cardiovascular disease risk assessment among PLHIV | What is the best method for assessing cardiovascular disease risk among PLHIV? |
| Impact of lifestyle counseling on cardiovascular disease among PLHIV |
How can cardiovascular disease risk scores be used to prioritize secondary prevention or treatment of PLHIV in LMICs given limited resources? What is the impact of lifestyle counseling on cardiovascular disease and its risk factors in PLHIV in LMICs? Does lower salt intake reduce hypertension and incidence of stroke among PLHIV in sub-Saharan Africa? What is the impact of a low cholesterol diet on cardiovascular disease outcomes among PLHIV? |
| Cervical cancer | |
| Effect of antiretroviral therapy on cervical disease | Does the early initiation of, and improved adherence to, combination antiretroviral therapy reduce cervical disease among HIV-infected women? |
| Development and evaluation of women-centric prevention and treatment | What is the impact of women-centric and women-operated methods (e.g. microbicides and topical agents) for prevention and treatment of HPV related disease? |
| Development of cervical cancer treatment protocols specific to HIV-infected women | What are the best approaches for treatment of cervical cancer with immunosuppressive therapy in HIV-infected patients who may be at high risk for opportunistic illnesses? |
| Depression | |
| Outreach and educational efforts | How can we best utilize outreach and educational efforts in HIV care to integrate culturally competent community education about depression? |
| Focus on stigma reduction | How can we utilize the mental health and HIV-related evidence to enhance stigma reduction interventions for targeted communities and care settings? |
| Use of innovative technologies | What is the impact of innovative technologies (e.g. mobile phones or telehealth interventions, etc.) and information systems on the clinical management of people with co-morbid mental and chronic health conditions in LMICs? |
| Marginalized communities | What is the best approach for marginalized communities and vulnerable subpopulations (e.g. MSM, people with severe mental illness)? |
| Diabetes | |
| Mechanism of diabetes in PLHIV | What are the direct effects of HIV and indirect effects because of HIV-related therapy on metabolic function and glucose homeostasis? |
| Understanding of drug interactions | What are the most relevant drug interactions between glucose-lowering medications and antiretrovirals? Should specific populations be considered? |
| Education and awareness of diabetes and appropriate nutrition | What are themost effective ways to share culturally appropriate information related to diabetes risk factors with special emphasis to PLHIV (explaining, for example, how antiretroviral therapy may increase the risk for diabetes) and dietary information for those at risk for or having diabetes? |
| Lifestyle modification and access to healthy foods | What are the most effective models for community programs addressing diabetes risk factors (i.e. smoking, physical inactivity, and poor diet) and programs addressing poor nutrition (both undernutrition and overnutrition) could be integrated into healthcare facilities to encourage participation and provide access to healthy foods? |
LMICs, low-income and middle-income countries (LMICs); NCD, noncommunicable disease; PLHIV, people living with HIV.
Cardiovascular disease risk factors
The majority of data on cardiovascular disease (CVD) among PLHIV are from high-income countries (HICs) where more experience with ART exists. In LMICs, there is a paucity of data; however, reports of PLHIV developing CVD, such as heart failure [36], stroke [37], and venous thromboembolism [38], at a higher frequency than uninfected persons exist [39]. Studies have shown that HIV is an independent predictor of stroke [40,41]. A combination of potential cardiometabolic effects of HIV infection, including abnormal lipid and glucose metabolism, fat redistribution, a chronic inflammatory milieu, and vascular endothelial dysfunction, may contribute to cardiovascular end-organ disease [39,42,43]. In addition, ART has been associated with development of cardiovascular risk factors and poor cardiovascular outcomes [41,42,44–48], through similar underlying pathophysiologic mechanisms [43,49]. HIV infection is associated with higher triglycerides, and lower HDL, lower BMI, and higher blood pressure; however, ART use, particularly protease inhibitors, seems to increase both LDL and HDL and lower glycated hemoglobin (HbA1C) [46,50]. Therefore, among PLHIV, CVD events are attributed to higher prevalence of both modifiable risk factors, such as obesity, and nonmodifiable risk factors, such as age [51].
In addition, studies of PLHIV have found a high prevalence of several metabolic disorders, which can increase the risk for CVD [48,50–55]. A recent meta-analysis of the prevalence of metabolic syndrome among PLHIV reported estimates of 16–31% depending on the criteria used; these studies were predominantly from HICs [56]. Our pooled estimates for CVD risk factors are hypertension 21.2% (95% CI 16.3–27.1), hypercholesterolemia 22.2% (95% CI 14.7–32.1), elevated LDL 23.2% (95% CI 15.2–33.8), hypertriglyceridemia 27.2% (95% CI 20.7–34.8), low HDL 52.3% (95% CI 35.6–62.8), and obesity 7.8% (95% CI 4.3–13.9; Fig. 2).
Hypertension is the most prevalent risk factor for CVD globally [57,58] and can be detected by standardized methods using an automated sphygmomanometer. Additionally, body mass can be easily assessed using anthropometry. Given their low cost and potential to provide beneficial information for comprehensive HIV care, these screening modalities can be integrated into routine HIV care. Several efforts are underway to integrate hypertension and HIV care (Table, Supplemental Digital Content 2, http://links.lww.com/QAD/B294); however, consistent availability of medications and devices, trained staff, and advanced medical care remain a challenge [59,60]. Therefore, lifestyle counseling advocating for regular exercise, low salt and cholesterol diets, tobacco cessation, and moderate alcohol use, [61] should be prioritized as many CVD risks, notably hypertension, hyperlipidemia, and obesity, are potentially preventable with education and policies to ensure healthy food products and environments [61]. Additionally, measurement for lipid abnormalities are a routine part of HIV care in HICs and should become the standard of care from PLHIV in LMICs. CVD risk scores can facilitate stratification and treatment [62]. Therefore, a standardized assessment of CVD risk among PLHIV should be developed.
Cervical cancer
Women living with HIVare at-risk for human papilloma virus (HPV) disease, particularly cervical cancer [63]. The prevention of cervical cancer among HIV-infected women is becoming a widely recognized public health priority with initiatives like Pink Ribbon-Red Ribbon (http://pinkribbonredribbon.org/). Many studies from LMICs have documented the excess disease burden of HPV-related neoplastic disease among HIV-infected women, particularly in rates of HPV prevalence [64–87], cytologically detected and histologically confirmed precancerous lesions [70,86,88–113], and invasive cancers [84,86,90,94,104,106,107,112], as well as population-based or hospital-based registry-confirmed invasive cervical cancer incidence and mortality rates [63,114–123]. Between 30 and 80% HIV-infected women have prevalent carcinogenic HPV genotypes, 10–40% have prevalent cervical precancerous lesions, and invasive cervical cancer is detected among 1.3–1.7% (Table, Supplemental Digital Content 2, http://links.lww.com/QAD/B294) [64–114]. The wide ranges of these estimates are reflective of the heterogeneity in the underlying ages of the populations being studied, stage of HIV disease and immunosuppression, and methods of diagnosis of HPV infection, precancerous lesions, and cervical cancer.
HIV care and treatment programs have provided an important platform for implementing cervical cancer prevention programs. Visual inspection with acetic acid (VIA) and HPV-testing are currently in use and perform effectively among HIV-infected women in LIMCs [124–131]. The focus of most cervical cancer prevention initiatives in LMICs has been to screen and treat detected precancerous lesions by same-visit treatment approaches (‘screen-and-treat’) without the need for an intermediate pathologic confirmatory step [132]. The most commonly deployed treatment approach is cryotherapy [133], which is often not definitive and recurrences are common, particularly in women with advanced HIV disease, necessitating continued surveillance and follow-up [134]. Women who have large cervical lesions or have lesions extending into the endocervical canal are ineligible to be treated with cryotherapy and excisional approaches such as Loop Electrosurgical Excision Procedure (LEEP) or conization are necessary. The efficacy and feasibility of innovative approaches (e.g. thermocoagulation, nongaseous, and portable devices for cryotherapy) as well as LEEP as the frontline treatment instead of cryotherapy are currently being examined [132]. Given the preventable nature of cervical cancer, related deaths would be averted by expanding access to healthcare to provide life-saving screening and treatment services. The HIV platform has been leveraged over the past decade to offer cervical cancer prevention and treatment services [134–136].
Treatment and management approaches for cervical cancer differ by the stage of presentation. In most LMICs, treatment protocols are consistent with the available local medical resources. Management of locally advanced cervical cancers in HIV-infected women is challenging as it involves the dueling imperatives of curing the underlying malignancy with immunosuppressive chemoradiation while controlling for HIV-related opportunistic infections and minimizing treatment toxicities [136]. Very few studies have specifically examined treatment protocols among HIV-infected women in low-resource settings, and these have been retrospective in nature [137–140]. These studies have demonstrated that HIV-infected women form a large proportion of women seeking care, present with more advanced disease stages, have lower rates of treatment completion, and have higher rates of complications compared with HIV-uninfected women.
Cervical cancer prevention approaches for HIV-infected women continue to be refined. HIV-infected women should be screened more frequently (e.g. annual) according to most clinical guidelines given their increased risk. However, local resource requirements often guide the choice of protocols for screening intensity, triage, follow-up, and surveillance [141].
Depression
Globally, depressive disorders are the largest source of burden of mental health disease [142]. In 2010, an estimated 2.2 million excess deaths occurred among people with major depressive disorder [143]. Depression commonly co-occurs with HIV infection, contributing to greater morbidity and mortality [144,145]. Estimates of the prevalence among people with HIV vary, in part because of the heterogeneity of study methodologies. A recent meta-analysis reported that prevalence of major depressive disorder among PLHIV in SSA was 13.9% (95% CI 9.7–18.6) [16]. Our pooled estimate of moderate-to-severe depression was 24.4% (95% CI 12.5–42.1) (Fig. 2). Persons with preexisting mental disorders, including depression, are also at increased risk of HIV infection, frequently because of unsafe sexual behavior, and for women in particular, coercive sexual encounters [146,147].
Among PLHIV, depression and stigma can poorly affect health outcomes [148,149]. People who are depressed are three times more likely to be nonadherent to ART as compared with those who are not depressed [150]. Depressive symptoms are associated with attrition from care [151], increased sexual risk behavior [152], and some studies suggest a higher rate of mortality [144,153,154]. Screening and treatment for depression are critical to HIV prevention and treatment.
Although PLHIV may experience depressive disorders, these disorders may not be readily recognized by primary HIV care clinicians. Symptoms of major depression such as poor appetite, fatigue, disturbed sleep, psychomotor retardation can overlap with symptoms of HIV disease. Over the past decade, a considerable number of studies have validated tools for depression screening in PLHIV in LMICs. These include the Kessler mental distress scales (6 and 10), the Hopkins Symptom Checklist (HSCL), PHQ-9 [155–157], the Center for Epidemiological Studies Depression Scale (CES-D) [158,159], and the Edinburgh Post-Natal Depression Scale (EPDS) [160]. The EPDS has been programmed into mobile phones and used by community health workers in South Africa during their routine outreach as a means of case finding [16].
Screening for depression must be accompanied by delivery of effective treatment. Collaborative Care is an evidence-based, ‘best practice’ model of care that has been used to effectively treat depression in primary care settings worldwide [161–168]. Within the collaborative care framework [163], providers typically include psychological and/or psychopharmacologic interventions for depression. Three psychological interventions have shown efficacy in LMICs: interpersonal psychotherapy for depression [165], cognitive behavioral therapies [169], and problem-solving therapy [170].
In LMICs, where specialists for mental healthcare are scarce, less specialized providers can be used to effectively deliver evidence-based treatments for depression via task-shifting [160–168,171]. Task-shifting can also extend to treatment with antidepressant medications in some contexts [172]. Two classes of antidepressants are most commonly available in LMICs: tricyclic antidepressants and selective serotonin reuptake inhibitors. Despite the well documented interactions between certain ART and these medications, both classes can effectively reduce depressive symptoms among PLHIV [173].
Diabetes, type 2
The wide range of prevalence of diabetes among PLHIV (1.3–18%; Table, Supplemental Digital Content 2, http://links.lww.com/QAD/B294) reflects actual variation between populations as well as the lack of standardization in criteria used to assess diabetes. Diabetes is a significant cardiovascular disease risk factor and contributes substantial morbidity because of microvascular complications such as blindness, lower limb amputations, and renal failure [174,175]. Increasing age, family history, urbanization, overweight/obesity, and physical inactivity are recognized risk factors for diabetes, but PLHIV are exposed to additional diabetes risk factors as discussed earlier, such as inflammation, which can directly and indirectly affect hormones that mediate insulin sensitivity [176]. Moreover, certain antiretrovirals may be associated with altered fat redistribution, dysglycemia, diabetes, and a predisposition to cardiometabolic disease has been shown to increase with cumulative exposure [176–179].
PLHIV, similar to others in the general population, are exposed to different cultural and socioeconomic factors that increase risk for diabetes. These include deterrents to exercise in some communities [180,181], diets that are rich in carbohydrates [182,183], or the high price and limited accessibility of healthy foods [184–186]. Therefore, the screening and diagnosis of diabetes among PLHIV should be considered in LMICs.
According to the World Health Organization (WHO) diabetes guidelines [187], the recommended laboratory plasma measurements are fasting glucose, random glucose or 2-h post oral glucose tolerance test (OGTT) on appropriately handled samples, and matched calibration of portable devices for diagnostic purposes. A variety of blood glucose meters are available in LMICs [188,189] but protocols to ensure calibration and correct cut-points are necessary. Importantly, the use of HbA1C as a diagnostic test for diabetes cannot be endorsed at present as the recommended cut-point has not been validated for PLHIV; several studies report that in PLHIV, HbA1C levels underestimate glycemic levels, largely because of abacavir use and high mean corpuscular volume of red blood cells [190–193]. Issues regarding ability to obtain appropriate samples, for example, fasting, sample handling, administering OGTT, and implementing standing operating procedures for analytic measurements in clinical laboratories, also impact the ability to diagnose diabetes.
Recent guidelines for diabetes management should be applicable to LMICs [194]. The treatment algorithm contains drugs that are on the WHO essential medicines list [195] and available in most LIMCs but importantly not in the public sector.
Challenges and gaps
Although there are published estimates of prevalence of the four selected NCDs among PLHIV in LMICs (see Table, Supplemental Digital Content 2, http://links.lww.com/QAD/B294), such assessments are often not standardized and the degree of multimorbidity is frequently unknown. Robust data about the prevention and management of NCDs in these resource-limited settings is lacking. Under-resourced health systems with inadequate NCD care infrastructure, diagnostics, interrupted supplies of NCD medications despite their presence on the WHO Essential Medicines List [59], inadequate numbers of NCD specialists to treat complicated cases, and inadequate numbers of trained nonspecialist healthcare workers to deliver evidence-based therapies [196,197] contribute to suboptimal NCD care. These system-level barriers are further complicated by limited health literacy and demand for NCD care [198]. Social stigma, discrimination, and exclusion associated with HIV may hinder provision and seeking of care for NCDs. For depression, specifically, a dearth of culturally competent care [199] and shared cultural beliefs about traditional medicine [200] may also hinder demand. Although numerous studies are underway, main gaps are the lack of cost [201] and outcome data, including mortality, from existing programs in LMICs that integrate NCD and HIV care. Many additional gaps that have been identified through this literature review have determined the research priorities for this field moving forward (Table 2).
Discussion
The present systematic review comes at an unprecedented time in global health when syndemics and their effect on population health are gaining recognition. Public health programs need to consider syndemics to effectively control diseases and improve the health of populations. The syndemic of substance use, violence, and HIV risk has been well characterized and as a result, a public health response has been developed to address all three issues concomitantly [202]. We provide evidence of the emerging syndemic of NCDs and HIV, which would benefit from a response that addresses multimorbidity with integrated care. With the advance of urbanization and globalization in LMICs, an epidemiological transition is occurring; our findings clearly show high prevalence of four NCDs among PLHIV. Ecologically, increases in NCD burden have been noted with increasing HIV prevalence [58]. The resulting double-burden of disease has significant potential for adversely affecting population health and current health systems [58].
To adequately address the needs of PLHIV in whom NCDs and its risk factors co-exist, we propose a research agenda to facilitate NCD and HIV care integration (Table 2). This agenda focuses on research at the population and individual levels and includes an epidemiological, behavioral, and health systems focus [203–205]. It also addresses the call for focus in four main areas: defining the burden of NCDs among PLHIV, understanding the impact of modifiable risk factors, evaluating effective and efficient care strategies at individual and health systems levels, and evaluating cost-effective prevention strategies [206].
Saving the lives of PLHIV but then losing them prematurely to NCDs would be disastrous. Providing NCD care as part of existing and functioning HIV care systems could be logistically simple and inexpensive but requires an evidence-based minimum package for NCD prevention, screening, and management [207] that is appropriate for PLHIV. Many of the health system interventions that were used to scale up ART in resource poor countries, such as standardized treatment protocols and task-shifting, can facilitate effective management of NCDs [208,209]. Also, implementing elements of the ‘DOTS’ framework for tuberculosis control for PLHIV such as registries, which collect clinical information from all patients diagnosed with a certain condition, and cohort monitoring, assessing whether interventions are effective and tracking performance, is prudent. These interventions will improve our understanding of the burden of NCDs among PLHIV and generate information to improve the management of NCDs [210–212]. The HIV platform is well positioned to collect clinical data on PLHIV and implement evidence-based NCD/HIV integrated care, which are needed as PLHIV age and live longer. A shift towards NCD/HIV-integrated care will need continued investments in supply-chain management and the development of point-of-care diagnostics in addition to ensuring that NCD treatments are consistently available [59]. Health systems will need to be adapted for comprehensive chronic care and training of personnel to deliver integrate care will be necessary.
HIV treatment is equally important in the prevention and management of NCDs among PLHIV [213,214]. Therefore, continued focus on test and treat with rapid viral suppression are necessary to improve HIV/NCD outcomes [213,214]. Moreover, integration of NCD and HIV screening and management helps address challenges in controlling the HIV epidemic by providing access to otherwise hard-to-reach populations, such as adult men [215], by decreasing the risk for poor ART adherence, reducing the stigma of HIV, and strengthening health systems to evolve from providing acute care to providing preventive, chronic care. NCD and HIV integration should be prioritized for PLHIV who are stable on ART because effective treatment can render individuals’ a near-normal lifespan with the resulting opportunity of developing chronic comorbidities associated with aging [216]. Use of the differentiated service delivery model – a responsive, client-centered approach that simplifies and adapts HIV services across the HIV care continuum to better serve individual needs and reduce unnecessary burdens on the health system [217] could facilitate the efficient integration and delivery of NCD care among those who would benefit most by focusing on PLHIV stable on ART.
The current review is subject to limitations. We specifically sought to summarize the burden of four NCDs among PLHIV. Therefore, the literature search and subsequent review may have missed data from articles that did not focus on these NCDs. We recognize the increased risk among certain sub-populations, such as persons who had exposure to specific ART, but did not include articles that focused on these persons in our meta-analyses because the pooled estimate would then be inherently biased and overestimated. In addition, we focused on the most prominent risk factors with available prevention intervention. Other risk factors are not included in this summary, given the intent to focus on those that would require medical management and integrated care.
Leveraging past investments into building functional NCD/HIV care systems in LMICs increases the chances of healthy aging among PLHIV and contributes to the targets of the sustainable development goals [218]. Hopefully, these integrated health systems can eventually improve NCD care for the entire population.
Supplementary Material
Acknowledgements
Author contributions:
Conception or design of the work: P.P., C.R., N.L.
Data collection: P.P., S.V.
Data analysis and interpretation: C.R.
Drafting the article: P.P., PC., B.N.-B., V.S., E.P., S.P., N.L.
Critical revision of the article: P.P.
Final approval of the version to be published: P.P., C.R., P.C., B.N.-B., V.S., E.P., S.V., S.P., D.R., N.L.
P.C. completed this work while at the National Institute of Mental Health.
The NIH HIV/NCD Project Disease Condition Technical Operating Group are:
Technical Operating Group (TOG) Leads: P.P. and Linda Kupfer
Depression: Pamela Collins and Dianne Rausch
Diabetes: Naomi S. Levitt, Caroline A. Macera, Bernardo Nuche-Berenguer, Joel Dave, Andrew Bremer
Cardiovascular disease: Emmanuel Peprah, Gerald Bloomfield, Michael Engelgau, Fleetwood Loustalot, Sonak Pastakia
Cervical cancer: Vikrant Sahasrabuddhe, Catherine Godfrey, Geraldina Dominguez, Carol Langley, Doreen Ramogola-Masire, Mona Saraiya
Support staff: Lindsey Templin, S.V., Blythe Beecroft, and Alicia Livinski
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US government.
Financial support: Fogarty International Center, National Institutes of Health.
Source of support: this article as part of the Research to Guide Practice: Enhancing HIV/AIDS Platform to Address Non-Communicable Diseases in sub-Saharan Africa was supported by the U.S. National Institutes of Health Fogarty International Center.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS). Global AIDS Update 2016. Available at: http://www.unaids.org/en/resources/documents/2016/Global-AIDS-update-2016. [Accessed 20 July 2017]
- 2.The HIV-CAUSAL Collaboration. Ray M, Logan R, Sterne JA, Hernandez-Díaz S, Robins JM, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS 2010; 24:123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 2008; 372:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhaskaran K, Hamouda O, Sannes M, Boufassa F, Johnson AM, Lambert PC, et al. , CASCADE Collaboration. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA 2008; 300:51–59. [DOI] [PubMed] [Google Scholar]
- 5.Buchacz K, Baker RK, Palella FJ, Chmiel JS, Lichtenstein KA, Novak RM, et al. , HOPS Investigators. AIDS-defining opportunistic illnesses in US patients, 1994–2007: a cohort study. AIDS 2010; 24:1549–1559. [DOI] [PubMed] [Google Scholar]
- 6.Ferry T, Raffi F, Collin-Filleul F, Dupon M, Dellamonica P, Waldner A, et al. , ANRS CO8 (APROCO-COPILOTE) Study Group. Uncontrolled viral replication as a risk factor for non-AIDS severe clinical events in HIV-infected patients on long-term antiretroviral therapy: APROCO/COPILOTE (ANRS CO8) cohort study. J Acquir Immune Defic Syndr 2009; 51:407–415. [DOI] [PubMed] [Google Scholar]
- 7.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ 2009; 338:a3172. [DOI] [PubMed] [Google Scholar]
- 8.Goulet JL, Fultz SL, Rimland D, Butt A, Gibert C, Rodriguez-Barradas M, et al. Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis 2007; 45:1593–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore RD, Gebo KA, Lucas GM, Keruly JC. Rate of comorbidities not related to HIV infection or AIDS among HIV-infected patients, by CD4 cell count and HAART use status. Clin Infect Dis 2008; 47:1102–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mocroft A, Reiss P, Gasiorowski J, Ledergerber B, Kowalska J, Chiesi A, et al. Serious fatal and nonfatal non-AIDS-defining illnesses in Europe. J Acquir Immune Defic Syndr 2010; 55:262–270. [DOI] [PubMed] [Google Scholar]
- 11.French AL, Gawel SH, Hershow R, Benning L, Hessol NA, Levine AM, et al. Trends in mortality and causes of death among women with HIV in the United States: a 10-year study. J Acquir Immune Defic Syndr 2009; 51:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onen NF, Overton ET, Seyfried W, Stumm ER, Snell M, Mondy K, et al. Aging and HIV infection: a comparison between older HIV-infected persons and the general population. HIV Clinical Trials 2010; 11:100–109. [DOI] [PubMed] [Google Scholar]
- 13.Kingsley LA, Cuervo-Rojas J, Munoz A, Palella FJ, Post W, Witt MD, et al. Subclinical coronary atherosclerosis, HIV infection and antiretroviral therapy: Multicenter AIDS Cohort Study. AIDS 2008; 22:1589–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bing EG, Burnam MA, Longshore D, Fleishman JA, Sher-bourne CD, London AS, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry 2001; 58: 721–728. [DOI] [PubMed] [Google Scholar]
- 15.Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry 2001; 158:25–30. [DOI] [PubMed] [Google Scholar]
- 16.Tsai AC. Reliability and validity of depression assessment among persons with HIV in sub-Saharan Africa: systematic review and meta-analysis. J Acquir Immune Defic Syndr 2014; 66:503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crum-Cianflone NF, Huppler Hullisiek K, Marconi V. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS 2009; 23:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, et al. , Adult and Adolescent Spectrum of Disease Project and HIV Outpatient Study Investigators. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med 2008; 148:728–736. [DOI] [PubMed] [Google Scholar]
- 19.Worm SW, De Wit S, Weber R, Sabin CA, Reiss P, El Sadr W, et al. Diabetes mellitus, preexisting coronary heart disease, and the risk of subsequent coronary heart disease events in patients infected with human immunodeficiency virus: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D Study). Circulation 2009; 119:805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neuhaus J, Jacobs DR, Baker JV, Calmy A, Duprez D, La Rosa A, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 2010; 201:1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wand H, Calmy A, Carey DL, Samaras K, Carr A, Law MG, et al. , INITIO Trial International Coordinating Committee. Metabolic syndrome, cardiovascular disease and type 2 diabetes mellitus after initiation of antiretroviral therapy in HIV infection. AIDS 2007; 21:2445–2453. [DOI] [PubMed] [Google Scholar]
- 22.Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med 2009; 17:118–123. [PubMed] [Google Scholar]
- 23.Lau B, Gange SJ, Moore RD. Risk of non-AIDS-related mortality may exceed risk of AIDS-related mortality among individuals enrolling into care with CD4R counts greater than 200 cells/mm3. J Acquir Immune Defic Syndr 2007; 44: 179–187. [DOI] [PubMed] [Google Scholar]
- 24.DAD Study Group. Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte A, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007; 356:1723–1735. [DOI] [PubMed] [Google Scholar]
- 25.Carr A HIV lipodystrophy: risk factors, pathogenesis, diagnosis and management. AIDS 2003; 17:S141–S148. [PubMed] [Google Scholar]
- 26.Anastos K, Lu D, Shi Q, Tien PC, Kaplan RC, Hessol NA, et al. Association of serum lipid levels with HIV serostatus, specific antiretroviral agents, and treatment regimens. J Acquir Immune Defic Syndr 2007; 45:34–42. [DOI] [PubMed] [Google Scholar]
- 27.Hessol NA, Kalinowski A, Benning L, Mullen J, Young M, Palella F, et al. Mortality among participants in the Multi-center AIDS Cohort Study and the Women’s Interagency HIV Study. Clin Infect Dis 2007; 44:287–294. [DOI] [PubMed] [Google Scholar]
- 28.Lichtenstein KA, Armon C, Buchacz K, Chmiel JS, Buckner K, Tedaldi EM, et al. , HIV Outpatient Study (HOPS) Investigators. Low CD4(R) T cell count is a risk factor for cardiovascular disease events in the HIV Outpatient Study. Clin Infect Dis 2010; 51:435–447. [DOI] [PubMed] [Google Scholar]
- 29.Saves M, Chene G, Ducimetiere P, Leport C, Le Moal G, Amouyel P, et al. , French WHO MONICA Project and the APROCO (ANRS EP11) Study Group. Risk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general population. Clin Infect Dis 2003; 37:292–298. [DOI] [PubMed] [Google Scholar]
- 30.The DAD. Study Group. Friis-Møller N, Reiss P, Sabin CA, Weber R, Monforte Ad, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007; 356:1723–1735. [DOI] [PubMed] [Google Scholar]
- 31.Holmberg SD, Tong TC, Ward DJ, Tong TC, Ward DJ, Wood KC, et al. , HIV Outpatient Study (HOPS) investigators. Protease inhibitor drug use and adverse cardiovascular outcomes in ambulatory HIV-infected persons. Lancet 2002; 360: 1747–1748. [DOI] [PubMed] [Google Scholar]
- 32.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med 2005; 352:48–62. [DOI] [PubMed] [Google Scholar]
- 33.Heaton LM, Bouey PD, Fu J, Stover J, Fowler TB, Lyerla R, Mahy M. Estimating the impact of the US President’s Emergency Plan for AIDS Relief on HIV treatment and prevention programmes in Africa. Sex Transm Infect 2015; 91:615–620. [DOI] [PubMed] [Google Scholar]
- 34.Council on Foreign Relations. The emerging global health crisis: noncommunicable diseases in low- and middle-income countries. Available at: http://www.cfr.org/diseases-noncommunicable/emerging-global-health-crisis/p33883?co=C007301. [Accessed 3 July 2015]
- 35.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Ann Intern Med 2009; 151:W65–W94. [DOI] [PubMed] [Google Scholar]
- 36.Bloomfield GS, Alenezi F, Barasa FA, Lumsden RH, Mayosi BM, Velazquez EJ. Human immunodeficiency virus and heart failure in low- and middle-income countries. JACC Heart Fail 2015; 3:579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bain LE, Kum AP, Ekukwe NC, Clovis NC, Enowbeyang TE. HIV, cardiovascular disease, and stroke in sub-Saharan Africa. Lancet (3):2016:341–342. [DOI] [PubMed] [Google Scholar]
- 38.Ogeng’o JA, Obimbo MM, Olabu BO, Gatonga PM, Ong’era D. Pulmonary thromboembolism in an East African tertiary referral hospital. J Thromb Thrombolysis 2011; 32:386–391. [DOI] [PubMed] [Google Scholar]
- 39.Currier JS, Lundgren JD, Carr A, Klein D, Sabin CA, Sax PE, et al. , Working Group 2. Epidemiological evidence for cardiovascular disease in HIV-infected patients and relationship to highly active antiretroviral therapy. Circulation 2008; 118:e29–e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stroke risk factors in an incident population in urban and rural Tanzania: a prospective, community-based, case-control study. Lancet Glob Health 2013; 1:e282–e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benjamin LA, Corbett EL, Connor MD, Mzinganjira H, Kampondeni S, Choko A, et al. HIV, antiretroviral treatment, hypertension, and stroke in Malawian adults: a case-control study. Neurology 2016; 86:324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin A, Emery S. Metabolic disorders and cardiovascular consequences of HIV infection and antiretroviral therapy. Expert Rev Clin Pharmacol 2009; 2:381–390. [DOI] [PubMed] [Google Scholar]
- 43.Dube MP, Lipshultz SE, Fichtenbaum CJ, Greenberg R, Schecter AD, Fisher SD, Working Group 3. Effects of HIV infection and antiretroviral therapy on the heart and vasculature. Circulation 2008; 118:e36–e40. [DOI] [PubMed] [Google Scholar]
- 44.D:A:D Study Group. Sabin CA, Worm SW, Weber R, Reiss P, El-Sadr W, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV infected patients enrolled in the D:A:D study: a multicohort collaboration. Lancet 2008; 371:1417–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crane HM, Van Rompaey SE, Kitahata MM. Antiretroviral medications associated with elevated blood pressure among patients receiving highly active antiretroviral therapy. AIDS 2006; 20:1019–1026. [DOI] [PubMed] [Google Scholar]
- 46.Dillon DG, Gurdasani D, Riha J, Ekoru K, Asiki G, Mayanja BN, et al. , African Partnership for Chronic Disease Research (APCDR). Association of HIV and ART with cardiometabolic traits in sub-Saharan Africa: a systematic review and meta-analysis. Int J Epidemiol 2013; 42:1754–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Islam FM, Wu J, Jansson J, Wilson DP. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med 2012; 13:453–468. [DOI] [PubMed] [Google Scholar]
- 48.Malaza A, Mossong J, Bärnighausen T, Newell ML. Hypertension and obesity in adults living in a high HIV prevalence rural area in South Africa. PLoS One 2012; 7:e47761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lipshultz SE, Lipshultz SE, Mas CM, Henkel JM, Franco VI, Fisher SD, Miller TL. HAART to heart: highly active antiretroviral therapy and the risk of cardiovascular disease in HIV-infected or exposed children and adults. Expert Rev Anti Infect Ther 2012; 10:661–674. [DOI] [PubMed] [Google Scholar]
- 50.Lake JE, Currier JS. Metabolic disease in HIV infection. Lancet Inf Dis 2013; 13:964–975. [DOI] [PubMed] [Google Scholar]
- 51.Sabin CA, Worm SW. Conventional cardiovascular risk factors in HIV infection: how conventional are they? Curr Opin HIV AIDS 2008; 3:214–219. [DOI] [PubMed] [Google Scholar]
- 52.Ali MK, Magee MJ, Dave JA, Ofotokun I, Tungsiripat M, Jones TK. HIV and metabolic, body, and bone disorders: what we know from low- and middle-income countries. J Acquir Immune Defic Syndr 2014; 67:S27–S39. [DOI] [PubMed] [Google Scholar]
- 53.Julius H, Basu D, Ricci E, Wing J, Basu JK, Pocaterra D, Bonfanti P. The burden of metabolic diseases amongst HIV positive patients on HAART attending The Johannesburg Hospital. Curr HIV Res 2011; 9:247–252. [DOI] [PubMed] [Google Scholar]
- 54.Wrottesley SV, Micklesfield LK, Hamill MM, Goldberg GR, Prentice A, Pettifor JM, et al. Dietary intake and body composition in HIV-positive and -negative South African women. Public Health Nutr 2014; 17:1603–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.George JA, Venter WD, Van Deventer HE, Crowther NJ. A longitudinal study of the changes in body fat and metabolic parameters in a South African population of HIV-positive patients receiving an antiretroviral therapeutic regimen containing stavudine. AIDS Res Hum Retroviruses 2009; 25:771–781. [DOI] [PubMed] [Google Scholar]
- 56.Nguyen KA, Peer N, Mills EJ, Kengne AP. A meta-analysis of the metabolic syndrome prevalence in the global HIV-infected population. PLoS One 2016; 11:e0150970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.GBD 2015 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388:1659–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Angkurawaranon C, Nitsch D, Larke N, Rehman AM, Smeeth L, Addo J. Ecological study of HIV infection and hypertension in sub-Saharan Africa: is there a double burden of disease? PLoS One 2016; 11:e0166375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pastakia SD, Tran DN, Manji I, Wells C, Kinderknect K, Ferris R. Building reliable supply chains for noncommunicable disease commodities: lessons learned from HIV and evidence needs. AIDS 2018; 32 (suppl 1):S55–S62. [DOI] [PubMed] [Google Scholar]
- 60.Juma K, Reid M, Roy M, Vorkoper S, Temu TM, Levitt NS, et al. From HIV prevention to non-communicable disease health promotion efforts in sub-Saharan Africa: A Narrative Review. AIDS 2018; 32 (Suppl 1):S63–S73. [DOI] [PubMed] [Google Scholar]
- 61.Fitch KV, Anderson EJ, Hubbard JL, Carpenter SJ, Waddell WR, Caliendo AM, et al. Effects of a lifestyle modification program in HIV-infected patients with the metabolic syndrome. AIDS 2006; 20:1843–1850. [DOI] [PubMed] [Google Scholar]
- 62.Rabkin M, Mutiti A, Chung C, Zhang Y, Wei Y, El-Sadr WM. Missed opportunities to address cardiovascular disease risk factors amongst adults attending an urban HIV clinic in South Africa. PLoS One 2015; 10:e0140298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Denny LA, Franceschi S, de Sanjose S, Heard I, Moscicki AB, Palefsky J. Human papillomavirus, human immunodeficiency virus and immunosuppression. Vaccine 2012; 30 (Suppl 5):F168–F174. [DOI] [PubMed] [Google Scholar]
- 64.Clifford GM, Goncalves MA, Franceschi S, HPV and HIV Study Group. Human papillomavirus types among women infected with HIV: a meta-analysis. AIDS 2006; 20:2337–2344. [DOI] [PubMed] [Google Scholar]
- 65.Sahasrabuddhe VV, Mwanahamuntu MH, Vermund SH, Huh WK, Lyon MD, Stringer JS, Parham GP. Prevalence and distribution of HPV genotypes among HIV-infected women in Zambia. Br J Cancer 2007; 96:1480–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramogola-Masire D, McGrath CM, Barnhart KT, Friedman HM, Zetola NM. Subtype distribution of human papilloma-virus in HIV-infected women with cervical intraepithelial neoplasia stages 2 and 3 in Botswana. Int J Gynecol Pathol 2011; 30:591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Odida M, Sandin S, Mirembe F, Kleter B, Quint W, Weiderpass E. HPV types, HIV and invasive cervical carcinoma risk in Kampala,;1; Uganda: a case-control study. Infect Agent Cancer 2011; 6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Aardt MC, Dreyer G, Pienaar HF, Karlsen F, Hovland S, Richter KL, Becker P. Unique human papillomavirus-type distribution in South african women with invasive cervical cancer and the effect of human immunodeficiency virus infection. Int J Gynecol Cancer 2015; 25:919–925. [DOI] [PubMed] [Google Scholar]
- 69.McDonald AC, Tergas AI, Kuhn L, Denny L, Wright TC Jr. Distribution of human papillomavirus genotypes among HIV-positive and HIV-negative women in Cape Town, South Africa. Front Oncol 2014; 4:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adler DH, Wallace M, Bennie T, Mrubata M, Abar B, Meiring TL, et al. Cervical dysplasia and high-risk human papilloma-virus infections among HIV-infected and HIV-uninfected adolescent females in South Africa. Infect Dis Obstet Gynecol 2014; 2014:498048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Musa J, Taiwo B, Achenbach C, Olugbenga S, Berzins B, Sagay AS, et al. High-risk human papillomavirus among HIV-infected women with normal cervical cytology: a pilot study in Jos, Nigeria. Arch Gynecol Obstet 2013; 288:1365–1370. [DOI] [PubMed] [Google Scholar]
- 72.Dartell M, Rasch V, Kahesa C, Mwaiselage J, Ngoma T, Junge J, et al. Human papillomavirus prevalence and type distribution in 3603 HIV-positive and HIV-negative women in the general population of Tanzania: the PROTECT study. Sex Transm Dis 2012; 39:201–208. [DOI] [PubMed] [Google Scholar]
- 73.De Vuyst H, Mugo NR, Chung MH, McKenzie KP, Nyongesa-Malava E, Tenet V, et al. Prevalence and determinants of human papillomavirus infection and cervical lesions in HIV-positive women in Kenya. Br J Cancer 2012; 107: 1624–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Vuyst H, Gichangi P, Estambale B, Njuguna E, Franceschi S, Temmerman M. Human papillomavirus types in women with invasive cervical carcinoma by HIV status in Kenya. Int J Cancer 2008; 122:244–246. [DOI] [PubMed] [Google Scholar]
- 75.Icenogle JP, Laga M, Miller D, Manoka AT, Tucker RA, Reeves WC. Genotypes and sequence variants of human papilloma-virus DNAs from human immunodeficiency virus type 1-infected women with cervical intraepithelial neoplasia. J Infect Dis 1992; 166:1210–1216. [DOI] [PubMed] [Google Scholar]
- 76.Reddy D, Njala J, Stocker P, Schooley A, Flores M, Tseng CH, et al. High-risk human papillomavirus in HIV infected women undergoing cervical cancer screening in Lilongwe, Malawi: a pilot study. Int J STD AIDS 2015; 26:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zohoncon TM, Bisseye C, Djigma FW, Yonli AT, Compaore TR, Sagna T, et al. Prevalence of HPV high-risk genotypes in three cohorts of women in Ouagadougou (Burkina Faso). Mediterr J Hematol Infect Dis 2013; 5:e2013059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Akarolo-Anthony SN, Al-Mujtaba M, Famooto AO, Dareng EO, Olaniyan OB, Offiong R, et al. HIV associated high-risk HPV infection among Nigerian women. BMC Infect Dis 2013; 13:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ndiaye C, Alemany L, Ndiaye N, Kamate B, Diop Y, Odida M, et al. Human papillomavirus distribution in invasive cervical carcinoma in sub-Saharan Africa: could HIV explain the differences? Trop Med Int Health 2012; 17:1432–1440. [DOI] [PubMed] [Google Scholar]
- 80.Macleod IJ, O’Donnell B, Moyo S, Lockman S, Shapiro RL, Kayembe M, et al. Prevalence of human papillomavirus genotypes and associated cervical squamous intraepithelial lesions in HIV-infected women in Botswana. J Med Virol 2011; 83:1689–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luque AE, Hitti J, Mwachari C, Lane C, Messing S, Cohn SE, et al. Prevalence of human papillomavirus genotypes in HIV-1-infected women in Seattle, USA and Nairobi, Kenya: results from the Women’s HIV Interdisciplinary Network (WHIN). Int J Infect Dis 2010; 14:e810–e814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luchters SM, Vanden Broeck D, Chersich MF, Nel A, Delva W, Mandaliya K, et al. Association of HIV infection with distribution and viral load of HPV types in Kenya: a survey with 820 female sex workers. BMC Infect Dis 2010; 10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blossom DB, Beigi RH, Farrell JJ, Mackay W, Qadadri B, Brown DR. Human papillomavirus genotypes associated with cervical cytologic abnormalities and HIV infection in Ugandan women. J Med Virol 2007; 79:758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Didelot-Rousseau MN, Nagot N, Costes-Martineau V, Valles X, Ouedraogo A, Konate I, et al. , Yerelon Study Group. Human papillomavirus genotype distribution and cervical squamous intraepithelial lesions among high-risk women with and without HIV-1 infection in Burkina Faso. Br J Cancer 2006; 95:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baay MF, Kjetland EF, Ndhlovu PD, Deschoolmeester V, Mduluza T, Gomo E, et al. Human papillomavirus in a rural community in Zimbabwe: the impact of HIV co-infection on HPV genotype distribution. J Med Virol 2004; 73:481–485. [DOI] [PubMed] [Google Scholar]
- 86.Firnhaber C, Zungu K, Levin S, Michelow P, Montaner LJ, McPhail P, et al. Diverse and high prevalence of human papillomavirus associated with a significant high rate of cervical dysplasia in human immunodeficiency virus infected women in Johannesburg, South Africa. Acta Cytol 2009; 53:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ng’andwe C, Lowe JJ, Richards PJ, Hause L, Wood C, Angeletti PC. The distribution of sexually-transmitted human papillomaviruses in HIV positive and negative patients in Zambia, Africa. BMC Infect Dis 2007; 7:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Parham GP, Sahasrabuddhe VV, Mwanahamuntu MH, Shepherd BE, Hicks ML, Stringer EM, Vermund SH. Prevalence and predictors of squamous intraepithelial lesions of the cervix in HIV-infected women in Lusaka, Zambia. Gynecol Oncol 2006; 103:1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Firnhaber C, Westreich D, Schulze D, Williams S, Siminya M, Michelow P, et al. Highly active antiretroviral therapy and cervical dysplasia in HIV-positive women in South Africa. J Int AIDS Soc 2012; 15:17382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Temmerman M, Tyndall MW, Kidula N, Claeys P, Muchiri L, Quint W, et al. Risk factors for human papillomavirus and cervical precancerous lesions, and the role of concurrent HIV-1 infection. Int J Gynaecol Obstet 1999; 65:171–181. [DOI] [PubMed] [Google Scholar]
- 91.Leroy V, Ladner J, De Clercq A, Meheus A, Nyiraziraje M, Karita E, Dabis F. Cervical dysplasia and HIV type 1 infection in African pregnant women: a cross sectional study, Kigali, Rwanda. The Pregnancy and HIV Study Group (EGE). Sex Transm Infect 1999; 75:103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.La Ruche G, Ramon R, Mensah-Ado I, Bergeron C, Diomande M, Sylla-Koko F, et al. Squamous intraepithelial lesions of the cervix, invasive cervical carcinoma, and immunosuppression induced by human immunodeficiency virus in Africa. Dyscer-CI Group. Cancer 1998; 82:2401–2408. [PubMed] [Google Scholar]
- 93.Motti PG, Dallabetta GA, Daniel RW, Canner JK, Chiphangwi JD, Liomba GN, et al. Cervical abnormalities, human papillomavirus, and human immunodeficiency virus infections in women in Malawi. J Infect Dis 1996; 173:714–717. [DOI] [PubMed] [Google Scholar]
- 94.Langley CL, Benga-De E, Critchlow CW, Ndoye I, Mbengue-Ly MD, Kuypers J, et al. HIV-1, HIV-2, human papillomavirus infection and cervical neoplasia in high-risk African women. AIDS 1996; 10:413–417. [DOI] [PubMed] [Google Scholar]
- 95.Maggwa BN, Hunter DJ, Mbugua S, Tukei P, Mati JK. The relationship between HIV infection and cervical intraepithelial neoplasia among women attending two family planning clinics in Nairobi. Kenya AIDS 1993; 7:733–738. [DOI] [PubMed] [Google Scholar]
- 96.ter Meulen J, Eberhardt HC, Luande J, Mgaya HN, Chang-Claude J, Mtiro H, et al. Human papillomavirus (HPV) infection, HIV infection and cervical cancer in Tanzania, east Africa. Int J Cancer 1992; 51:515–521. [DOI] [PubMed] [Google Scholar]
- 97.Kreiss JK, Kiviat NB, Plummer FA, Roberts PL, Waiyaki P, Ngugi E, et al. Human immunodeficiency virus, human papillomavirus, and cervical intraepithelial neoplasia in Nairobi prostitutes. Sex Transm Dis 1992; 19:54–59. [DOI] [PubMed] [Google Scholar]
- 98.Chama CM, Nggada H, Gashau W. Cervical dysplasia in HIV infected women in Maiduguri, Nigeria. J Obstet Gynaecol 2005; 25:286–288. [DOI] [PubMed] [Google Scholar]
- 99.Hawes SE, Critchlow CW, Faye Niang MA, Diouf MB, Diop A, Tour e P, et al. Increased risk of high-grade cervical squamous intraepithelial lesions and invasive cervical cancer among African women with human immunodeficiency virus type 1 and 2 infections. J Infect Dis 2003; 188:555–563. [DOI] [PubMed] [Google Scholar]
- 100.Chirenje ZM, Loeb L, Mwale M, Nyamapfeni P, Kamba M, Padian N. Association of cervical SIL and HIV-1 infection among Zimbabwean women in an HIV/STI prevention study. Int J STD AIDS 2002; 13:765–768. [DOI] [PubMed] [Google Scholar]
- 101.Hawes SE, Critchlow CW, Sow PS, Toure P, N’Doye I, Diop A, et al. Incident high-grade squamous intraepithelial lesions in Senegalese women with and without human immunodeficiency virus type 1 (HIV-1) and HIV-2. J Natl Cancer Inst 2006; 98:100–109. [DOI] [PubMed] [Google Scholar]
- 102.Kafuruki L, Rambau PF, Massinde A, Masalu N. Prevalence and predictors of cervical intraepithelial neoplasia among HIV infected women at Bugando Medical Centre, Mwanza-Tanzania. Infect Agent Cancer 2013; 8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gedefaw A, Astatkie A, Tessema GA. The prevalence of precancerous cervical cancer lesion among HIV-infected women in southern Ethiopia: a cross-sectional study. PLoS One 2013; 8:e84519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Atashili J, Adimora AA, Ndumbe PM, Ikomey GM, Rinas AC, Myers E. High prevalence of cervical squamous intraepithelial lesions in women on antiretroviral therapy in Cameroon: Istargeted screening feasible? Cancer Epidemiol 2012; 36:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Firnhaber C, Van Le H, Pettifor A, Schulze D,Michelow P, Sanne IM, et al. Association between cervical dysplasia and human papillomavirus in HIV seropositive women from Johannesburg South Africa. Cancer Causes Control 2010; 21:433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Anastos K, Hoover DR, Burk RD, Cajigas A, Shi Q, Singh DK, et al. Risk factors for cervical precancer and cancer in HIV-infected, HPV-positive Rwandan women. PLoS One 2010; 5:e13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gaym A, Mashego M, Kharsany AB, Walldorf J, Frohlich J, Karim QA. High prevalence of abnormal Pap smears among young women co-infected with HIV in rural South Africa -implications for cervical cancer screening policies in high HIV prevalence populations. S Afr Med J 2007; 97:120–123. [PubMed] [Google Scholar]
- 108.Anorlu RI, Igwilo CI, Akanmu AS, Banjo AA, Odunukwe NN, Okany CC, et al. Prevalence of abnormal cervical smears among patients with HIV in Lagos, Nigeria. West Afr J Med 2007; 26:143–147. [PubMed] [Google Scholar]
- 109.Ononogbu U, Almujtaba M, Modibbo F, Lawal I, Offiong R, Olaniyan O, et al. Cervical cancer risk factors among HIV-infected Nigerian women. BMC Public Health 2013; 13:582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu E, McCree R, Mtisi E, Fawzi WW, Aris E, Lema IA, et al. Prevalence and risk factors of cervical squamous intraepithelial lesions among HIV-infected women in Dar es Salaam, Tanzania. Int J STD AIDS 2016; 27:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Okonda S, Wright C, Michelow P. The status of cervical cytology in Swaziland, Southern Africa: a descriptive study. Cytojournal 2009; 6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jaquet A, Horo A, Ekouevi DK, Toure B, Coffie PA, Effi B, et al. , IeDEA West Africa Collaboration. Risk factors for cervical intraepithelial neoplasia in HIV-infected women on antiretroviral treatment in Cote d’Ivoire, West Africa. PLoS One 2014; 9:e90625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jaquet A, Horo A, Charbonneau V, Ekouevi DK, Roncin L, Toure B, et al. , IeDEA West Africa collaboration. Cervical human papillomavirus and HIV infection in women of child-bearing age in Abidjan, Cote d’Ivoire. Br J Cancer 2012; 107:556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mungo C, Cohen CR, Maloba M, Bukusi EA, Huchko MJ. Prevalence, characteristics, and outcomes of HIV-positive women diagnosed with invasive cancer of the cervix in Kenya. Int J Gynaecol Obstet 2013; 123:231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mutyaba I, Phipps W, Krantz EM, Goldman JD, Nambooze S, Orem J, et al. A Population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999 – 2008. J Acquir Immune Defic Syndr 2015; 69:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Coghill AE, Newcomb PA, Madeleine MM, Richardson BA, Mutyaba I, Okuku F, et al. Contribution of HIV infection to mortality among cancer patients in Uganda. AIDS 2013; 27:2933–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chokunonga E, Borok MZ, Chirenje ZM, Nyakabau AM, Parkin DM. Trends in the incidence of cancer in the black population of Harare, Zimbabwe 1991–2010. Int J Cancer 2013; 133:721–729. [DOI] [PubMed] [Google Scholar]
- 118.Mbulaiteye SM, Katabira ET, Wabinga H, Parkin DM, Virgo P, Ochai R, et al. Spectrum of cancers among HIV infected persons in Africa: the Uganda AIDS-Cancer Registry Match Study. Int J Cancer 2006; 118:985–990. [DOI] [PubMed] [Google Scholar]
- 119.Wabinga H, Ramanakumar AV, Banura C, Luwaga A, Nam-booze S, Parkin DM. Survival of cervix cancer patients in Kampala, Uganda: 1995–1997. Br J Cancer 2003; 89:65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sitas F, Pacella-Norman R, Carrara H, Patel M, Ruff P, Donde B, et al. The spectrum of HIV-1 related cancers in South Africa. Int J Cancer 2000; 88:489–492. [DOI] [PubMed] [Google Scholar]
- 121.Wabinga HR, Parkin DM, Wabwire-Mangen F, Mugerwa JW. Cancer in Kampala, Uganda, in 1989–1991: changes in incidence in the era of AIDS. Int J Cancer 1993; 54: 26–36. [DOI] [PubMed] [Google Scholar]
- 122.Akarolo-Anthony SN, Maso LD, Igbinoba F, Mbulaiteye SM, Adebamowo CA. Cancer burden among HIV-positive persons in Nigeria: preliminary findings from the Nigerian AIDS-cancer match study. Infect Agent Cancer 2014; 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tanon A, Jaquet A, Ekouevi DK, Akakpo J, Adoubi I, Diomande I, IeDEA West Africa Collaboration. The spectrum of cancers in West Africa: associations with human immunodeficiency virus. PLoS One 2012; 7:e48108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mabeya H, Khozaim K, Liu T, Orango O, Chumba D, et al. Comparison of conventional cervical cytology versus visual inspection with acetic acid among human immunodeficiency virus-infected women in Western Kenya. J Low Genit Tract Dis 2012; 16:92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chung MH, McKenzie KP, De Vuyst H, Richardson BA, Rana F, Pamnani R, et al. Comparing Papanicolau smear, visual inspection with acetic acid and human papillomavirus cervical cancer screening methods among HIV-positive women by immune status and antiretroviral therapy. AIDS 2013; 27:2909–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Firnhaber C, Mayisela N, Mao L, Williams S, Swarts A, Faesen M, et al. Validation of cervical cancer screening methods in HIV positive women from Johannesburg South Africa. PLoS One 2013; 8:e53494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dartell MA, Rasch V, Iftner T, Kahesa C, Mwaiselage JD, Junge J. Performance of visual inspection with acetic acid and human papillomavirus testing for detection of high-grade cervical lesions in HIV positive and HIV negative Tanzanian women. Int J Cancer 2014; 135:896–904. [DOI] [PubMed] [Google Scholar]
- 128.Huchko MJ, Sneden J, Sawaya G, Smith-McCune K, Maloba M, Abdulrahim N, et al. Accuracy of visual inspection with acetic acid to detect cervical cancer precursors among HIV-infected women in Kenya. Int J Cancer 2015; 136:392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bateman AC, Parham GP, Sahasrabuddhe VV, Mwanahamuntu MH, Kapambwe S, Katundu K, et al. Clinical performance of digital cervicography and cytology for cervical cancer screening in HIV-infected women in Lusaka, Zambia. J Acquir Immune Defic Syndr 2014; 67:212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kuhn L, Wang C, Tsai WY, Wright TC, Denny L. Efficacy of human papillomavirus-based screen-and treat for cervical cancer prevention among HIV-infected women. AIDS 2010; 24:2553–2561. [DOI] [PubMed] [Google Scholar]
- 131.Bansil P, Lim J, Byamugisha J, Kumakech E, Nakisige C, Jeronimo JA. Performance of cervical cancer screening techniques in HIV-infected women in Uganda. J Low Genit Tract Dis 2015; 19:215–219. [DOI] [PubMed] [Google Scholar]
- 132.Forhan SE, Godfrey CC, Watts DH, Langley CL. A systematic review of the effects of visual inspection with acetic acid, cryotherapy, and loop electrosurgical excision procedures for cervical dysplasia in HIV-infected women in low- and middle-income countries. J Acquir Immune Defic Syndr 2015; 68 (Suppl 3):S350–S356. [DOI] [PubMed] [Google Scholar]
- 133.Mwanahamuntu MH, Sahasrabuddhe VV, Pfaendler KS, Mudenda V, Hicks M, Vermund SH, et al. Implementation of ‘seeand-treat’ cervical cancer prevention services linked to HIV care in Zambia. AIDS 2009; 23:N1–N5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mwanahamuntu MH, Sahasrabuddhe VV, Kapambwe S, Pfaendler KS, Chibwesha C, Mkumba FF G, et al. Advancing cervical cancer prevention initiatives in resource-constrained settings: insights from the Cervical Cancer Prevention Program in Zambia. PLoS Med 2011; 8:e1001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Oluwole D, Kraemer J. Innovative public-private partnership: a diagonal approach to combating women’s cancers in Africa. Bull World Health Organ 2013; 91:691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mbulaiteye SM, Bhatia K, Adebamowo C, Sasco AJ. HIV and cancer in Africa: mutual collaboration between HIV and cancer programs may provide timely research and public health data. Infect Agent Cancer 2011; 6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Simonds HM, Neugut AI, Jacobson JS. HIV status and acute hematologic toxicity among patients with cervix cancer undergoing radical chemoradiation. Int J Gynecol Cancer 2015; 25:884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Simonds HM, Wright JD, du Toit N, Neugut AI, Jacobson JS. Completion of and early response to chemoradiation among human immunodeficiency virus (HIV)-positive and HIV-negative patients with locally advanced cervical carcinoma in South Africa. Cancer 2012; 118:2971–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Adewuyi SA, Shittu OS, Rafindadi AH, Zayyan MS, Samaila MO, Oguntayo AO. Cisplatin chemotherapy for haemostasis in bleeding cervical cancer: experience from a resource-poor setting. Niger Postgrad Med J 2010; 17:122–127. [PubMed] [Google Scholar]
- 140.Moodley M Radical hysterectomy for cervical cancer amongst women infected with the human immunodeficiency virus. Int J Gynecol Cancer 2007; 17:1264–1265. [DOI] [PubMed] [Google Scholar]
- 141.Sahasrabuddhe VV, Parham GP, Mwanahamuntu MH, Vermund SH. Cervical cancer prevention in low-and middle-income countries: feasible, affordable, essential. Cancer Prev Res (Phila) 2012; 5:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Whiteford HA, Degenhardt L, Rehm, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 2013; 382: 1575–1586. [DOI] [PubMed] [Google Scholar]
- 143.Patel V, Chisholm D, Parikh R, Charlson FJ, Degenhardt L, Dua T, et al. , DCP MNS authors group. Global priorities for addressing the burden of mental, neurological, and substance use disorders In: Patel V, Chisholm D, Dua T, Laxminarayan R, Medina-Mora ME,editors. Mental, neurological, and substance use disorders: disease control priorities. 3rd ed (Volume 4) Washington DC: 2016 International Bank for Reconstruction and Development/The World Bank; 2016. [PubMed] [Google Scholar]
- 144.Antelman G, Kaaya S, Wei R, Mbwambo J, Msamanga GI, Fawzi WW, Fawzi MCS. Depressive symptoms increase risk of HIV disease progression and mortality among women in Tanzania. J Acquir Immune Defic Syndr 2007; 44:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chibanda D, Benjamin L, Weiss HA, Abas M. Mental, neurological, and substance use disorders in people living with HIV/AIDS in low-and middle-income countries. J Acquir Immune Defic Syndr 2014; 67:S54–S67. [DOI] [PubMed] [Google Scholar]
- 146.Collins PY, Holman AR, Freeman MC, Patel V. What is the relevance of mental health to HIV/AIDS care and treatment programs in developing countries? A systematic review. AIDS 2006; 20:1571–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hughes E, Bassi S, Gilbody S, Bland M, Martin F. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness: a systematic review and meta-analysis. Lancet Psychiatry 2016; 3:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Uthman OA, Magidson JF, Safren SA, Nachega JB. Depression and adherence to antiretroviral therapy in low-, middle-and high-income countries: a systematic review and meta-analysis. Curr HIV/AIDS Rep 2014; 11:291–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mayston R, Kinyanda E, Chishinga N, Prince M, Patel V. Mental disorder and the outcome of HIV/AIDS in low-income and middle-income countries: a systematic review. AIDS 2012; 26 (Suppl 2):S117–S135. [DOI] [PubMed] [Google Scholar]
- 150.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 2000; 160:2101–2107. [DOI] [PubMed] [Google Scholar]
- 151.Krumme AA, Kaigamba F, Binagwaho A, Murray MB, Rich ML, Franke MF. Depression, adherence and attrition from care in HIV-infected adults receiving antiretroviral therapy. J Epidemiol Community Health 2015; 69:284–289. [DOI] [PubMed] [Google Scholar]
- 152.Musisi S, Wagner GJ, Ghosh-Dastidar B, Nakasujja N, Dickens A, Okello E. Depression and sexual risk behaviour among clients about to start HIV antiretroviral therapy in Uganda. Int J STD AIDS 2014; 25:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sudfeld CR, Kaaya S, Gunaratna NS, Mugusi F, Fawzi WW, Aboud S, et al. Depression at antiretroviral therapy initiation and clinical outcomes among a cohort of Tanzanian women living with HIV. AIDS 2017; 31:263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Todd JV, Cole SR, Pence BW, Lesko CR, Bacchetti P, Cohen MH, et al. Effects of antiretroviral therapy and depressive symptoms on all-cause mortality among HIV-infected women. Am J Epidemiol 2017; 185:869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cholera R, Gaynes BN, Pence BW, Bassett J, Qangule N, Macphail C, Miller WC. Validity of the patient health questionnaire-9 to screen for depression in a high-HIV burden primary healthcare clinic in Johannesburg, South Africa. J Affect Disord 2014; 167:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bhana A, Rathod SD, Selohilwe O, Kathree T, Petersen I. The validity of the Patient Health Questionnaire for screening depression in chronic care patients in primary healthcare in South Africa. BMC psychiatry 2015; 15:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Monahan PO, Shacham E, Reece M, Kroenke K, Ong’or WO, Omollo O, Ojwang C. Validity/reliability of PHQ-9 and PHQ-2 depression scales among adults living with HIV/AIDS in western Kenya. J Gen Intern Med 2009; 24:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Akena D, Joska J, Obuku EA, Stein DJ. Sensitivity and specificity of clinician administered screening instruments in detecting depression among HIV-positive individuals in Uganda. AIDS Care 2013; 25:1245–1252. [DOI] [PubMed] [Google Scholar]
- 159.Chishinga N, Kinyanda E, Weiss HA, Patel V, Ayles H, Seedat S. Validation of brief screening tools for depressive and alcohol use disorders among TB and HIV patients in primary care in Zambia. BMC Psychiatry 2011; 11:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sweetland AC, Belkin GS, Verdeli H. Measuring depression and anxiety in sub-Saharan Africa. Depress anxiety 2014; 31:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Araya R, Alvarado R, Minoletti A. Chile: an ongoing mental health revolution. Lancet 2009; 374:597–598. [DOI] [PubMed] [Google Scholar]
- 162.Araya R, Rojas G, Fritsch R, Gaete J, Rojas M, Simon G, Peters TJ. Treating depression in primary care in low-income women in Santiago, Chile: a randomised controlled trial. Lancet 2003; 361:995–1000. [DOI] [PubMed] [Google Scholar]
- 163.Archer J, Bower P, Gilbody S, Lovell K, Richards D, Gask L, Coventry P. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012; 10:CD006525. [DOI] [PubMed] [Google Scholar]
- 164.Bass J, Neugebauer R, Clougherty KF, Verdeli H, Wickramaratne P, Ndogoni L, Bolton P. Group interpersonal psychotherapy for depression in rural Uganda: 6-month outcomes. Br J Psychiatry 2006; 188:567–573. [DOI] [PubMed] [Google Scholar]
- 165.Bolton P, Bass J, Neugebauer R, Verdeli H, Clougherty KF, Wickramaratne P, Weissman M. Group interpersonal psychotherapy for depression in rural Uganda: a randomized controlled trial. JAMA 2003; 289:3117–3124. [DOI] [PubMed] [Google Scholar]
- 166.Ngo D, Gibbons JL, Scire G, Le D. Mental health needs in Vietnamese American Communities affected by the Gulf Oil Spill. Psychology 2014; 5:109. [Google Scholar]
- 167.Honikman S, van Heyningen T, Field S, Baron E, Tomlinson M. Stepped care for maternal mental health: a case study of the perinatal mental health project in South Africa. PLoS Med 2012; 9:e1001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rahman A, Malik A, Sikander S, Roberts C, Creed F. Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised controlled trial. Lancet 2008; 372:902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nakimuli-Mpungu E, Wamala K, Okello J, Alderman S, Odokonyero R, Mojtabai R, Musisi S. Group support psychotherapy for depression treatment in people with HIV/AIDS in northern Uganda: a single-centre randomised controlled trial. Lancet HIV 2015; 2:e190–e199. [DOI] [PubMed] [Google Scholar]
- 170.Chibanda D, Cowan FM, Healy JL, Abas M, Lund C. Psychological interventions for common mental disorders for people living with HIV in low-and middle-income countries: Systematic review. Trop Med Int Health 2015; 21:198–1208. [DOI] [PubMed] [Google Scholar]
- 171.Singla DR, Weobong B, Nadkarni A, Chowdhary N, Shinde S, Anand A, Patel V. Improving the scalability of psychological treatments in developing countries: An evaluation of peer-led therapy quality assessment in Goa, India. Behav Res Ther 2014; 60:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wagner GJ, Ngo V, Goutam P, Glick P, Musisi S, Akena D. A structured protocol model of depression care versus clinical acumen: a cluster randomized trial of the effects on depression screening, diagnostic evaluation, and treatment uptake in Ugandan HIV Clinics. PLoS One 2016; 11:e0153132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at: http://www.aidsinfo.nih.gov/Content-Files/AdultandAdolescentGL.pdf. [Accessed 30 April 2017]
- 174.Levitt NS. Diabetes in Africa: epidemiology, management and healthcare challenges. Heart 2008; 94:1376–1382. [DOI] [PubMed] [Google Scholar]
- 175.Dalal S, Beunza JJ, Volmink J, Adebamowo C, Bajunirwe F, Njelekela M, et al. Noncommunicable diseases in sub-Saharan Africa: what we now know. Int J Epidemiol 2011; 40:885–901. [DOI] [PubMed] [Google Scholar]
- 176.Alencastro PR, Fuchs SC, Wolff FH, Ikeda ML, Brandão AB, Barcellos NT. Independent predictors of metabolic syndrome in HIV-infected patients. AIDS Patient Care STDS 2011; 25:627–634. [DOI] [PubMed] [Google Scholar]
- 177.Dave JA, Lambert EV, Badri M, West S, Maartens G, Levitt NS. Effect of nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy on dysglycemia and insulin sensitivity in South African HIV-infected patients. J Acquired Immune Defic Syndr 2011; 57:284–289. [DOI] [PubMed] [Google Scholar]
- 178.Tesfaye DY, Kinde S, Medhin G, Megerssa YC, Tadewos A, Tadesse E, Shimelis T. Burden of metabolic syndrome among HIV-infected patients in Southern Ethiopia. Diabetes Metab Syndr 2014; 8:102–107. [DOI] [PubMed] [Google Scholar]
- 179.Karamchand S, Leisegang R, Schomaker M, Maartens G, Walters L, Hislop M, et al. Risk factors for incident diabetes in a cohort taking first-line nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy. Medicine (Baltimore) 2016; 95:e2844–e2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Van Rooijen AJ, Rheeder P, Eales CJ, Becker PJ. Effect of exercise versus relaxation on haemoglobin A1C in black females with type 2 diabetes mellitus. QJM 2004; 97:343–351. [DOI] [PubMed] [Google Scholar]
- 181.Kiawi E, Edwards R, Shu J, Unwin N, Kamadjeu R, Mbanya JC. Knowledge, attitudes, and behavior relating to diabetes and its main risk factors among urban residents in Cameroon: a qualitative survey. Ethn Dis 2006; 16:503–509. [PubMed] [Google Scholar]
- 182.Baumann LC, Opio CK, Otim M, Olson L, Ellison S. Self-care beliefs and behaviors in Ugandan adults with type 2 diabetes. Diabetes Educ 2010; 36:293–300. [DOI] [PubMed] [Google Scholar]
- 183.BeLue R, Diaw M, Ndao F, Okoror T, Degboe A, Abiero B. A cultural lens to understanding daily experiences with type 2 diabetes self-management among clinic patients in M’bour, Senegal. Int Q Community Health Educ 2012–2013; 33: 329–347. [DOI] [PubMed] [Google Scholar]
- 184.Muhihi A, Gimbi D, Njelekela M, Shemaghembe E, Mwambene K, Chiwanga F, et al. Consumption and acceptability of whole grain staples for lowering markers of diabetes risk among overweight and obese Tanzanian adults. Global Health 2013; 9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Frank LK, Kröger J, Schulze MB, Bedu-Addo G, Mockenhaupt FP, Danquah I. Dietary patterns in urban Ghana and risk of type 2 diabetes. Br J Nutr 2014; 112:89–98. [DOI] [PubMed] [Google Scholar]
- 186.Delisle H, Ntandou-Bouzitou G, Agueh V, Sodjinou R, Fayomi B. Urbanisation, nutrition transition and cardiometabolic risk: The Benin study. Br J Nutr 2012; 107:1534–1544. [DOI] [PubMed] [Google Scholar]
- 187.WHO. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Report of a WHO/IDF consultation.: http://who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf. [Accessed 12 April 2017]
- 188.Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Kirk-man MS, et al. , National Academy of Clinical Biochemistry; Evidence-Based Laboratory Medicine Committee of the American Association for Clinical Chemistry. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care 2011; 34:e661–e699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sacks DB. A1C versus glucose testing: a comparison. Diabetes Care 2011; 34:518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Polgreen P, Putz D, Stapleton JT. Inaccurate glycosylated hemoglobin A1C measurements in human immunodeficiency virus-positive patients with diabetes mellitus. Clin Infect Dis 2003; 37:e53–e56. [DOI] [PubMed] [Google Scholar]
- 191.Diop ME, Bastard JP, Meunier N, Th evenet S, Maachi M, Capeau J, et al. Inappropriately low glycated hemoglobin values and hemolysis in HIV-infected patients. AIDS Res Hum Retroviruses 2006; 22:1242–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kim P, Woods C, Georgoff P, Crum D, Rosenberg A, Smith M, Hadigan C. A1C underestimates glycemia in HIV infection. Diabetes Care 2009; 32:1591–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Glesby M, Hoover D, Shi Q, Danoff A, Howard A, Tien P, et al. Glycated haemoglobin in diabetic women with and without HIV infection: Data from the Women’s Interagency HIV Study. Antivir Ther 2010; 15:571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.European AIDS Clinical Society guidelines. Available at: http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.htmlguideline. [Accessed 13 April 2017]
- 195.WHO Model List of Essential Medicines. Available at: http://www.who.int/medicines/publications/essentialmedicines/EML_2015_FINAL_amended_NOV2015.pdf?ua=1. [Accessed 13 April 2017]
- 196.Charlson FJ, Diminic S, Lund C, Degenhardt L, Whiteford HA. Mental and substance use disorders in Sub-Saharan Africa: predictions of epidemiological changes and mental health workforce requirements for the next 40 years. PLoS One 2014; 9:e110208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rabkin M, de Pinho H, Michaels-Strasser S, Naitore D, Rawat A, Topp SM. Strengthening the health workforce to support integration of HIV and noncommunicable disease services in sub-Saharan Africa. AIDS 2018; 32 (Suppl 1): S47–S54. [DOI] [PubMed] [Google Scholar]
- 198.Mall S, Sorsdahl K, Swartz L, Joska J. I understand just a little. . .’ Perspectives of HIV/AIDS service providers in South Africa of providing mental healthcare for people living with HIV/AIDS. AIDS care 2012; 24:319–323. [DOI] [PubMed] [Google Scholar]
- 199.Collins PY, Musisi S, Frehywot S, Patel V. The core competencies for mental, neurological, and substance use disorder care in sub-Saharan Africa. Glob Health Action 2015; 8:26682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mascayano F, Armijo JE, Yang LH. Addressing stigma relating to mental illness in low-and middle-income countries. Front Psychiatry 2015; 6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nugent R, Barnabas RV, Golovaty I, Osetinsky B, Roberts DA, Bisson C, Courtney L, et al. Costs and cost-effectiveness of HIV/noncommunicable disease integration in Africa: from theory to practice. AIDS 2018; 32 (Suppl 1):S83–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Menderhall E, Kohrt BA, Norris SA, Ndetei D, Prabhakaran D. Noncommunicable disease syndemics: poverty, depression, and diabetes among low-income populations. Lancet 2017; 389:951–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Geldsetzer P, Manne-Goehler J, Barnighausen T, Davies JI. What research us needed to address the co-epidemics of HIV and cardiometabolic disease in SSA. Lancet Diabetes Endocrinol 2017; 6:7–9. [DOI] [PubMed] [Google Scholar]
- 204.Ebrahim S, Pearce N, Smeeth L, Casas JP, Jaffar S, Piot P. Tackling non-communicable diseases in low- and middle-income countries: is the evidence from high-income countries all we need? PLoS Med 2013; 10:e1001377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Vorkoper S, Kupfer LE, Anand N, Patel P, Beecroft B, Tierney WM, et al. Building on the HIV chronic care platform to address noncommunicable diseases in sub-Saharan Africa: a research agenda. AIDS 2018; 32 (Supp 1):S107–S113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Petersen M, Yiannoutsos CT, Justice A, Egger M. Observational research on NCDs in HIV positive populations: conceptual and methodological considerations. J Acquir Immune Defic Syndr 2014; 67 (Suppl 1):S8–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.WHO. Package of essential noncommunicable (PEN) disease interventions for primary healthcare in low-resource settings. Geneva: World Health Organization, 2010. Available at: http://www.who.int/nmh/publications/essential_ncd_interventions_lr_settings.pdf. [Google Scholar]
- 208.Angell SY, DeCock KM, Frieden TR. A public health approach to global management of hypertension. Lancet 2015; 385:825–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Patel P, Ordunez P, DiPette D, Escobar MC, Hassell T, Wyss F, et al. , Standardized Hypertension Treatment and Prevention Network. Improved blood pressure control to reduce cardiovascular disease morbidity and mortality: the Standardized Hypertension Treatment and Prevention Project. J Clin Hypertens (Greenwich) 2016; 18:1284–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Allain TJ, van Oosterhout JJ, Douglas GP, Joukes S, Gadabu OJ, Darts C, et al. Applying lessons learnt for ‘DOTS’ Tuberculosis Model to monitoring and evaluating persons with diabetes mellitus in Blantyre, Malawi. Tropical Med Int Health 2011; 16:1077–1084. [DOI] [PubMed] [Google Scholar]
- 211.Harries AD, Zachariah R, Jahn A, Schouten EJ, Kamoto K. Scaling up antiretroviral therapy in Malawi - implications for managing other chronic diseases in resource-limited countries. J Acquir Immune Defic Syndr 2009; 52: S14–S16. [DOI] [PubMed] [Google Scholar]
- 212.Mullins J Cohort reporting improves hypertension care for refugees. Lancet 2012; 380:552. [DOI] [PubMed] [Google Scholar]
- 213.Deeks SG, Lewin SR, Havir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013; 382: 1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Geldsetzer P, Feigl AB, Tanser F, Gareta D, Pillay D, Bärnighausen T. Population-level decline in BMI and systolic blood pressure following mass HIV treatment: evidence from rural KwaZulu-Natal. Obesity (Silver Spring) 2017; 25:200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sharma M, Ying R, Tarr G, Barnabas R. Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in SSA. Nature 2015; 528: S77–S85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.PEPAFR. Differentiated care. Available at: http://www.differ-entiatedcare.org/about. [Accessed 10 April 2017]
- 218.United Nations. Sustainable development goals. Available at: http://www.un.org/sustainabledevelopment/. [Accessed 13 April, 2017]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.